Abstract
Background
Epidemiologic and clinical data have suggested the existence of a biologic linkage between the bone system and the vascular system. Bisphosphonates (BPs) are effective inhibitors of bone resorption and are currently considered the drugs of choice for the prevention and treatment of osteoporosis and related fractures. Data from several publications have suggested that BPs may also be effective in reducing the atherosclerotic process and vascular calcification, but the results of these studies are contrasting. This review aimed to allow a better understanding of the relationships between BPs and atherosclerosis in humans.
Materials and methods
Electronic databases of Pubmed-Medline, Cochrane Library and SCOPUS from inception to June 30, 2016 were searched. The full texts of the articles potentially eligible were carefully assessed and reviewed. Finally, 20 studies were found to be eligible and were included in the systematic review. All included studies were published between 2000 and 2014.
Results
In several studies, etidronate limited the progression of aortic and coronary calcification in hemodialysis patients, whereas the nitrogen-containing-BPs given orally did not significantly reduce vascular calcifications in patients with chronic kidney disease, kidney trasplant or in those with osteoporosis. Nitrogen-containing-BPs present favorable effects both on vessel wall thickness and on arterial elasticity due to both a reduction in serum lipids and the interaction of BPs with the bone tissue, with the consequent release of bone turnover markers and cytokines into the bloodstream.
Conclusion
To sum up, the BPs seem to have the potential of influencing atherosclerosis and calcium homeostasis at the level of vascular walls with several possible mechanisms which may differ according to the type, potency, dosage and administration route of BPs. Additional studies are needed to specifically address the mechanism by which BP use could influence cardiovascular morbidity and mortality.
Introduction
In the past, osteoporosis and atherosclerosis were considered as separate entities with a similar increasing prevalence with aging. Recently, both epidemiologic and clinical studies have outlined that patients with low bone mineral density (BMD) are at significantly greater risk of developing cardiovascular disease (CVD) as well as unexpected cardiovascular events, more severe coronary atherosclerosis and vascular calcification.Citation1–Citation6 In addition, it is known that postmenopausal women with osteoporosis have an increased risk of developing cardiovascular events and that the increased risk is proportional to the severity of osteoporosis.Citation7 These data have also suggested a possible influence of drugs affecting bone metabolism on lipid and atherosclerosis mechanisms, or that drugs effective on the atherosclerosis process could also be efficacious in fracture prevention.Citation3,Citation4 Moreover, there is growing evidence that osteoporosis and atherosclerosis share not only common risk markers such as aging, smoking, reduced physical activity and menopause in women, but also some pathophysiologic mechanisms and some genetic causes. For example, mutations with loss of function of LRP6 in humans lead to increased plasma low-density lipoprotein-cholesterol (LDL-C), hypertension and osteoporosis.Citation8
An initial interesting theory was that CVD and osteoporosis were linked by a common denominator, such as serum lipid profile, which could act in parallel on both vascular and bone cells.Citation9 Some experimental data seemed to reinforce this concept showing an influence of lipids on osteoblasts and osteoclasts.Citation10–Citation12 However, an interesting observational study showed that in a multiple regression analysis, lipid profile did not predict osteoporosis or fracture risk, whereas aortic calcification severity significantly explained BMD at the hip.Citation13 On the other hand, low BMD at the distal radius was found to be associated with increased risk of stroke and CVD mortality.Citation6 Several studies have reported that the progression of arterial calcification is linked with concurrent bone loss and vertebral fractures, further supporting a relationship between osteoporosis and CVD.Citation14,Citation15 The common finding of simultaneous vascular calcification and osteoporosis in individual patients suggests that local tissue factors could have a crucial role in the regulation of mineralization and cell differentiation.Citation16 Cardiovascular calcification was conventionally viewed as an inevitable consequence of aging, but some landmark studies have demonstrated that it is a highly regulated process of mineralization which involves cellular and molecular signaling processes similar to those found in normal osteogenesis.Citation17–Citation20 The similarity of the molecular mechanisms in osteogenesis and vascular calcification has led to the knowledge that atherosclerotic calcification is an actively regulated process, not a passive mineralization.Citation21 In fact, the mineral matrix of the plaque (hydroxyapatite) is identical to that found in bone and the process of vascular calcification, similar to bone remodeling, is a regulated process which includes both inductive and inhibitory mechanisms.Citation22,Citation23 Moreover, mineral deposits in atherosclerotic plaque result from several different pathways involving metabolic and/or inflammatory processes.Citation24 The major factors influencing vascular atherosclerotic calcifications are listed in .
Table 1 Factors influencing vascular atherosclerotic calcification
The effects of bisphosphonates on atherosclerotic plaque and vascular calcifications: experimental studies
The growing evidence that atherosclerosis and osteoporosis share several pathophysiologic mechanisms reinforces the interest in pharmacologic agents which could inhibit bone loss and also provide benefits in terms of slowing the progression of atherosclerosis. At present, only bisphosphonates (BPs), currently considered the drug of choice for the prevention and treatment of osteoporosis, could have this potential.Citation50–Citation52 In a mouse model of glucocorticoid-induced osteoporosis, denosumab, a human monoclonal antibody targeting RANKL, reduced the progression of atherosclerosis.Citation41 Moreover, a more recent study reported that denosumab reduced spontaneous and induced calcification in an in vitro porcine valvular interstitial cell model.Citation53 However, the analysis of 2,363 postmenopausal women with osteoporosis selected from the participants in the FREEDOM study and at high risk of cardiovascular events showed a 3-year denosumab treatment had no effect on the progression of aortic calcifications or on the incidence of cardiovascular adverse events, compared to placebo.Citation54 Also, odanacatib, a cathepsin K inhibitor, may have an anti-atherosclerotic effect; but in 2016, the development of odanacatib was discontinued after analysis discovered an increased risk of cardiovascular events.Citation55 BPs are grouped into two classes according to their chemical structure and the molecular mechanism by which they inhibit osteoclast activity (). Members of the first generation of BPs are called simple BPs (S-BPs; clodronate and etidronate), while those of the second or newer generation are called nitrogen-containing BPs (N-BPs). The latter have a high affinity for the bone tissue where they bind to and inhibit the activity of farnesyl pyrophosphate synthase, a key regulatory enzyme in the mevalonic acid pathway, leading to osteoclast apoptosis.Citation56 The first report of the effects of BPs on vascular calcification was published in the 1970s, with experiments showing inhibition of soft tissue calcification.Citation57 The accumulation of BPs in atherosclerotic arteries may be due to either their binding to calcified atheromatous lesions or their internalization into phagocytosing cells (namely, the macrophages).Citation52 Etidronate was the first BP to demonstrate the suppression of atherosclerotic lesion formation in the arteries of both rats and pigs, although there was no reduction in serum calcium and cholesterol.Citation58,Citation59 Lomashvili et alCitation50 found that etidronate significantly reduced the calcium content in rat aortae cultured in a medium containing warfarin to induce calcification, and suggested that S-BPs may prevent calcifications by blocking the apatite crystalsformation in the vessels as they do in the bone.Citation50 Clodronate at high dosages, when administered to rabbits, also significantly reduced the area of atherosclerotic lesions in the aorta, total cholesterol and total calcium concentration in the aorta.Citation52 S-BPs may directly inhibit metalloproteinasesCitation60 and the expression of tumor necrosis factor-α, a cytokine that promotes osteoblastic differentiation of vascular cells and also inhibits calcium deposition in the atherosclerotic lesions.Citation61 Moreover, S-BPs can be metabolized by the phagocytes into non-hydrolyzable adenine-containing analogs of adenosine triphosphate,Citation62 which inhibit adenine nucleotide translocase, thus promoting the activation of caspase-3 and thereby leading to apoptosis of macrophages and osteoclasts.Citation63 Several animal studies suggest that N-BPs also may have an inhibitory effect on vascular calcification and atherosclerosis process. Alendronate and ibandronate are reported to inhibit calcification of arteries and cardiac valves in rat models of warfarin-related calcification at doses comparable to those that inhibit bone resorption, without affecting serum calcium and phosphate levels.Citation64 Moreover, in uremic rats fed with a low-protein diet, artery calcifications were prevented by treatment with ibandronate.Citation65 Another study reported that high doses of vitamin D were lethal to rats and caused excessive calcification of arteries, lungs, kidneys and cartilage; however, when subjects were given vitamin D plus ibandronate, soft tissue calcification was inhibited in all organs and death was prevented.Citation66 Moreover, in monkeys, a 2-year treatment with pamidronate inhibited the development of diet-induced atherosclerosis without significant changes in serum cholesterol and calcium.Citation67 The exact mechanism by which N-BPs inhibit vascular calcification is not fully clear. It may be by the inhibition of bone resorption, with reduced efflux of calcium and phosphate, thus limiting their availability for deposition in the vessels or their ability to influence the activity of the vascular smooth muscle cells NaPi cotransporter.Citation68 Alternatively, N-BPs may have direct effects on the vessel wall and/or crystal formation. Another possible mechanism of action of N-BPs may be a reduction in serum cholesterol levels. To sum up, the experimental studies have confirmed the ability of BPs to reduce the formation of atherosclerotic plaques and to inhibit vascular calcifications. In animal studies, etidronate was the more potent inhibitor of vascular calcifications.
Table 2 Different potential effects of simple bisphosphonates and nitrogen-containing bisphosphonates on vascular calcification
The effects of BPs on atherosclerotic plaque and vascular calcifications: a systematic review of clinical studies
This review aimed to collect and synthesize the available data, in order to allow a better understanding of the relationships between BPs and atherosclerosis/vascular calcifications in humans.
Materials and methods
A literature review was conducted from inception to June 30, 2016. Pubmed-Medline, Cochrane Library and SCOPUS databases were searched using the following search terms (“bisphosphonates” or “etidronate” or “alendronate” or “risedronate” and so on) AND (“atherosclerosis” or “atherosclerotic plaque” or “cholesterol” or “vascular calcification”).
Study selection
Studies were excluded if they were not available; they were on children, animals, in vitro or experimental studies, case reports, case series, letters to editor, comments, review articles; they were published in languages other than English; and they did not fulfill the objective of this review. Original studies were included if they met the following inclusion criteria: 1) being a clinical study; 2) investigating the impact of BPs on atherosclerosis, vascular calcification or serum cholesterol and 3) presentation of sufficient information on the study parameters at baseline and at the end of follow-up. Exclusion criteria were: 1) non-interventional studies; 2) observational and cross-sectional studies and 3) study duration of <6 months.
Eligible studies were reviewed and the following data were extracted: 1) first author’s name; 2) year of publication; 3) study design; 4) study duration; 5) number of subjects in BP and control groups; 6) intervention assigned to the control group (placebo, non-active treatment or active treatment); 7) outcome measurements evaluated and 8) baseline and end-study values for the study parameters.
Characteristics of included studies
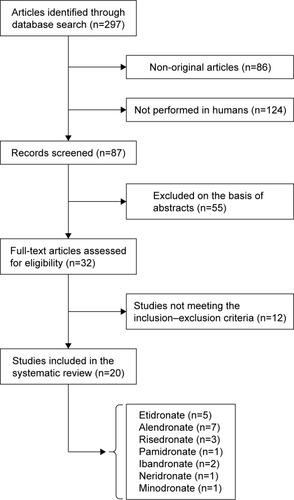
After the multiple database search, 297 published studies were identified and the abstracts reviewed. The study selection process is shown in . The full text of the 32 articles potentially eligible were carefully assessed and reviewed. Finally, 20 studies were found to be eligible and were included in the systematic review.
Results
The characteristics of the 20 studies included in the systematic review are presented in . The included studies were published between 2000 and 2014. Similar to animal experiments, which reported conflicting results for BP treatment of atherosclerosis and related vascular calcification, there have been varying responses in clinical studies.
Table 3 Main characteristics of the 20 studies included in the review
Effects of BPs an intima–media thickening and serum cholesterol
More than a decade ago, Adami et al firstly reported a significant reduction in LDL-C and an increase in high-density lipoprotein-cholesterol (HDL-C) in postmenopausal women treated with intravenous (i.v.) neridronate, whereas no significant changes were observed in total cholesterol.Citation69 Significant reductions in LDL-C were also reported by other two clinical studies carried out, respectively, in patients treated with i.v. pamidronate for Paget’s bone disease and in patients with smouldering multiple myeloma treated with zoledronic acid.Citation70,Citation71 More recently, Gonnelli et al reported that in postmenopausal osteoporotic women, a 1-year treatment with both zoledronate (a single yearly injection) and ibandronate (a 3-monthly injection) induced similar changes in lipid profile by increasing HDL-C and reducing LDL-C and resulted in a reduction of intima–media thickness (IMT) at the carotid artery; however, in the latter study, no significant changes in total cholesterol were observed.Citation72 These positive effects of i.v. N-BPs on lipids have not yet been confirmed by the majority of studies carried out with N-BPs or S-BPs given orally. In particular, a study by Luckish et al carried out on osteoporotic women with at least one cardiovascular risk factor treated with risedronate 35 mg weekly for 6 months reported a significant improvement in artery elasticity, but no change in HDL-C and LDL-C levels.Citation73 Koshiyama et alCitation74 investigated the effect of etidronate on carotid IMT in patients with type 2 diabetes and reported that at the end of a 12-month treatment, IMT was significantly reduced in the etidronate group with respect to control participants. Also, the study by Celiloglu et al reported that in postmenopausal osteoporotic women, a 1-year treatment with alendronate significantly reduced carotid IMT with respect to controls.Citation75 Moreover, in a study carried out on Japanese women with diabetes and postmenopausal osteoporosis, a 1-year therapy with risedronate (2.5 mg/day) along with alfacalcidiol (1 mg/day) significantly reduced the progression of atherosclerotic plaques at the carotid arteries and abdominal aorta.Citation76 On the contrary, the study by Delibasi et al reported that in postmenopausal osteoporotic women, a 13-month treatment with alendronate did not evidence any changes in carotid IMT or serum levels of lipids.Citation77 Also, the study by Igase et al reported that in a Japanese population, a 1-year treatment with alendronate (35 mg/week) did not improve arterial stiffness.Citation78 Some studies reported that a 12-month treatment with etidronateCitation74,Citation79 or alendronateCitation75 induced a nonsignificant reduction in total and LDL-C, along with a nonsignificant increase in HDL-C. Moreover, Kawahara et al, in a prospective randomized study carried out in hypercholesterolemic patients, reported that atorvastatin plus etidronate combination therapy was significantly more effective (P<0.001) in reducing atherosclerotic plaques in the abdominal aorta than both atorvastatin and etidronate monotherapy.Citation79 In the same study, the atorvastatin plus etidronate combination therapy showed a reduction of the atherosclerotic plaques in thoracic aorta similar to that of atorvastatin monotherapy.Citation79 On the contrary, a Japanese study carried out on osteoporotic women treated with a reduced dose of alendronate (5 mg/day) did not find any changes in cholesterol levels.Citation80 To sum up, the majority of human studies have reported a reduction of IMT at the carotid artery, whereas data on arterial stiffness and the atherosclerotic plaques are inconclusive. Moreover, N-BPs seem to improve the lipid profile only when given i.v.
Effects of BPs on vascular calcification
Nitta et al reported that in hemodialysis patients, etidronate at a dose of 200 mg/day for 2 weeks every 90 days significantly reduced coronary artery calcifications, assessed by spiral computed tomography, and serum levels of osteoprotegerin.Citation81 In another study carried out on hemodialysis patients, etidronate (400 mg/day for 6 months) decreased aortic calcifications by 65%, whereas aortic calcifications markedly increased in controls.Citation82 Another Japanese study carried out on hemodialysis patients reported that etidronate (200 mg on days of dialysis for 23 months) protected against progression of aortic calcifications, whereas these significantly increased in the control group.Citation83 A 2-year weekly treatment with alendronate in kidney transplant recipients was effective in preventing the progression of aortic calcifications with respect to control patients (1.4% vs 5%, P=ns).Citation84 Moreover, in a study carried out in end-stage chronic kidney disease (CKD) patients, an 18-month treatment with alendronate significantly decreased the progression of aortic calcification with respect to placebo.Citation85 Similarly, Torregrosa et al reported that 1-year treatment with residronate (35 mg/week) did not significantly influence the progression of vascular calcifications in kidney transplant patients.Citation86 Another clinical study involved osteoporotic women receiving oral or i.v. ibandronate for 3 years; at the end of the study period, the effect on BMD was positive, whereas no difference was detected in the rate of aortic calcification change.Citation87 Moreover, the study by Hill et al assessing coronary artery calcification, measured by spiral computed tomography scans, in patients treated with alendronate 10 mg daily for 2 years matched to a cohort of reference subjects did not find any significant differences between the two groups.Citation88 To sum up, etidronate markedly reduced progression of vascular calcifications, but most of these studies were carried out in patients with CKD or in hemodialysis. The effects of N-BPs given orally on the vascular calcifications were modest and inconclusive.
Discussion
The interest in the relationships between BPs and atherosclerosis has recently shown a further increase after the publication of the results of the HORIZON study which reported a 28% reduction in mortality in hip fracture patients treated with an annual i.v. dose of zoledronic acid.Citation89 In another study, Kang et al revealed that patients who received BP therapy for osteoporotic fracture had a lower hazard of myocardial infarction during the 2-year follow-up period with respect to controls.Citation90 Moreover, two recent studies have reported that oral BPs reduce mortality in osteoporotic patients and that the reduction in mortality could be mainly due to cardiovascular and cerebrovascular deaths.Citation91,Citation92 On the contrary, a recent meta-analysis by Kim et alCitation93 indicated that commonly prescribed BPs do not provide any clinically important benefits or harm with regard to cardiovascular events. The analysis of the studies included in this systematic review seems to suggest a positive effect of BPs on the progression of both atherosclerotic plaque and vascular calcifications. First of all, it is important to emphasize that this review has some limitations primarily resulting from the small sample size of most studies. Thus, insufficient data were available to allow separate analysis of the effects of BPs in different patient subgroups. Moreover, most of these studies were of suboptimal quality in terms of providing adequate description of allocation concealment and the lack of the use of double blinding, thus leading to possible overestimation of BP benefit.
Several studies have shown that etidronate limited the progression of aortic and coronary calcification in hemodialysis patients.Citation81–Citation83 Instead, the N-BPs given orally (alendronate, risedronate and ibandronate) did not significantly reduce vascular calcifications either in patients with CKDCitation85 and kidney trasplantCitation86 or in patients with osteoporosisCitation88 and hypercholesterolemia.Citation79 However, at present, the clinical use of etidronate should be considered with caution because this agent can cause impaired bone mineralization, which may lead to osteomalacia and to an increased risk of stress fractures. Moreover, particular attention is required when using BPs in CKD and hemodialysis patients because in these patients, BPs may excessively reduce bone turnover, ultimately aggravating adynamic bone disease. Several studies have reported that BPs, especially N-BPs, present favorable effects both on vessel wall thickness and on the parameters of arterial elasticity and stiffness.Citation72–Citation76,Citation79 A possible mechanism of action of N-BPs may be a slowing down of the formation of atherosclerotic plaques due to a reduction in serum lipids, which are considered responsible for the triggering of atherosclerosis progression. In fact, the majority of studies found a reduction in total and LDL-C along with a mild increase in HDL-C,Citation69–Citation72,Citation74 whereas other studies did not report any changes.Citation77,Citation80 These controversial data may suggest that the effect of BPs on lipids depends on the administration route, with a more favorable effect found when given i.v. However, literature data suggest that the changes in lipids alone are not sufficient to explain the positive effect on atherosclerosis. Moreover, BPs, especially N-BPs, could have an indirect effect on atherosclerotic manifestations due to the interaction of BPs with the bone tissue, with the consequent release of bone turnover markers, bone-related hormones and cytokines (such as osteocalcin, fibroblast growth factor 23, osteoprotegerin and so on) into the bloodstream. As confirmation, a recent study reported that osteocalcin and fibroblast growth factor 23 were independent predictors of carotid IMT in postmenopausal women treated with zoledronate.Citation72
To sum up, the BPs seem to have the potential of influencing atherosclerosis and calcium homeostasis at the level of vascular walls with several possible mechanisms which may differ according to the type, potency, dosage and administration route of BPs. However, until the present time, it is not yet clear which of these above-mentioned mechanisms may be the most important in humans and additional studies are needed to specifically address the mechanism by which BPs use could influence cardiovascular morbidity and mortality.
Disclosure
The authors report no conflicts of interest in this work.
References
- SinnottBSyedISevrukovABarengoltsECoronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with agingCalcif Tissue Int200678419520216604285
- Von der ReckePHansenMAHassagerCThe association between low bone mass at the menopause and cardiovascular mortalityAm J Med1999106327327810190374
- EspositoKCapuanoASportielloLGiustinaAGiuglianoDShould we abandon statins in the prevention of bone fractures?Endocrine201344232633323526261
- SantosLLCavalcantiTBBandeiraFAVascular effects of bisphosphonates-A systematic reviewClin Med Insights Endocrinol Diabetes20125475423133318
- DanileviciusCFLopesJBPereiraRMBone metabolism and vascular calcificationBraz J Med Biol Res200740443544217401486
- KielDPKauppilaLICupplesLAHannanMTO’DonnellCJWilsonPWBone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart StudyCalcif Tissue Int200168527127611683533
- TankoLBChristiansenCCoxDAGeigerMJMcNabbMACummingsSRRelationship between osteoporosis and cardiovascular disease in postmenopausal womenJ Bone Miner Res200520111912192016234963
- WilliamsBOInsognaKLWhere Wnts went: the exploding field of Lrp5 and Lrp6 signaling in boneJ Bone Miner Res200924217117819072724
- BaldiniVMastropasquaMFrancucciCMD’ErasmoECardiovascular disease and osteoporosisJ Endocrinol Invest200528 Suppl6972
- ParhamiFBasseriBHwangJTintutYDemerLLHigh-density lipoprotein regulates calcification of vascular cellsCirc Res200291757057612364384
- ParhamiFGarfinkelADemerLLRole of lipids in osteoporosisArterioscler Thromb Vasc Biol200020112346234811073836
- TintutYMoronySDemerLLHyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivoArterioscler Thromb Vasc Biol2004242e6e1014670933
- BaggerYZRasmussenHBAlexandersenPWergeTChristiansenCTankóLBPERF study groupLinks between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se?Osteoporos Int200718450551217109061
- VogtMTSan ValentinRForrestKYNevittMCCauleyJABone mineral density and aortic calcification: the study of osteoporotic fracturesJ Am Geriatr Soc19974521401459033510
- HofbauerLCBrueckCCShanahanCMSchoppetMDobnigHVascular calcification and osteoporosis from clinical observation towards molecular understandingOsteoporos Int200718325125917151836
- ParhamiFMorrowADBalucanJLipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patientsArterioscler Thromb Vasc Biol19971746806879108780
- AikawaENahrendorfMSosnovikDMultimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve diseaseCirculation2007115337738617224478
- AikawaEAikawaMLibbyPArterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal diseaseCirculation2009119131785179419307473
- BostromKWatsonKEStanfordWPDemerLLAtherosclerotic calcification: relation to developmental osteogenesisAm J Cardiol199575688B91B7801876
- DuerMJFriscicTProudfootDMineral surface in calcified plaque is like that of bone: further evidence for regulated mineralizationArterioscler Thromb Vasc Biol200828112030203418703777
- DemerLLTintutYMineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lectureArterioscler Thromb Vasc Biol200323101739174312958041
- LusisAJAtherosclerosisNature2000407680123324111001066
- BostromKDemerLLRegulatory mechanisms in vascular calcificationCrit Rev Eukaryot Gene Expr200010215115811186330
- SageAPTintutYDemerLLRegulatory mechanisms in atherosclerotic calcificationNat Rev Cardiol20107952853620664518
- BostromKWatsonKEHornSWorthamCHermanIMDemerLLBone morphogenetic protein expression in human atherosclerotic lesionsJ Clin Invest1993914180018098473518
- SteitzSASpeerMYCuringaGSmooth muscle cell phenotypic transition associated with calcification: up regulation of Cbfa1 and down regulation of smooth muscle lineage markersCirc Res200189121147115411739279
- IkedaTShirasawaTEsakiYYoshikiSHirokawaKOsteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aortaJ Clin Invest1993926281428208254036
- MurshedMSchinkeTMcKeeMDKarsentyGExtracellular matrix mineralization is regulated locally; different roles of two gla-containing proteinsJ Cell Biol2004165562563015184399
- SchaferCHeissASchwarzAThe serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcificationJ Clin Invest2003112335736612897203
- KettelerMBongartzPWestenfeldRAssociation of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional studyLancet2003361936082783312642050
- LaceyDLTimmsETanHLOsteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activationCell19989321651769568710
- SimonetWSLaceyDLDunstanCROsteoprotegerin: a novel secreted protein involved in the regulation of bone densityCell19978923093199108485
- BucayNSarosiIDunstanCROsteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcificationGenes Dev1998129126012689573043
- MoronySTintutYZhangZOsteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr (−/−) miceCirculation2008117341142018172035
- BennettBJScatenaMKirkEAOsteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− miceArterioscler Thromb Vasc Biol20062692117212416840715
- JonoSIkariYShioiASerum osteoprotegerin levels are associated with the presence and severity of coronary artery diseaseCirculation2002106101192119412208791
- KiechlSSchettGWenningGOsteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular diseaseCirculation200410982175218015117849
- OzkokACaliskanYSakaciTOsteoprotegerin/RANKL axis and progression of coronary artery calcification in hemodialysis patientsClin J Am Soc Nephrol20127696597322490874
- SchoppetMSchaeferJRHofbauerLCLow serum levels of soluble RANK ligand are associated with the presence of coronary artery disease in menCirculation200310711e7612654623
- KiechlSSchettGSchwaigerJSoluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular diseaseCirculation2007116438539117620507
- HelasSGoettschCSchoppetMInhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in miceAm J Pathol2009175247347819590040
- ScatenaMLiawLGiachelliCMOsteopontin: a multifunctional molecule regulating chronic inflammation and vascular diseaseArterioscler Thromb Vasc Biol200727112302230917717292
- WolakTOsteopontin – a multi-modal marker and mediator in atherosclerotic vascular diseaseAtherosclerosis2014236232733725128758
- ShimadaTKakitaniMYamazakiYTargeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolismJ Clin Invest2004113456156814966565
- SciallaJJWolfMRoles of phosphate and fibroblast growth factor 23 in cardiovascular diseaseNat Rev Nephrol201410526827824686452
- SomjenDWeismanYKohenF25-hydroxyvitamin D3–1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compoundsCirculation2005111131666167115795327
- OhJWengSFeltonSK1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitusCirculation2009120868769819667238
- GiovannucciELiuYHollisBWRimmEB25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective studyArch Intern Med2008168111174118018541825
- BearMButcherMShaughnessySGOxidized low-density lipoprotein acts synergistically with beta-glycerophosphate to induce osteoblast differentiation in primary cultures of vascular smooth muscle cellsJ Cell Biochem2008105118519318461557
- LomashviliKAMonier-FaugereMCWangXMallucheHHO’NeillWCEffect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failureKidney Int200975661762519129793
- TamuraKSuzukiYHashibaHTamuraHAizawaSKogoHEffect of etidronate on aortic calcification and bone metabolism in calcitriol treated rats with subtotal nephrectomyJ Pharmacol Sci2005991899416141638
- YlitaloROksalaOYlä-HerttualaSYlitaloPEffects of clodronate (dichloromethylene bisphosphonate) on the development of experimental atherosclerosis in rabbitsJ Lab Clin Med199412357697768195683
- LermanDAPrasadSAlottiNDenosumab could be a potential inhibitor of valvular interstitial cells calcification in vitroInt J Cardiovasc Res2016511
- SamelsonEJMillerPDChristiansenCRANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular riskJ Bone Miner Res201429245045723873632
- MullardAMerck &Co. drops osteoporosis drug odanacatibNat Rev Drug Discov20161510669
- LuckmanSPHughesDECoxonFPGrahamRRussellGRogersMJNitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including RasJ Bone Miner Res19981345815899556058
- FleischHARussellRGBisazSMühlbauerRCWilliamsDAThe inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivoEur J Clin Invest19701112184319371
- RosenblumIYFloraLEisensteinRThe effect of disodium ethane-1-hydroxy-1,1-diphosphonate (EHDP) on a rabbit model of atheroarteriosclerosisAtherosclerosis1975223411424812508
- DaoudASFrankASJarmolychJFritzKEThe effect of ethane-1-hydroxy-1,1-d iphosphonate (EHDP) on necrosis of atherosclerotic lesionsAtherosclerosis198767141483118892
- TeronenOHeikkiläPKonttinenYTMMP inhibition and downregulation by bisphosphonatesAnn N Y Acad Sci199987845346510415748
- TintutYPatelJParhamiFDemerLLTumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathwayCirculation2000102212636264211085968
- RogersMJGordonSBenfordHLCellular and molecular mechanisms of action of bisphosphonatesCancer20008812 Suppl2961297810898340
- LehenkariPPKellinsalmiMNäpänkangasJPFurther insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metaboliteMol Pharmacol20026151255126211961144
- PricePAFausSAWilliamsonMKBisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorptionArterioscler Thromb Vasc Biol200121581782411348880
- PricePARoublickAMWilliamsonMKArtery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronateKidney Int20067091577158316955099
- PricePABuckleyJRWilliamsonMKThe amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in ratsJ Nutr2001131112910291511694617
- KramschDMAspenAJRozlerLJAtherosclerosis: prevention by agents not affecting abnormal levels of blood lipidsScience19812134515151115126792706
- PersyVDe BroeMKettelerMBisphosphonates prevent experimental vascular calcification: Treat the bone to cure the vessels?Kidney Int20067091537153817051257
- AdamiSBragaVGuidiGGattiDGerardiDFracassiEChronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterolJ Bone Miner Res200015359960410750576
- MontagnaniAGonnelliSCepollaroCChanges in serum HDL and LDL cholesterol in patients with Paget’s bone disease treated with pamidronateBone2003321151912584031
- GozzettiAGennariLMerlottiDThe effects of zoledronic acid on serum lipids in multiple myeloma patientsCalcif Tissue Int200882425826218418538
- GonnelliSCaffarelliCTanzilliLEffects of intravenous zoledronate and ibandronate on carotid intima-media thickness, lipids and FGF-23 in postmenopausal osteoporotic womenBone201461273224389416
- LuckishACernesRBoazMEffect of long-term treatment with risedronate on arterial compliance in osteoporotic patients with cardiovascular risk factorsBone200843227928318515205
- KoshiyamaHNakamuraYTanakaSMinamikawaJDecrease in carotid intima-media thickness after 1-year therapy with etidronate for osteopenia associated with type 2 diabetesJ Clin Endocrinol Metab20008582793279610946883
- CelilogluMAydinYBalciPKolamazTThe effect of alendronate sodium on carotid artery intima-media thickness and lipid profile in women with postmenopausal osteoporosisMenopause200916468969319240658
- KanazawaIYamaguchiTHayashiKTakaseHShimizuTSugimotoTEffects of treatment with risedronate and alfacalcidol on progression of atherosclerosis in postmenopausal women with type 2 diabetes mellitus accompanied with osteoporosisAm J Med Sci2010339651952420400887
- DelibasiTEmralRErdoganMFKamelNEffects of alendronate sodium therapy on carotid intima media thickness in postmenopausal women with osteoporosisAdv Ther200724231932517565922
- IgaseMKoharaKTabaraYChange in arterial stiffness associated with monthly bisphosphonate treatment in women with postmenopausal osteoporosisMenopause201421996296624552979
- KawaharaTNishikawaMKawaharaCInazuTSakaiKSuzukiGAtorvastatin, etidronate, or both in patients at high risk for atherosclerotic aortic plaques a randomized, controlled trialCirculation2013127232327233523658438
- IwamotoJSatoYUzawaMTakedaTMatsumotoHComparison of effects of alendronate and raloxifene on lumbar bone mineral density, bone turnover, and lipid metabolism in elderly women with osteoporosisYonsei Med J200849111912818306478
- NittaKAkibaTSuzukiKEffects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysisAm J Kidney Dis200444468068815384019
- AriyoshiTEishiKSakamotoIMatsukumaSOdateTEffect of etidronic acid on arterial calcification in dialysis patientsClin Drug Investig2006264215222
- HashibaHAizawaSTamuraKKogoHInhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effectsTher Apher Dial2006101596416556138
- OkamotoMYamanakaSYoshimotoWShigematsuTAlendronate as an effective treatment for bone loss and vascular calcification in kidney transplant recipientsJ Transplant2014201426961324696777
- ToussaintNDLauKKStraussBJPolkinghorneKRKerrPGEffect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trialAm J Kidney Dis2010561576820347511
- TorregrosaJVFusterDGentilMAOpen-label trial: effect of weekly risedronate immediately after transplantation in kidney recipientsTransplantation201089121476148120393402
- TankóLBQinGAlexandersenPBaggerYZChristiansenCEffective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic womenOsteoporos Int200516218419015197541
- HillJAGoldinJGGjertsonDProgression of coronary artery calcification in patients taking alendronate for osteoporosisAcad Radiol20029101148115212385509
- LylesKWColon-EmericCSMagazinerJSZoledronic acid in reducing clinical fracture and mortality after hip fractureN Engl J Med20073571799180917878149
- KangJHKellerJJLinHCBisphosphonates reduced the risk of acute myocardial infarction: a 2-year follow-up studyOsteoporos Int201324127127723152093
- CenterJRBliucDNguyenNDNguyenTVEismanJAOsteoporosis medication and reduced mortality risk in elderly women and menJ Clin Endocrinol Metab20119641006101421289270
- WolfeFBolsterMBO’ConnorCMMichaudKLylesKWColoń-EmericCSBisphosphonate use is associated with reduced risk of myocardial infarction in patients with rheumatoid arthritisJ Bone Miner Res201328598499123074131
- KimDHRogersJRFulchinoLAKimCASolomonDHKimSCBisphosphonates and risk of cardiovascular events: a meta-analysisPLoS One2015104e012264625884398