Abstract
Purpose
To estimate the discriminative value of serum P1NP/βCTX ratio and albumin levels in hospitalized orthogeriatric patients with and without nonvertebral fractures.
Methods
In 1,239 orthogeriatric patients (mean age 78.1±9.52 years, 69.1% women) including 854 (68.9%) with osteoporotic nonvertebral fractures (455 [36.7%] with hip fracture [HF]) and 385 (31.1%) without fractures, markers of bone formation (procollagen type 1 N-terminal propeptide [P1NP], osteocalcin [OC], and bone resorption (beta-C-terminal cross-linking telopeptide of type 1 collagen [βCTX]), indices of mineral metabolism, and parameters of liver and renal functions were assessed; data on clinical and laboratory characteristics were collected prospectively.
Results
Both lower serum P1NP/βCTX ratio and albumin concentration (as continuous or categorical variables) were independently associated with fracture presence in multivariate logistic regressions. Compared with the highest P1NP/βCTX tertile, the prevalence of HF, after adjustment for multiple covariates, was 3-fold higher in the lowest tertile and 1.5 times higher in the middle tertile; presence of any fracture was 2.3- and 1.6-fold higher, respectively; patients with albumin levels in the lowest tertile had multivariate odds ratio (OR) of 4.6 for HF and 2.8 for any fracture, in the middle tertile the ORs were 2.2 and 1.3, respectively. The P1NP/βCTX <100.0 (median) and hypoalbuminemia (<33 g/L) demonstrated area under the curve values for HF of 0.802 and 0.806, respectively, and for any fractures of 0.711 and 0.706, respectively. When both characteristics were combined, the ORs for HF or any fracture, compared with the nonfractured group, were 7.8 and 3.2, respectively, with an accuracy of 79.6% and 71.6%, respectively.
Conclusions
In orthogeriatric patients, both serum P1NP/βCTX ratio and albumin levels demonstrated an inverse dose–effect relationship with the prevalence of nonvertebral fractures and independently indicated fracture presence with acceptable discriminatory power. Lower P1NP/βCTX (<100) and hypoalbuminemia could be useful simple additive prognostic tools for fracture risk stratification in the elderly.
Introduction
The ability to predict and prevent fragility fractures is limited.Citation1–Citation3 Currently, the predicting is largely based on bone mineral density (BMD) testing and several clinical risk factors (the World Health Organization’s fracture risk assessment tool FRAX,Citation4,Citation5 Garvan and QFracture).Citation1 However, BMD indicated osteoporosis only in 30%–50% of patients with major fragility fractureCitation6 and in 4% of women with a distal radial fracture.Citation7 The prognostic value of clinical risk factors alone in FRAX is comparable to that of BMD alone.Citation8 There is an obvious need of identifying additional fracture risk factors not included in currently available strategies.
Numerous studies on the prognostic value of bone turnover markers (BTMs) yielded conflicting results.Citation9–Citation17 BTMs display significant analytical and biological variabilityCitation18,Citation19 and currently are recommended only for monitoring the efficacy of osteoporosis treatment and compliance.Citation11,Citation20,Citation21 Bone formation and resorption are coupled but not always remain in balance: in the elderly bone is lost because remodeling becomes unbalanced.Citation22 However, most of the reports focused on separate BTMs. One way to overcome the existing discrepancies between studies would be to characterize the balance between total bone formation and resorption. In the only prognostic study, which assessed such index, the ratio of a urinary resorption (N-telopeptide of type 1 collagen [u-NTx]) to serum formation marker (osteocalcin, OC) (u-NTx/OC) predicted fractures independently of FRAX, but did not significantly improve the accuracy of fracture risk prediction in addition to FRAX.Citation23 Recently the joint international working group proposed serum procollagen type 1 N-terminal propeptide (P1NP) and beta-C-terminal cross-linking telopeptide of type 1 collagen (βCTX) as the reference BTMs to evaluate bone formation and bone resorption, respectively.Citation11 To our knowledge, no studies of P1NP/βCTX ratio measurements have been undertaken.
Albumin, one of the major proteins synthesized in the liver and the most abundant protein in the circulatory system, has pleotropic physiologic effects.Citation24,Citation25 Hypoalbuminemia is strongly associated with inflammation–malnutrition complexCitation25 and various systemic disorders (liver, kidneys, cardiovascular, diabetes, and malignancy), many of which are particularly common in the elderly, increases the risk of falls and fractures,Citation26,Citation27 and is linked to poor prognosis and mortality in the general populationCitation28,Citation29 as well as in the orthopedic patients.Citation30,Citation31 Only limited information with conflicting results is available regarding the role of hypoalbuminemia in osteoporosis,Citation32,Citation33 and its value in predicting fractures has not been sufficiently investigated.Citation34,Citation35
We hypothesized that in orthogeriatric patients lower serum P1NP/βCTX ratio and/or hypoalbuminemia, two indices that current algorithms do not take into consideration, may be associated with the presence of fracture, indicating a greater osteoporosis-related fracture risk. In this study, we aimed to investigate the relationship between serum P1NP/βCTX ratio, an index of bone turnover balance, and hypoalbuminemia with the presence of osteoporotic hip or other nonvertebral fractures in a cohort of hospitalized orthogeriatric patients, which reflects the real world in regard to the prevalence of major fractures.
Methods
Patients
This was an observational study using prospectively collected data on 1,899 consecutive patients >60 years of age who were admitted to the Department of Orthopedic Surgery at the Canberra Hospital (a 500-bed university-affiliated tertiary care center, Australian Capital Territory, Australia) between 1 January 2012 and 31 December 2014. After excluding patients with high-trauma fractures, vertebral and periprostetic fractures, primary hyperparathyroidism, Paget’s disease, metastatic cancer to bone, or who lacked adequate laboratory data, 1,239 patients were evaluated for the study. Of these 1,239 hospitalized patients (mean age 78.1±9.52 years, 69.1% women), 854 (68.9%) had a low-energy trauma (falls from standing height) which resulted in a nonvertebral bone fracture: 455 (36.7%) had a hip fracture (HF) (52.0% cervical and 48.0% trochanteric) and 399 (32.2%) had other nonvertebral fractures (humerus −79, femur −75, ankle −68, tibia or/and fibula −27, knee −16, wrist −16, forearm −15, others −103). There were 385 (31.1%) patients without fractures (elective hip or knee replacement −343, suspected surgical site infections not confirmed by further investigation −15, and 27 patients with a prosthetic joint infection following total hip [n=20] or knee [n=7] arthroplasty). Data were collected on demographics, orthopedic and medical diagnoses, procedures performed, laboratory characteristics, medication used, and outcomes.
The study was approved by the Australian Capital Territory Ethical Review Board and performed in accordance with the principles of the Helsinki declaration. All study patients or the legally authorized carers gave their informed consent.
Validation dataset
A retrospective analysis of a second cohort included data obtained from electronic medical and administrative records from 417 consecutive orthogeriatric patients (mean age 78.9±8.7 years, 68.2% women) admitted to the same department between October 2011 and August 2012. Among these patients, there were 152 (36.5%) subjects with an HF, 103 (24.7%) with other nonvertebral fractures, and 162 (38.8%) without fractures.
Laboratory evaluation
In each patient, fasting venous blood samples were collected within 24 hours of admission and the following tests were performed: serum concentrations of P1NP, OC, and βCTX using an automated electrochemiluminescent immunoassay (Elecsys 2010, Roche Diagnostics, Ltd Corp., Indianapolis, IN, USA), 25 (OH) vitamin D [25(OH)D] by a radioimmunoassay kit (DiaSorin, Stillwater, MN, USA), intact PTH by 2-site chemiluminescent enzyme-linked immunoassay on DPC Immulite 2000 (Diagnostic Products, Los Angeles, CA, USA), total calcium, phosphate and magnesium, as well as routine laboratory investigations, including complete blood count, electrolytes, renal (creatinine, urea), liver (alanine aminotransferase [ALT], gamma-glutamyltransferase [GGT], alkaline phosphatase [ALP], albumin, and total bilirubin) and thyroid function tests (thyroid-stimulating hormone [TSH]; free thyroxine [fT4]), by standard automated laboratory methods. Intra- and inter-assay coefficients of variation (CV) for P1NP were 2.6% and 4.1%, respectively, for OC 3.6% and 6.6%, respectively, and for βCTX 3.2% and 6.5%, respectively; for 25(OH)D and PTH, the intra- and interassay CV ranged from 2.1% to 12.7%. Calcium concentrations were corrected for serum albumin. Vitamin D status was defined as deficient for circulating 25(OH)D concentration <25 nmol/L and as insufficient for 25–50 nmol/L. Secondary hyperparathyroidism (SHPT) was defined as elevated serum PTH (>6.8 pmol/L, the upper limit of the laboratory reference range). The glomerular filtration rate (GFR) was estimatedCitation36 and chronic kidney disease (CKD ≥stage 3) was defined as GFR <60 mL/min/1.73 m2. Anemia was defined as hemoglobin <120 g/L. Similar laboratory tests, equipment, methods, and definitions were used in the validation cohort.
Statistical analyses
The statistical analysis was performed with Stata software version 10 (StataCorp, College Station, TX, USA). Continuous variables are expressed as mean ± SD and compared using analysis of variance. Categorical variables are presented as numeral/percentages and compared by chi-square and Fisher exact tests. The correlations between the variables were determined by Pearson’s coefficients. For Pearson correlations and regressions, values of all continuous laboratory parameters were logarithmically transformed to account for the skewed nature of most of these variables. The admission P1NP/βCTX ratio was analyzed as both a continuous and a categorical variable; in the latter, P1NP/βCTX ratio was categorized into three groups (tertiles) or as the median value. Univariate and multivariate logistic analyses were performed to identify factors associated with the presence of HF or of any nonvertebral fracture. Multivariate forward stepwise procedures (covariates with P≤0.100 in univariate analysis were selected for entry) were performed. To quantify the significance of multicollinearity phenomena in regression analyses, the variance inflation factor was calculated. To quantify the discriminative utility for serum P1NP/βCTX ratio, albumin concentration, other parameters of interest, and their combination receiver operating characteristic (ROC), analysis was used and the predictive accuracy was expressed as area under curve (AUC). All statistical tests were two tailed and P-values <0.05 was considered statistically significant.
Results
Patient characteristics
In the entire cohort, patients averaged 2.7 chronic diseases per person, and the most common were hypertension (60.0%), osteoarthritis (42.5%), abnormal gait with the use of an assistive device (42.0%), diabetes mellitus type 2 (DM, 22.0%), CKD (21.3%), coronary artery disease (CAD, 17.1%), chronic obstructive airway disease (COPD, 15.4%), atrial fibrillation (AF, 14.8%), dementia (14.4%), cerebrovascular disease (12.2%), malignancy (10.4%), and chronic heart failure (7.8%). Four and more chronic conditions were identified in total in 28.5% of patients with the greatest burden, as expected, among the HF patients (36.0% vs 29.4% in the nonfracture group, P=0.040). Prior to admission, osteoporosis has been diagnosed in 239 (19.3%) patients and 182 (14.7%) subjects were receiving antiresorptive treatment.
Regarding laboratory parameters, both groups with fracture (HF and other nonvertebral fractures), compared to the nonfracture group, had significantly higher mean levels of βCTX, lower P1NP/βCTX ratios, and concentrations of calcium and higher prevalence of hyperparathyroidism (). Subjects with HF in addition exhibited higher levels of PTH, lower OC/βCTX ratios, phosphate, magnesium, hemoglobin, albumin concentrations and GFR, as well as a higher prevalence of hypoalbuminemia, vitamin D deficiency, and anemia. On admission, hypoalbuminemia (<33 g/L) was observed in 688 (55.6%) patients including 342 (75.2%) with HF, 185 (46.4%) with other fractures, and 161 (42.2%) without fractures. Of note, mean serum levels of P1NP, OC, P1NP/OC ratio, 25(OH)D, as well as creatinine, ALP, TSH, and fT4 did not differ between the three groups.
Table 1 Demographic, clinical, and laboratory characteristics of orthogeriatric patients by fracture status
Patients receiving antiosteoporotic therapy (a bisphosphonate plus vitamin D and calcium supplements) at least for 3 months prior to hospital admission (n=182) compared to those who were not treated (n=1,057) had significantly higher mean levels of serum P1NP/βCTX ratio (+16.7%: 141.8± 120.7 vs 121.5±90.8) and 25(OH)D (+19.6%: 73.1±24.4 vs 61.1±26.3 nmol/L) and lower βCTX (−7.3%: 0.38±0.27 vs 0.52±0.36 µg/mL) (all P<0.01), whereas the P1NP, PTH, and albumin levels were not different.
Osteoporotic fractures and laboratory parameters (correlation analyses)
These relationships have been evaluated for laboratory parameters expressed as both continuous and categorical variables. Among 13 studied laboratory parameters (P1NP, OC, βCTX, P1NP/βCTX ratio, OC/βCTX ratio, PTH, 25(OH)D, calcium, phosphate, magnesium, ALP, albumin, and hemoglobin), analyzed as continuous log-transformed variables adjusted for age and gender, the highest Pearson correlation coefficients in relation to the presence of HF or any fracture demonstrated the PINP/βCTX ratio and albumin: −0.3015 and −0.3667, respectively, for HF, and −0.1971 and −0.2133, respectively, for any fracture (all P=0.000). Only age showed higher correlation coefficients (r=0.4736, P=0.000 for HF; r=0.2902, P=0.000 for any fracture). Other clinical (female, dementia, AF, CAD, anemia, arthritis, osteoporosis, history of stroke or transient ischemic attack (TIA), use of walking aid) and laboratory (P1NP, OC, βCTX, OC/βCTX ratio, PTH, calcium, phosphate, magnesium) parameters were also significantly but weaker associated with the presence of fracture (r ranged between 0.2930 for dementia and HF, and 0.075 for stroke and any fracture).
Multivariate logistic regressions performed with HF or any fracture as a dependent variable and all clinical and laboratory characteristics with P≤0.100 in univariate analysis as independent variables after adjusting for age and gender revealed that both lower serum P1NP/βCTX ratio and lower albumin concentration (as continuous variables) are independent and significant factors associated with these conditions (β coefficients 0.897 and 0.869, respectively, P=0.000 for both variables). These models explained 28.5% and 22.3% of variance among patients with an HF or any fracture, respectively, correctly classifying 77.4% and 70.5% of cases, respectively. For HF, the model’s sensitivity was 79.4%, specificity 74.9%, positive predictive value (PPV) 79.2% and negative predictive value (NPV) 75.1%, and for any fracture, 89.9%, 26.7%, 73.5%, and 53.8%, respectively. For the presence of HF, the AUC was 0.799 for P1NP/βCTX and 0.816 for albumin, for the presence of any fracture, the AUC was 0.701 and 0.713, respectively.
Because of practical considerations, we further examined the impact and clinical usefulness of the P1NP/βCTX ratio and albumin level as categorical variables. First, we examined the association of fracture presence and the serum P1NP/βCTX ratio divided into tertiles. shows the tertile groupings and the percentage of individuals in each tertile, as well as the ORs for fracture presence. Proportion of patients with fractures decreased sharply from tertile 1 (lowest) to tertile 3 (highest). With tertile 3 (P1NP/βCTX >129.2, mean 219.3±112.6) used as the reference, the odds of fracture were significantly higher in tertiles 2 (P1NP/βCTX 78.6–129.2, mean 100.7±14.3) and 1 (P1NP/βCTX <78.6, mean 53.9±15.8). After adjusting for age and gender, patients in tertile 2 had a 1.6-fold higher risk of HF or any fracture, while for those in tertile 1, the risk of HF was 3.4-fold higher and the risk of any fracture was 2.5-fold higher. In other words, the ORs for the presence of fracture linearly increased across decreasing P1NP/βCTX ratio tertiles, indicating a dose–response effect.
Table 2 Presence of fracture in hospitalized orthogeriatric patients according to serum P1NP/βCTX ratio tertiles
We further performed multivariate forward stepwise logistic regression analyses for the presence of HF or of any fracture according to serum P1NP/βCTX ratio tertiles including in the models the following variables: age, gender, 25(OH)D, PTH, calcium (corrected for albumin), phosphate, magnesium, OC, albumin, alkaline phosphatase, presence of dementia, cardiovascular diseases (CAD, AF, CVA, and CHF), diabetes mellitus, history of smoking, and alcohol consumption status. The adjustment for all these confounding factors did not significantly alter the results. Lower P1NP/βCTX ratio remained an independent and powerful indicator for fracture presence. The ORs for HF and any fracture demonstrated a similar linearly increased pattern across decreasing P1NP/βCTX ratio tertiles (, model 2). Compared with the highest tertile, the presence of HF among patients in the lowest tertile was more than 3-fold higher and among patients in the middle tertile 1.5 times higher, whereas the presence of any fracture was 2.3-fold and 1.6-fold higher, respectively.
Albumin levels on admission analyzed in tertiles and adjusted for age and gender also demonstrated a dose–response relationship with fracture presence. Compared to tertile 3 (the highest: >34 g/L, mean 37.3±2.2 g/L), the HF patients in tertile 2 (16–34 g/L, mean 32.4±1.04 g/L) had an OR of 2.2 (95% CI: 1.4–3.3, P=0.000) and in tertile 1 (the lowest: <31 g/L, mean 27.6±2.5 g/L), an OR of 4.6 (95% CI: 3.0–7.1, P=0.000); similarly, patients with any fracture had ORs of 1.3 (95% CI: 0.98–1.8, P=0.065) and 2.8 (95% CI: 2.0–3.8, P=0.000), respectively.
Next, we dichotomized subjects using the median value of the P1NP/βCTX ratio (100.0) in our cohort. Of 612 orthogeriatric patients with the P1NP/βCTX <100.0 (under median level) on admission, 484 (79.1%) presented with a fracture. In stepwise multiple linear regression analyses which included all laboratory indices along with sociodemographic and clinical characteristics, P1NP/βCTX <100.0 and hypoalbuminemia (<33 g/L) were independent indicators of an HF (OR 2.8, 95% CI: 2.0–3.8, P=0.000 and OR 3.1, 95% CI: 2.2–4.3, P=0.000, respectively) or any fracture (OR 2.1, 95% CI: 1.6–2.8, P=0.000 and OR 1.7, 95% CI: 1.3–2.3, P=0.000, respectively).
Determinants of lower PINP/βCTX ratio and hypoalbuminemia
In multivariate forward stepwise regression analyses, which included all univariate clinical (dementia, CAD, AF, history of stroke, TIA, CKD, osteoporosis, antiosteoporotic medications use, smoking status), sociodemographic (age, gender, residence type, and use of walking aids), laboratory parameters with P≤0.100 and the presence of an HF or any fracture (separate analyses), the PINP/βCTX ratio as a continuous variable was independently predicted by the presence of HF (β=−28.602, P=0.000) or of any fracture (β=−23.4845, P=0.000), age (β=−1.3032, P=0.000), OC (β=2.574, P=0.000), GFR (β=0.482, P=0.002), and the use of antiosteoporotic medications (β=29.761, P=0.000). These data indicate that lower PINP/βCTX ratio is largely determined by increasing age, lower OC, and decline of renal function, and is strongly associated with any nonvertebral fracture and the nonuse of antiosteoporotic therapy.
Independent determinants of P1NP/βCTX <100.0 (under median level) were also assessed in a similar stepwise multiple linear regression analyses which included the laboratory, sociodemographic, and clinical characteristics. The probability of P1NP/βCTX <100.0 increased with increment in each year of age by 4% (OR 1.04, 95% CI: 1.02–1.06, P=0.000), the presence of HF by 2.8-fold (OR 2.8, 95% CI: 2.0–3.8, P=0.000), and the presence of any fracture by 2.1-fold (OR 2.1, 95% CI: 1.6–2.8, P=0.000), and decreased with the use of antiosteoporotic medications by 34.6% for HF (OR 0.65, 95% CI: 0.43–1.00, P=0.050) and 32.4% for any fracture (OR 0.68, 95% CI: 0.48–0.96, P=0.027).
With regard to hypoalbuminemia (<33 g/L), a similar multiple regression revealed that the presence of HF (OR 2.8, 95% CI: 2.0–3.9, P=0.000) or of any fracture (OR 1.5, 95% CI: 1.2–2.0, P=0.002) is an independent determinant of this condition and its probability with each year of age increases by 3% in HF patients (OR 1.03, 95% CI: 1.01–1.05, P=0.005) and by 4% in the group with any fracture (OR 1.04, 95% CI: 1.02–1.05, P=0.000).
Taken together, these data suggest that among the studied laboratory parameters both lower serum PINP/βCTX ratio and hypoalbuminemia are strongly associated with and are the best to indicate a nonvertebral osteoporotic fracture.
Informative/predictive values of lower P1NP/βCTX ratio and hypoalbuminemia
In the attempt to give to practicing physicians a simple tool, we focused on the median P1NP/βCTX ratio (<100.0) and low albumin (<33 g/L) as cutoff values. We additionally evaluated the discriminative values of recently recommended treatment targets for antiosteoporotic therapies: P1NP >62 µg/L for bone-forming agentsCitation37 and βCTX <0.250 µg/L for antiresorptive therapy.Citation20,Citation38 In our cohort, there were only nine (0.73%) patients (including five without fractures, one with an HF, and three with other fractures) in whom both these markers were within the targeted zone; none of them had P1NP/βCTX <100.0 and eight subjects (including all four with fractures) have been receiving antiosteoporotic treatment. In other words, both markers were in the desired zone only in 1.3% of patients without fractures and in 0.47% of patients with any fracture (0.22% among HF). However, P1NP <62 µg/L was found in 942 (76.6%) patients (in 368 without fracture, 304 with HF and 270 with other fractures), and βCTX >0.250 µg/L in 955 (77.7%) patients (in 376, 302 and 277, respectively).
The results of the analyses performed with five explanatory variables are displayed in and . AUC values after adjustment for age and gender for all markers were between 0.691 and 0.855, indicating a mild-to-moderate discriminatory ability. The P1NP/βCTX ratio <100.0 and albumin <33 g/L yielded the best AUC measures for HF (0.802 and 0.806, respectively) or for any fractures (0.711 and 0.706, respectively).
Table 3 Bone turnover markers and hypoalbuminemia as indicators of nonvertebral osteoporotic fractures in orthogeriatric patients
Figure 1 Discriminative information on nonvertebral fracture presence according to serum P1NP/βCTX ratio and albumin concentrations in orthogeriatric patients.
Abbreviations: βCTX, cross-linked carboxy-terminal telopeptide of type 1 collagen; HF, heart failure; P1NP, amino-terminal propeptide of type 1 procollagen.
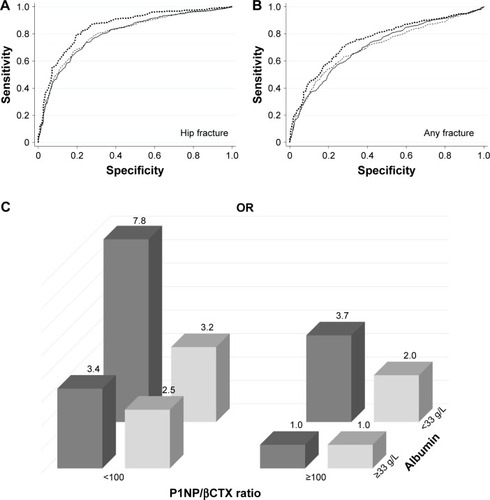
Interestingly, among patients with only P1NP/βCTX <100.0, the ORs were 3.4-fold (95% CI: 2.0–5.7, P=0.000) and 2.5-fold (95% CI: 1.7–3.7, P=0.000) higher in subjects with HF or any nonvertebral fracture, respectively, and among patients with only albumin <33 g/L, the ORs were 3.7-fold (95% CI: 2.7–6.0, P=0.000) and 2.0-fold (95% CI: 1.4–2.8, P=0.000) higher, respectively (). The data further suggest the independent and strong association of each of these two factors with nonvertebral fractures.
When P1NP/βCTX ratio <100.0 and albumin <33 g/L were combined, the ORs for HF or for any fracture, compared with the nonfractured group, were 7.8 and 3.2, respectively, and the AUC improved (0.855 and 0.754, respectively). The presence of both these characteristics was a more sensitive (83.0% for HF and 85.7% for any fracture) and accurate indicator of fracture presence (79.6% and 71.6%, respectively) compared to other variables.
Validation of serum P1NP/βCTX ratio and albumin levels as indicators of nonvertebral fracture
Patients in the validation dataset comparing to those in the test dataset did not show significant differences in sociodemographics, comorbidities, and antiresorptive medication use. When the P1NP/βCTX ratio cutoff of <100.0 and albumin <33 g/L were applied to the validation dataset, they showed significant and similar discriminative values. P1NP/βCTX <100: for HF AUC 0.810 (sensitivity 78.7%, PPV 75.9%), for any fracture AUC 0.710 (sensitivity 89.3%, PPV 73.8%); albumin <33 g/L: for HF AUC 0.811 (sensitivity 78.3%, PPV 71.7%), for any fracture AUC 0.705 (sensitivity 88.3%, PPV 71.7%); both factors combined: for HF AUC 0.861 (sensitivity 86.2%, PPV 81.1%), for any fracture AUC 0.765 (sensitivity 88.0%, PPV 76.7%).
Discussion
In this study, in a large cohort of consecutive hospitalized orthogeriatric patients, lower levels of serum P1NP/βCTX ratio and albumin concentration were 1) strong independent indicators of HF or of any nonvertebral fracture, 2) showed a dose-dependent relationship with the prevalence of fractures, and 3) demonstrated a discrimination ability of acceptable precision that exceeded the discrimination ability of other studied laboratory parameters. To the best of our knowledge, this is the first study to demonstrate the clinical utility of lower serum P1NP/βCTX ratio and hypoalbuminemia as promising biomarkers for predicting osteoporotic fractures in older adults.
The prevalence of both lower P1NP/βCTX ratio (reflects an imbalance between total bone formation and resorption in favour of the latter) and hypoalbuminaemia increase with age, and both factors are independently associated with osteoporotic fractures; lower PINP/βCTX ratio is also largely determined by decline of renal function and the nonuse of antiosteoporotic therapy. It appears that these two characteristics – serum PINP/βCTX ratio and albumin – accumulate key determinants of fracture risk incorporating the effects of multiple clinical and metabolic abnormalities reported in the literature and observed in our univariate analysis. Although there are no prior studies using P1NP/βCTX ratio, our results are consistent with the only previous report showing that the ratio of a urinary resorption to serum formation marker (u-NTx/OC) was predictive of fractures independent of FRAX.Citation23 A number of reports, but not all,Citation11,Citation20,Citation39,Citation40 indicated a link between abnormalities in BTMs, bone loss, and increased risk of fracture independent of BMD.Citation10,Citation12,Citation13,Citation41–Citation44
The pathophysiological mechanism(s) underlying the relationship between altered albumin homeostasis and osteoporotic fractures is not fully understood. Hypoalbuminemia could be caused and/or aggravated by numerous chronic diseases associated with increased risk of falls and fractures.Citation26,Citation27 Hypoalbuminemia may directly and indirectly influence bone status, shifting the balance toward bone resorption via its effects on the nuclear factor-kB, disturbed inflammatory and antioxidant responses, reduced flux of minerals to and from the bone, decreased formation of calcium phosphate apatite crystals, as well as affecting the metabolism of PTH, vitamin D binding protein, and Gla-protein.Citation32,Citation45
Our data showed that among hospitalized orthogeriatric patients, the serum P1NP/βCTX <100 or/and albumin <33 g/L at admission outweighed other laboratory parameters in its discriminatory ability of fracture presence, especially for HF, and the combination of both signs doubles the ORs. The fact that near equal proportions of patients admitted with a fracture had only one of these characteristics () reflects the complexity, multifactorial nature, and heterogeneity of metabolic mechanisms underlying osteoporotic fractures and indicates the usefulness to include in the screening strategy measuring of both parameters, each of which demonstrated a strong independent association with fractures.
Taken together, in older patients, serum BTMs and albumin may perhaps help distinguish subgroups with different prognoses for osteoporotic fracture: 1) high risk if P1NP/βCTX <100.0 and albumin <33 g/L (OR 7.8 for HF and 3.2 for any fracture), 2) intermediate risk if P1NP/βCTX <100.0 (OR 3.4 and 2.5, respectively) or albumin <33 g/L (OR 3.7 and 2.0), and 3) low risk (<0.5%) in older adults with βCTX <0.250 µg/L and P1NP >62.0 µg/L. Although the discriminative ability of these markers is only moderate (but higher when compared with other currently available indices), they may be particularly useful in persons who have negative BMD test. It should be, however, emphasized that the fracture risk remains substantial in subjects with P1NP/βCTX >100.0 and albumin >33 g/L; such characteristics demonstrated 58.1% of orthogeriatric patients without fracture but also 18.7% of all fracture patients including 9.0% with HF, indicating that in near 1/5 of subjects with fragility fractures other factors rather than the total balance between bone formation and resorption and/or albumin homeostasis are important in the development of fractures.
Limitations of the study include: 1) cross-sectional design (results describe associations rather than causation), 2) comparison with nonfractured elderly orthopedic patients (not a healthy control group), a significant proportion of which may have undiagnosed/undocumented osteoporosis, 3) reliance on single measurement, and 4) data from one medical center, mostly on white older adults, limiting the generalizability of the results. Of note, within 26 hours after fracture, BTMs are not altered from the preinjury levels,Citation46 but both bone formation and bone resorption markers significantly increase within 6 weeks to 6 months after fracture, reflecting the fracture healing process, and these changes may persist for up to a year.Citation46–Citation49 As in all our patient, fasting venous blood samples were collected within 24 hours of admission to the hospital it is unlikely that the fracture per se contributed to the observed changes in BTMs.
This study also has several strengths: the relatively large number of patients, adjustment for a wide range of confounding factors, and the use of validation cohort. In multivariate regression analyses, the variance inflation factor was between 1.07 and 1.18, indicating that the amount of multicollinearity was not significant.
Conclusion
In an unselected cohort of hospitalized consecutive orthogeriatric patients, both serum P1NP/βCTX ratio and albumin levels demonstrated an inverse dose–effect relationship with the prevalence of nonvertebral fractures and independently indicated fracture presence with acceptable discriminatory power. Lower P1NP/βCTX (<100) and hypoalbuminemia (<33 g/L) could be useful simple and inexpensive tools to obtain additive prognostic information on fracture risk in the elderly. However, confirmation in other cohorts is needed to further support the applicability of these characteristics to the total population.
Summary
In a cohort of unselected orthogeriatric patients (n=1,239), serum P1NP/βCTX ratio and albumin levels demonstrated an inverse dose–effect relationship with the prevalence of nonvertebral fractures. P1NP/βCTX <100 and hypoalbuminemia (<33 g/L) could be useful additive prognostic tools for fracture risk stratification in the elderly.
Author contributions
AF was the coordinator of the study and together with LF participated in the study design, data collection, analysis, interpretation, and article writing. PS operated on the patients and contributed to data interpretation. WS performed statistical analysis and took part in interpretation of data. The final version of the manuscript was approved by all authors. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- MarquesAFerreiraRJSantosELozaECarmonaLda SilvaJAThe accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysisAnn Rheum Dis201574111958196726248637
- LeslieWDLixLMComparison between various fracture risk assessment toolsOsteoporos Int201425112123797847
- DaganNCohen-StaviCLeventer-RobertsMBalicerRDExternal validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort studyBMJ2017356i675528104610
- KanisJAHarveyNCCooperCAdvisory Board of the National Osteoporosis Guideline GroupA systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis FoundationArch Osteoporos20161112527465509
- KanisJAOdenAJohanssonHBorgströmFStrömOMcCloskeyEFRAX and its applications to clinical practiceBone200944573474319195497
- NguyenNDEismanJACenterJRNguyenTVRisk factors for fracture in nonosteoporotic men and womenJ Clin Endocrinol Metab200792395596217164302
- RozentalTDHerderLMWalleyKC25-Hydroxyvitamin-D and bone turnover marker levels in patients with distal radial fractureJ Bone Joint Surg Am201597201685169326491133
- KanisJAHarveyNCJohanssonHOdénALeslieWDMcCloskeyEVFRAX and fracture prediction without bone mineral densityClimacteric201518Suppl 229
- BurchJRiceSYangHSystematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groupsHealth Technol Assess201418111180
- JohanssonHOdenAKanisJAA meta-analysis of reference markers of bone turnover for prediction of fractureCalcif Tissue Int201494556056724590144
- VasikaranSEastellRBruyereOIOF-IFCC Bone Marker Standards Working GroupMarkers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standardsOsteoporos Int201122239142021184054
- Arceo-MendozaRMCamachoPPrediction of fracture risk in patients with osteoporosis: a brief reviewWomens Health (Lond)201511447748226236988
- ChubbSAByrnesEManningLReference intervals for bone turnover markers and their association with incident hip fractures in older men: the Health in Men studyJ Clin Endocrinol Metab20151001909925322270
- ShigdelROsimaMAhmedLABone turnover markers are associated with higher cortical porosity, thinner cortices, and larger size of the proximal femur and non-vertebral fracturesBone2015811626112819
- ShiehAHanWIshiiSGreendaleGACrandallCJKarlamanglaASQuantifying the balance between total bone formation and total bone resorption: an index of net bone formationJ Clin Endocrinol Metab201610172802280927336357
- HannemannAWallaschofskiHReference intervals for serum concentrations of three bone turnover markers for men and womenBone20169321626123593
- MarquesEAGudnasonVSigurdssonGAre bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES-Reykjavik studyOsteoporos Int20162751765177626630978
- BiverEUse of bone turnover markers in clinical practiceCurr Opin Endocrinol Diabetes Obes201219646847323128576
- GarneroPNew developments in biological markers of bone metabolism in osteoporosisBone201466465524909537
- VasikaranSDChubbSAThe use of biochemical markers of bone turnover in the clinical management of primary and secondary osteoporosisEndocrine201652222222526906711
- EastellRRogersANiXKregeJHEffects of raloxifene and alendronate on bone turnover as assessed by procollagen type I N-terminal propeptideOsteoporos Int20112261927193420838771
- SeemanENguyenTVBone remodeling markers: so easy to measure, so difficult to interpretOsteoporos Int20162713335
- MeltonLJ3rdAtkinsonEJAchenbachSJPotential extensions of the US FRAX algorithmJ Osteoporos2012201252879022934235
- AscenziPdi MasiAFanaliGFasanoMHeme-based catalytic properties of human serum albuminCell Death Discov201511502527551458
- FanaliGdi MasiATrezzaVMarinoMFasanoMAscenziPHuman serum albumin: from bench to bedsideMol Aspects Med201233320929022230555
- DrevetSBioteauCMaziereSPrevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fractureOrthop Traumatol Surg Res2014100666967424998085
- GoisserSSchraderESinglerKMalnutrition according to mini nutritional assessment is associated with severe functional impairment in geriatric patients before and up to 6 months after hip fractureJ Am Med Dir Assoc201516866166725864084
- CabrerizoSCuadrasDGomez-BustoFArtaza-ArtabeIMarín-CiancasFMalafarinaVSerum albumin and health in older people: review and meta analysisMaturitas2015811172725782627
- LevittDGLevittMDHuman serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurementsInt J Gen Med2016922925527486341
- FisherLSrikusalanukulWFisherASmithPLiver function parameters in hip fracture patients: relations to age, adipokines, comorbidities and outcomesInt J Med Sci201512210011525589886
- YuwenPChenWLvHAlbumin and surgical site infection risk in orthopaedics: a meta-analysisBMC Surg2017171728093079
- AfshinniaFPennathurSAssociation of hypoalbuminemia with osteoporosis: analysis of the National Health and Nutrition Examination SurveyJ Clin Endocrinol Metab201610162468247427144935
- NakamuraKOyamaMSaitoTNutritional and biochemical parameters associated with 6-year change in bone mineral density in community-dwelling Japanese women aged 69 years and older: the Muramatsu StudyNutrition201228435736121917422
- FormosaMMXuereb-AnastasiABiochemical predictors of low bone mineral density and fracture susceptibility in Maltese postmenopausal womenCalcif Tissue Int2016981284126400554
- MaMKYapDYYipTPLuiSLLoWKCharlson co-morbidity index and albumin significantly associated with fracture risk in peritoneal dialysis patientsNephrology (Carlton)201318536536823600370
- LeveyASde JongPECoreshJThe definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference reportKidney Int2011801172821150873
- EastellRChristiansenCGrauerAEffects of denosumab on bone turnover markers in postmenopausal osteoporosisJ Bone Miner Res201126353053720839290
- EastellRBartonIHannonRAChinesAGarneroPDelmasPDRelationship of early changes in bone resorption to the reduction in fracture risk with risedronateJ Bone Miner Res20031861051105612817758
- SzulcPMontellaADelmasPDHigh bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: the prospective MINOS studyAnn Rheum Dis20086791249125518065499
- FinnesTELofthusCMMeyerHEProcollagen type 1 amino- terminal propeptide (P1NP) and risk of hip fractures in elderly Norwegian men and women. A NOREPOS studyBone2014641724667179
- GarneroPBiomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoringMol Diagn Ther200812315717018510379
- IvaskaKKGerdhemPVaananenHKAkessonKObrantKJBone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 yearsJ Bone Miner Res201025239340319961336
- DaiZWangRAngLWYuanJMKohWPBone turnover biomarkers and risk of osteoporotic hip fracture in an Asian populationBone20168317117726555636
- MorrisHAEastellRJorgensenNRIFCC-IOF Working Group for Standardisation of Bone Marker Assays (WG-BMA)Clinical usefulness of bone turnover marker concentrations in osteoporosisClin Chim Acta20174973441
- Abu-AmerYNF-kappaB signaling and bone resorptionOsteoporos Int20132492377238623468073
- IvaskaKKGerdhemPAkessonKGarneroPObrantKJEffect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly womenJ Bone Miner Res200722811561164
- AkessonKKakonenSMJosefssonPOKarlssonMKObrantKJPeterssonKFracture-induced changes in bone turnover: a potential confounder in the use of biochemical markers in osteoporosisJ Bone Miner Metab20052313035
- ObrantKJIvaskaKKGerdhemPAlataloSLPeterssonKVaananenHKBiochemical markers of bone turnover are influenced by recently sustained fractureBone200536578679215804493
- IkegamiSKamimuraMNakagawaHComparison in bone turnover markers during early healing of femoral neck fracture and trochanteric fracture in elderly patientsOrthop Rev (Pavia)200912e2121808683
