Abstract
Osteoporosis is a condition causing significant morbidity and mortality in the elderly population worldwide. Age-related testosterone deficiency is the most important factor of bone loss in elderly men. Androgen can influence bone health by binding to androgen receptors directly or to estrogen receptors (ERs) indirectly via aromatization to estrogen. This review summarized the direct and indirect effects of androgens on bone derived from in vitro, in vivo, and human studies. Cellular studies showed that androgen stimulated the proliferation of preosteoblasts and differentiation of osteoblasts. The converted estrogen suppressed osteoclast formation and resorption activity by blocking the receptor activator of nuclear factor k-B ligand pathway. In animal studies, activation of androgen and ERα, but not ERβ, was shown to be important in acquisition and maintenance of bone mass. Human epidemiological studies demonstrated a significant relationship between estrogen and testosterone in bone mineral density and fracture risk, but the relative significance between the two remained debatable. Human experimental studies showed that estrogen was needed in suppressing bone resorption, but both androgen and estrogen were indispensable for bone formation. As a conclusion, maintaining optimal level of androgen is essential in preventing osteoporosis and its complications in elderly men.
Introduction
Osteoporosis is a progressive metabolic bone disease characterized by reduced bone mass and destructive bone microstructural changes, resulting in bone fragility and increased fracture risk.Citation1,Citation2 It is a significant global health care issue with an expanding prevalence of nine million patients suffering from osteoporotic fractures, in which 1.6 million are suffering from hip fractures, 1.7 million from lower arm fractures, and 1.4 million from vertebral fractures.Citation3 Osteoporotic fracture-associated morbidity and mortality have significant societal impact due to the various medical, social, and financial implications on the patients.Citation4
Osteoporosis is a common condition that afflicts both genders, with the lifetime risk of fracture at ≥50 years being 50% for women and 20% for men.Citation5–Citation7 The major cause of osteoporosis in women is estrogen deficiency due to menopause, while in men, age-related testosterone deficiency.Citation8 Despite the higher prevalence in women, men are more prone to be associated with disability and death due to osteoporotic fractures compared to women.Citation9 The Dubbo Osteoporosis study demonstrated that death rate 5 years after fracture and second fracture in men aged 60 years (standardized mortality ratio [SMR]: 1.78, 95% confidence interval [CI]: 0.96–3.31) was higher compared to their women counterpart (SMR: 1.41, 95% CI: 1.01–1.97).Citation10 The incidence of osteoporotic fractures in men increases exponentially later in their life.Citation11 This is accompanied by a parallel decrease in their bioavailable testosterone level.Citation12 Osteoporosis is also an important side effect of androgen deprivation therapy aimed to treat prostate cancer in men.Citation13,Citation14 These observations highlight the importance of testosterone in maintaining optimum bone health in men. This review gathered important evidence from cellular, animal, and human studies to present a comprehensive view on the role of testosterone in maintaining bone health, particularly in elderly men.
Androgen and androgen receptor
“Androgen” is a broad term encompassing testosterone and its precursors which are C19 metabolites of cholesterol. The predominant gonadal androgen in men is testosterone, 95% of which is secreted by the testes. The remaining 5% is produced by the adrenals by the conversion of dehydroepiandrosterone.Citation15 Testosterone is bound by sex hormone-binding globulin (SHBG) and albumin with strong and weak bonding in blood subsequently.Citation16 Effects of testosterone on the body are mediated by its local conversion to 5α-dihydrotestosterone by peripheral tissues, which has a greater affinity for androgen receptors (ARs).Citation17,Citation18
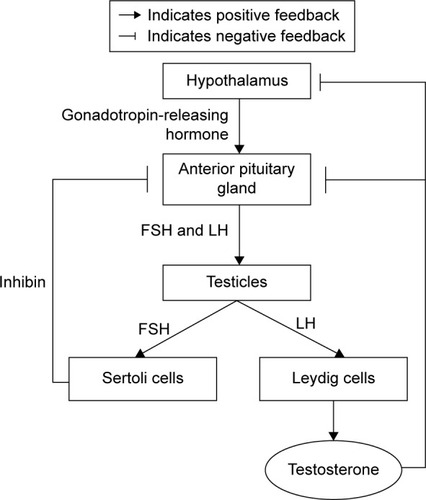
Androgen synthesis is controlled by the pulsatile release of hypothalamic gonadotropin-releasing hormone (GnRH). At the pituitary gland, GnRH stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the general circulation. Both FSH and LH are released by the anterior pituitary gland. FSH stimulates Sertoli cells to support spermatogenesis and secrete inhibin B, which negatively regulates FSH secretion. Meanwhile, LH is needed for the Leydig cells in the testes to produce testosterone. Testosterone stimulates sperm production and virilization, in addition to providing feedback to the hypothalamus and pituitary to regulate GnRH secretion via negative feedback mechanism ().Citation19
Figure 1 The synthesis of testosterone.
Testosterone possesses strong androgenic and anabolic effects that are important for both women and men, despite that men produce significantly more testosterone than women.Citation20 For example, bone growth and maintenance are significantly influenced by testosterone. Previous studies showed that testosterone administration increased the width of epiphyseal growth plate of growing rats directly.Citation21,Citation22 This effect was independent of growth hormone and insulin-like growth factor-1.Citation21,Citation22 The effects of testosterone in maintaining bone mineral density (BMD) in elderly men are well known and have been summarized by previous authors.Citation16,Citation23,Citation24 Thus, androgens take part in building the skeleton of young men and help to prevent bone loss in the elderly men.Citation25 Furthermore, testosterone is metabolized via the cytochrome P450 aromatase enzyme into 17β-estradiol. The aromatization process is also important for bone.Citation26 Several case reports indicated that men with mutations in the estrogen receptor (ER) or aromatase genes suffered from severe osteoporosis,Citation27,Citation28 suggesting that at least part of the activities of testosterone on the male skeleton are exerted by its aromatization to estradiol.Citation29
Sex steroid hormones act on their target cells by binding to members of the nuclear hormone receptor superfamily. Androgens bind to the AR, while estrogens bind to ERα or ERβ. ARs are expressed in bone marrow cell and growth plate.Citation30,Citation31 In a study, ARs were expressed in the proliferative and early hypertrophic chondrocytes of sexually mature rats, and only in prehypertrophic chondrocytes in older rats.Citation32 Male rats had increased AR expression in the growth plate and metaphyseal bone with higher mRNA- and protein-staining intensities, as well as preferential nuclear staining during sexual maturation, compared to female rats.Citation32 This suggests that direct actions of androgens in chondrocytes and in bone-forming cells may be involved in establishing the gender differences in the skeleton. However, there is no difference in receptor expression in the growth plate chondrocytes of human males or females.Citation33,Citation34 ERs α and β are expressed in the human growth plate. This suggested that androgens possibly affect pubertal growth or epiphyseal closure indirectly via aromatization to estrogen.Citation35,Citation36
The effects of testosterone on bone cells
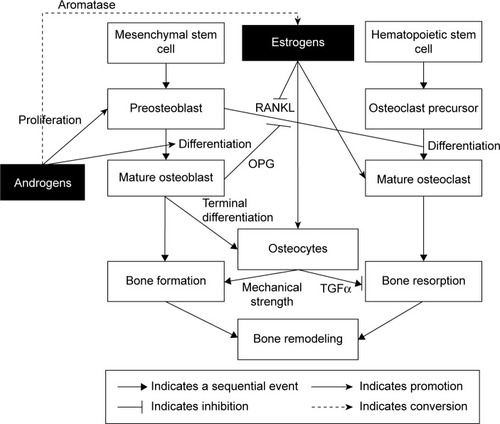
Integrity of our skeletal system is maintained by an intricate process named remodeling which in turn is governed by three major types of bone cells: bone-forming osteoblasts, bone-resorbing osteoclasts, and mechanosensor/mediator osteocytes. These bone cells are sensitive to signaling conveyed through hormones, cytokines, minerals, and dietary components. Some of these cells express ARs and ERs, and thus are responsive to both sex hormones. Dysfunction of these cells leads to dysregulation of the remodeling process. In the case of osteoporosis, the rate of bone resorption is greater than bone formation, leading to net bone loss.Citation37,Citation38
Osteoblasts
Osteoblasts are specialized bone-forming cells that have an essential role in bone remodeling, such as production of bone matrix proteins as well as bone mineralization.Citation39 Osteoblasts are developed from pluripotent mesenchymal stem cells under the direction of a characterized suite of regulatory transcription factors, such as osterix and runt-related factor 2.Citation40 The term “osteoblasts” actually refers to a diverse cellular population that includes immature osteoblast lineage cells as well as differentiating and mature matrix-producing osteoblasts. The mature osteoblasts are responsible for matrix mineralization, while the immature ones regulate formation of osteoclasts.Citation41–Citation43
ARs have been identified in cultured human fetal osteoblasts utilizing a nuclear-binding assay.Citation44 Subsequent studies identified AR mRNA and protein in osteoblasts. Almost all studies demonstrated that androgen upregulated the expression of ARs in osteoblasts.Citation40,Citation45 Stromal cells (precursors of osteoblasts), megakaryocytes, and endothelial cells in the bone marrow express ARs.Citation30,Citation31 Studies showed that both testosterone and 5α-dihydrotestosterone stimulated proliferation of cultured osteoblast precursors in distinctive species.Citation46,Citation47 In addition, androgens have been shown to suppress the apoptosis of osteoblasts.Citation48,Citation49 Androgens stimulated interleukin-1β production and enhanced the mitogenic effect of fibroblast growth factor in cultured osteoblasts.Citation50,Citation51 Further studies demonstrated that androgens exerted stimulatory, inhibitory, or no effects on the expression of osteoblast markers, such as osteocalcin, collagen type 1 alpha 1, and alkaline phosphatase, and mineralization of extracellular bone matrix. However, the evidence generally suggested that androgens stimulate differentiation of osteoblasts.Citation52–Citation55
Osteoclasts
Osteoclasts originate from the colony-forming unit-granulocyte macrophage hematopoietic cell lineage in the bone marrow. Osteoclast differentiation entails contact with stromal cells of the osteoblastic lineage in bone marrow microenvironment, and stimulation by receptor activator of nuclear factor k-B ligand (RANKL) released by immature osteoblasts, which binds to RANK on osteoclasts.Citation56 The effects of RANKL on osteoclasts are tightly regulated by osteoprotegerin (OPG), a decoy receptor of RANKL, also secreted by osteoblast precursors.Citation57
The proliferation of osteoclasts after orchidectomy is most likely due to androgen deficiency. Androgens exert their bone-defensive effects indirectly through osteoblastic cells. Orchidectomy will cause the proliferation of osteoblast precursors which secrete RANKL that stimulates osteoclast proliferation and activation, thus resulting in bone loss.Citation58 In vitro studies showed that dihydrotestosterone interacted with ARs on osteoclasts and inhibited bone resorption in human, murine, and avian osteoclasts.Citation59 In other cell culture studies, androgens were shown to regulate osteoclast formation and survival associated with RANKL pathway, and also to inhibit OPG mRNA levels and protein secretion by osteoblastic cells.Citation38,Citation57,Citation59
Osteocytes
During bone formation, a portion of osteoblasts undergoes terminal separation and entombment by mineralized osteoid, subsequently transforming to osteocytes.Citation60 Osteocytes are cased in liquid-filled holes (lacunae) inside the mineralized bone and are found in abundance, representing 90%–95% of the total bone cells.Citation61 Osteocytes possess long dendrite-like processes that cooperate with other osteocytes inside the mineralized bone and interact with osteoblasts on the bone surface.Citation62 Osteocytes modulate bone remodeling by responding to mechanical stimuli to prevent accumulation of bone microdamage.Citation63,Citation64
In vitro studies suggested that ERα or ERβ translated mechanical forces into prosurvival signals in osteocytes and osteoblasts, independent of estrogens.Citation65 Estradiol was shown to prevent osteocytes apoptosis and enhance the production of transforming growth factor-alpha which inhibits osteoclastic bone resorption.Citation66 Thus, estrogens converted from testosterone in male could contribute to the anabolic action of osteocytes.
The effects of androgens and estrogens on bone cells are summarized in .
The effects of testosterone on animals
Many animal studies have been performed to further investigate the relative effects of androgen and estrogen on bone. Orchidectomized rodent is a well-characterized animal model for osteoporosis. Following orchidectomy, bone resorption increases at cancellous and endocortical surfaces, thus reducing cancellous and cortical bone volume. Besides, periosteal bone formation during growth is decreased in orchidectomized rodents. These effects translate to a lower bone strength.Citation67
Several studies investigated the bone phenotypic changes induced by androgens, nonaromatizable androgens, and estrogens in orchidectomized rodents. These studies revealed that nonaromatizable androgens stimulated periosteal bone formation and inhibited cancellous bone resorption in orchidectomized rats, although the effect is lesser compared to testosterone and estradiol.Citation68 Despites this, it is unclear to what extent the effects of these hormones are pharmacological or physiological, and whether these physiological effects can be extrapolated to the human condition, as estradiol concentration is higher in mice.Citation69
Other animal studies on bone phenotype of transgenic male animals with knockout (KO) of AR, ERα or ERβ, or both have been investigated. These studies manipulated the genetic conditions of the mice, in combination with orchidectomy and replacement of androgens and estrogens. The evidence suggests that activation of ER but not AR is involved in longitudinal appendicular skeletal growth in male mice.Citation70 Besides, studies in knockout mice again suggest that both ERα and AR are involved in enhancing cortical radial bone growth. Both AR and ERα can independently mediate the cancellous bone-sparing effects of sex steroids in male mice, but not ERβ.Citation71,Citation72 These studies have greatly increased the understanding of the role of ERs and ARs in male skeletal growth. The dual mode of action of testosterone is exerted either directly by the AR or indirectly by the ERα through aromatization.
In conclusion, animal evidence suggests that testosterone exerts anabolic effects on different bone surfaces of AR with activation of both ERα and AR on cortical bone and muscle mass; ERβ, on the other hand, seems not to be significantly related to bone growth and maintenance in male mice.
The relationship between testosterone and bone in human studies
The effects of testosterone on bone health in humans can be measured in terms of BMD and fracture risk. On the other hand, bone formation and resorption markers offer insight on the individual processes that constitute bone remodeling. While association obtained in observational studies is hypothesis generating at best, some experimental studies have been performed to give us direct evidence on the importance of bone health in humans.
Many prospective studies have demonstrated that the risk of fragility fracture increases progressively as BMD decreases.Citation73 The Framingham cohort studies established that femoral and radial BMD decreased significantly with age in both men and women (n=1,154, aged 68–98 years).Citation74 The decline of BMD is probably due to age-related loss of trabecular and cortical bone.Citation75 However, prior to this slow phase of bone loss, degeneration of the cancellous bone is sexually dimorphic, in which rapid-accelerated bone loss after menopause only occurs in women.Citation76 In men, cancellous bone loss occurs much later and gradually in life, especially after the age of 70 years.Citation77
The associations between circulating testosterone and estrogen, and BMD and fracture in elderly men have been demonstrated in many studies. The US cohort of the Osteoporotic Fractures in Men Study examined the associations between nonvertebral fracture risk and bioavailable testosterone, bioavailable estradiol, and SHBG of elderly men (n=5,995, aged ≥65 years, follow-up duration =4.7 years). Each sex hormone was associated with fracture risk, but the combination of low bioavailable testosterone, low bioavailable estradiol, and high SHBG predicted fracture risk the best.Citation78 In another cohort study of elderly men in the People’s Republic of China (n=1,448, aged ≥65 years, follow-up duration =4 years), associations between serum total testosterone, free testosterone, estradiol, bioavailable estradiol, SHBG, BMD, and incident fractures were examined. The results showed that low serum estradiol levels were associated with elevated bone loss and increased risk of fractures.Citation79 Similar to the American cohort, the combination of the lowest quartile of free testosterone and bioavailable estradiol and the highest two quartiles of SHBG predicted incident fracture the best.Citation79 This suggests that the association between sex hormones and bone health is similar across different populations. The relative importance of androgens and estrogens in maintaining bone health in the elderly remains debatable. Serum testosterone and estrogens were found to be associated significantly with fracture in elderly men (n=609, median follow-up duration =5.8 years) in the Dubbo Osteoporosis Epidemiology Study. After adjustment for confounding factors, only the relationship between serum testosterone and fracture remained significant.Citation80 The Sweden cohort of the Osteoporotic Fractures in Men Study found otherwise, whereby low level of free estradiol, but not free testosterone, was significantly associated with fracture risk in older men (n=2,639, average follow-up duration =3.3 years).Citation81
Interestingly, the relationship between SHBG and bone is dependent on age in men. A study showed that increase in SHBG with age was associated with bone loss in elderly men.Citation78 However, in younger men, the relationship between peak bone mass and SHBG was positive rather than negative.Citation82–Citation84 We postulate that the higher level of SHBG in young men is reflective of higher circulating testosterone level, thus explaining the positive relationship. However, a higher SHBG level is not accompanied by a higher testosterone level in older men because the negative feedback mechanism is dysregulated. Thus, the ensuing decrease in the bioavailability of testosterone causes the deterioration of bone health. Results on testosterone concentrations were less consistent compared to estradiol level in relationship with bone loss or fractures in men, except for very low levels in hypogonadal men, especially those undergoing chemical and surgical castration. They had an increase in bone turnover, bone loss, and fracture risk.Citation85
While association obtained in observational studies is hypothesis generating at best, experimental studies in human have been performed to provide direct evidence on the importance of bone health in humans. In an experimental study, young men aged between 20 and 44 years were divided into three groups. The first group received only a GnRH analog to suppress endogenous sex hormone production, while the second group received a GnRH analog plus testosterone, and the third group, a GnRH analog plus an aromatase inhibitor, to prevent the conversion of testosterone to estrogen. The results showed that bone resorption markers were increased in the groups receiving GnRH analog alone and GnRH analog plus aromatase inhibitor, indicating that estrogen is necessary in suppressing bone resorption. The level of bone formation markers increased greatly in the group receiving GnRH analog alone than the group receiving GnRH analog and testosterone. Overall, these findings suggest that both estrogens and androgens play independent and fundamental roles in regulating bone resorption in men.Citation86
Another study showed that bone resorption markers increased significantly in the absence of both hormones and were unchanged in men when receiving both testosterone and estrogen. Although estrogen prevented the increase in bone resorption markers, testosterone did not exert similar effects. Meanwhile, the bone formation marker, serum osteocalcin, decreased in the absence of both hormones, and its level reverted back to normal with replenishment of either estrogen or testosterone. This study postulated that estrogen may be essential in regulating bone resorption, and both estrogen and testosterone may be critical in maintaining bone formation.Citation87
Besides, a study demonstrated that a 12-week administration of aromatase inhibitor in elderly men with hypogonadism increased testosterone and reduced estrogen levels but did not affect biochemical markers of bone formation (osteocalcin and amino-terminal propeptide of type 1 collagen), serum OPG, and total body BMD significantly.Citation88 However, administration of aromatase inhibitor for 12 months resulted in a decrease in BMD.Citation89 Thus, it proved the important role of estrogens and androgens in male bone metabolism (bone biochemical markers) when induced with aromatase inhibitor.
Conclusion
The current evidence suggests that circulating androgens and estrogens are protective of bone. However, the relative importance between androgens and their derived estrogens in bone is still debatable. Experimental data suggest that androgens influence bone directly via interactions with ARs, and indirectly via binding to ERα and ERβ after aromatization in adipose or different tissues. Androgens, directly or indirectly through estrogens, preserve trabecular bone principally by diminishing osteoclastogenesis, and both hormones counteract osteoblast apoptosis and stimulate osteoclast apoptosis.
Acknowledgments
The authors would like to thank Universiti Kebangsaan Malaysia for funding the study via GGPM-2015-036 and FF-2016-008.
Disclosure
The authors report no conflicts of interest in this work.
References
- DelmasPDTreatment of postmenopausal osteoporosisLancet200235993222018202612076571
- Tranquilli LealiPMuresuFMelisARuggiuAZachosACarlo DoriaCSkeletal fragility definitionClin Cases Miner Bone Metab201182111322461808
- JohnellOKanisJAn estimate of the worldwide prevalence and disability associated with osteoporotic fracturesOsteoporos Int200617121726173316983459
- OkaHYoshimuraNKinoshitaHSaigaAKawaguchiHNakamuraKDecreased activities of daily living and associations with bone loss among aged residents in a rural Japanese community: the Miyama StudyJ Bone Miner Metab200624430731316816925
- MeltonLJAtkinsonEJO’ConnorMKO’FallonWMRiggsBLBone density and fracture risk in menJ Bone Miner Res19981312191519239844110
- MeltonJLPerspectives: how many women have osteoporosis now?J Bone Miner Res19951021751777754796
- KanisJJohnellOOdenALong-term risk of osteoporotic fracture in MalmöOsteoporos Int200011866967411095169
- RiggsBLKhoslaSMeltonLJ3rdSex steroids and the construction and conservation of the adult skeletonEndocr Rev200223327930212050121
- CenterJRNguyenTVSchneiderDSambrookPNEismanJAMortality after all major types of osteoporotic fracture in men and women: an observational studyLancet1999353915687888210093980
- BliucDNguyenNDMilchVENguyenTVEismanJACenterJRMortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and womenJAMA2009301551352119190316
- LeeJ-KKhirASMThe incidence of hip fracture in Malaysians above 50 years of age: variation in different ethnic groupsAPLAR J Rheum2007104300305
- ChinK-YSoelaimanI-NMohamedINSex hormones in Malay and Chinese men in Malaysia: are there age and race differences?Clinics (Sao Paulo)201368215916623525310
- RossRWSmallEJOsteoporosis in men treated with androgen deprivation therapy for prostate cancerJ Urol200216751952195611956415
- DiamondTHHiganoCSSmithMRGuiseTASingerFROsteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapiesCancer2004100589289914983482
- VanderschuerenDLaurentMRClaessensFSex steroid actions in male boneEndocr Rev201435690696025202834
- ChinKYIma-NirwanaSSex steroids and bone health status in menInt J Endocrinol2012201220871923150727
- KaufmanJMVermeulenAThe decline of androgen levels in elderly men and its clinical and therapeutic implicationsEndocr Rev200526683387615901667
- LabrieFCusanLGomezJLComparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancerJ Steroid Biochem Mol Biol20091131525619073258
- FraiettaRZylberstejnDSEstevesSCHypogonadotropic hypogonadism revisitedClinics (Sao Paulo)201368Suppl 1818823503957
- TorjesenPASandnesLSerum testosterone in women as measured by an automated immunoassay and a RIAClin Chem200450367867914981046
- PhillipMMaorGAssaSSilbergeldASegevYTestosterone stimulates growth of tibial epiphyseal growth plate and insulin-like growth factor-1 receptor abundance in hypophysectomized and castrated ratsEndocrine20011611611822821
- RenSGMalozowskiSSanchezPSweetDELoriauxDLCassorlaFDirect administration of testosterone increases rat tibial epiphyseal growth plate widthActa Endocrinol198912134014052800918
- FinkelsteinJSNeerRMBillerBMCrawfordJDKlibanskiAOsteopenia in men with a history of delayed pubertyN Engl J Med199232696006041734250
- AndersonFFrancisRSelbyPCooperCSex hormones and osteoporosis in menCalcif Tissue Int19986231851889501948
- AlbrightFReifensteinECMetabolic bone disease: osteoporosisAlbrightFReifensteinECThe parathyroid glands and metabolic bone diseaseBaltimoreWilliams and Wilkins1948145204
- GennariLNutiRBilezikianJPAromatase activity and bone homeostasis in menJ Clin Endocrinol Metab200489125898590715579733
- KhoslaSMeltonLJIIIRiggsBLEstrogen and the male skeletonJ Clin Endocrinol Metab20028741443145011932262
- SmithRCorticosteroids and osteoporosisThorax19904585735782205946
- CaraniCQinKSimoniMEffect of testosterone and estradiol in a man with aromatase deficiencyN Engl J Med1997337291959211678
- BraidmanIPHaineyLBatraGSelbyPLSaundersPTHoylandJALocalization of estrogen receptor β protein expression in adult human boneJ Bone Miner Res200116221422011204421
- GruberRCzerwenkaKWolfFHoG-MWillheimMPeterlikMExpression of the vitamin D receptor, of estrogen and thyroid hormone receptor α- and β-isoforms, and of the androgen receptor in cultures of native mouse bone marrow and of stromal/osteoblastic cellsBone199924546547310321906
- van der EerdenBvan TilNBrinkmannALowikCWitJKarperienMGender differences in expression of androgen receptor in tibial growth plate and metaphyseal bone of the ratBone200230689189612052459
- CarrascosaAAudiLFerrandezMBallabrigaABiological effects of androgens and identification of specific dihydrotestosterone-binding sites in cultured human fetal epiphyseal chondrocytesJ Clin Endocrinol Metab19907011341402294127
- Ben-HurHTholeHMashiahAEstrogen, progesterone and testosterone receptors in human fetal cartilaginous tissue: immunohistochemical studiesCalcif Tissue Int19976065205269164826
- BordSHornerABeavanSCompstonJEstrogen receptors α and β are differentially expressed in developing human bone 1J Clin Endocrinol Metab20018652309231411344243
- NilssonLOBomanASävendahlLDemonstration of estrogen receptor-β immunoreactivity in human growth plate cartilageJ Clin Endocrinol Metab19998413703739920110
- FengXMcDonaldJMDisorders of bone remodelingAnnu Rev Pathol2011612114520936937
- HadjidakisDJAndroulakisIIBone remodelingAnn N Y Acad Sci20061092138539617308163
- KarsentyGTranscriptional control of skeletogenesisAnnu Rev Genomics Hum Genet2008918319618767962
- RaggattLJPartridgeNCCellular and molecular mechanisms of bone remodelingJ Biol Chem201028533251032510820501658
- CorralDAAmlingMPriemelMDissociation between bone resorption and bone formation in osteopenic transgenic miceProc Natl Acad Sci U S A1998952313835138409811887
- GoriFHofbauerLCDunstanCRSpelsbergTCKhoslaSRiggsBLThe expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated 1Endocrinology2000141124768477611108292
- KomoriTYagiHNomuraSTargeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblastsCell19978957557649182763
- ColvardDSEriksenEFKeetingPEIdentification of androgen receptors in normal human osteoblast-like cellsProc Natl Acad Sci U S A19898638548572915981
- KasperkCHelmboldtABörcsökISkeletal site-dependent expression of the androgen receptor in human osteoblastic cell populationsCalcif Tissue Int19976164644739383273
- DamienEPriceJLanyonLMechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as femalesJ Bone Miner Res200015112169217711092397
- NakanoYMorimotoIIshidaOThe receptor, metabolism and effects of androgen in osteoblastic MC3T3-E1 cellsBone Miner19942632452597819831
- AlmeidaMHanLAmbroginiEBartellSMManolagasSCOxidative stress stimulates apoptosis and activates NF-κB in osteoblastic cells via a PKCβ/p66shc signaling cascade: counter regulation by estrogens or androgensMol Endocrinol201024102030203720685851
- WirenKMToombsARSemiraleAAZhangXOsteoblast and osteocyte apoptosis associated with androgen action in bone: requirement of increased Bax/Bcl-2 ratioBone200638563765116413235
- KeetingPERifasLHarrisSAEvidence for interleukin-1β production by cultured normal human osteoblast-like cellsJ Bone Miner Res1991688278331785373
- KasperkCFitzsimmonsRStrongDStudies of the mechanism by which androgens enhance mitogenesis and differentiation in bone cellsJ Clin Endocrinol Metab1990715132213292229290
- KasperkCHWergedalJEFarleyJRLinkhartTATurnerRTBaylinkDJAndrogens directly stimulate proliferation of bone cells in vitroEndocrinology19891243157615782521824
- KasperkCHWakleyGKHierlTZieglerRGonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitroJ Bone Miner Res19971234644719076590
- HofbauerLCHicokKCKhoslaSEffects of gonadal and adrenal androgens in a novel androgen-responsive human osteoblastic cell lineJ Cell Biochem1998711961089736458
- ChinK-YSoelaimanINThe effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in ratsAging Male2015181606625166624
- SudaTTakahashiNUdagawaNJimiEGillespieMTMartinTJModulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand familiesEndocr Rev199920334535710368775
- HofbauerLCHicokKCChenDKhoslaSRegulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cellsEur J Endocrinol2002147226927312153751
- ClarkeBLKhoslaSAndrogens and boneSteroids200974329630518992761
- HuberDMBendixenACPathrosePAndrogens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factorEndocrinology200114293800380811517156
- PalumboCA three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryosCell Tissue Res198624611251313779795
- BonewaldLFOsteocytes as dynamic multifunctional cellsAnn N Y Acad Sci20071116128129017646259
- KamiokaHHonjoTTakano-YamamotoTA three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopyBone200128214514911182371
- VerborgtOTattonNAMajeskaRJSchafflerMBSpatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation?J Bone Miner Res200217590791412009022
- VerborgtOGibsonGJSchafflerMBLoss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivoJ Bone Miner Res2000151606710646115
- AguirreJIPlotkinLIGortazarARA novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransductionJ Biol Chem200728235255012550817609204
- TomkinsonAReeveJShawRNobleBThe death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone 1J Clin Endocrinol Metab1997829312831359284757
- VanderschuerenDVandenputLBoonenSLindbergMKBouillonROhlssonCAndrogens and boneEndocr Rev200425338942515180950
- VandenputLBoonenSVan HerckESwinnenJBouillonRVanderschuerenDEvidence from the aged orchidectomized male rat model that 17β-estradiol is a more effective bone-sparing and anabolic agent than 5α-dihydrotestosteroneJ Bone Miner Res200217112080208612412816
- ModderURiggsBLSpelsbergTCDose-response of estrogen on bone versus the uterus in ovariectomized miceEur J Endocrinol2004151450351015476452
- VidalOLindbergMKHollbergKEstrogen receptor specificity in the regulation of skeletal growth and maturation in male miceProc Natl Acad Sci U S A200097105474547910805804
- LindbergMKMovérareSSkrticSTwo different pathways for the maintenance of trabecular bone in adult male miceJ Bone Miner Res200217455556211918213
- KeHBrownTQiHThe role of estrogen receptor-b in the early age-related bone gain and later age-related bone loss in female miceJ Musculoskelet Neuronal Interact20022547948815758417
- KaufmanJJohnellOAbadieEBackground for studies on the treatment of male osteoporosis: state of the artAnn Rheum Dis2000591076577211005775
- HannanMTFelsonDTAndersonJJBone mineral density in elderly men and women: results from the Framingham osteoporosis studyJ Bone Miner Res1992755475531615761
- RiggsBLKhoslaSMeltonLJA unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging menJ Bone Miner Res19981357637739610739
- BurgerHde LaetCvan DaelePRisk factors for increased bone loss in an elderly population the rotterdam studyAm J Epidemiol199814798718799583718
- CawthonPMEwingSKMcCullochCELoss of hip BMD in older men: the osteoporotic fractures in men (MrOS) studyJ Bone Miner Res200924101728173519419308
- LeBlancESNielsonCMMarshallLMThe effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older menJ Clin Endocrinol Metab20099493337334619584177
- WooJKwokTLeungJCOhlssonCVandenputLLeungPCSex steroids and bone health in older Chinese menOsteoporos Int20122351553156221318439
- MeierCNguyenTVHandelsmanDJEndogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology StudyArch Intern Med20081681475418195195
- MellstromDVandenputLMallminHOlder men with low serum estradiol and high serum SHBG have an increased risk of fracturesJ Bone Miner Res200823101552156018518773
- LorentzonMSwansonCAnderssonNMellströmDOhlssonCFree testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD studyJ Bone Miner Res20052081334134116007330
- VanbillemontGLapauwBBogaertVSex hormone-binding globulin as an independent determinant of cortical bone status in men at the age of peak bone massJ Clin Endocrinol Metab20109541579158620133463
- KhoslaSAminSSinghRJAtkinsonEJMeltonLJ3rdRiggsBLComparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral densityOsteoporos Int200819101465147118338096
- DaniellHWDunnSRFergusonDWLomasGNiaziZStrattePTProgressive osteoporosis during androgen deprivation therapy for prostate cancerJ Urol2000163118118610604342
- LederBZLeBlancKMSchoenfeldDAEastellRFinkelsteinJSDifferential effects of androgens and estrogens on bone turnover in normal menJ Clin Endocrinol Metab200388120421012519853
- Falahati-NiniARiggsBLAtkinsonEJO’FallonWMEastellRKhoslaSRelative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly menJ Clin Invest2000106121553156011120762
- LederBZFinkelsteinJSEffect of aromatase inhibition on bone metabolism in elderly hypogonadal menOsteoporos Int200516121487149415856361
- Burnett-BowieS-AMMcKayEALeeHLederBZEffects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levelsJ Clin Endocrinol Metab200994124785479219820017