Abstract
Objective
The primary objective of this study was to evaluate whether the polymorphism of poly (ADP-ribose) polymerase-1 (PARP-1) is involved as a potential risk factor in the development of acute renal injury in elderly Chinese patients with diabetes mellitus.
Subjects and methods
In this pilot study, diabetic patients of either gender (aged ≥65 years) with a confirmed diagnosis of acute renal injury and individuals with no clinical symptoms of acute renal injury were enrolled at Nanxishan Hospital of Guangxi Zhuang Autonomous Region, China. Genetic polymorphism of PARP-1 was assessed using polymerase chain reaction-restriction fragment length polymorphism assay. Cytokine levels (interleukin [IL]-6, IL-1β and tumor necrosis factor-alpha) were measured in the serum samples by sandwich enzyme-linked immunosorbent assay technique.
Results
A total of 130 Chinese patients with acute renal injury and 130 Chinese individuals with no clinical symptoms of acute renal injury were included. We found that the patients with GG genotype and carriers of the G and C alleles of PARP-1 were at high risk of developing acute renal injury. Moreover, del/ins polymorphism of the NF-κB1 gene was also found to be associated with acute renal injury. In addition, the levels of IL-6, IL-1β and tumor necrosis factor-alpha were significantly increased in patients with acute renal injury (p<0.05).
Conclusion
Our findings showed the involvement of PARP-1 polymorphisms in the development of acute renal injury in Chinese individuals. This study identified the involvement of two SNPs of PARP-1 (C410T and G1672A) in development of acute renal injury among Chinese diabetic patients. Also, increased expression of C and G alleles of PARP-1 can be considered as one of the potential risk factors for developing acute renal injury. Increased serum cytokine levels can be considered as one of the potential risk factors for developing acute renal injury.
Introduction
Diabetes-induced renal injury is one of the most common chronic microvascular complications in patient of type 2 diabetes mellitus. In patients with uncontrolled diabetes, diabetic nephropathy is known to cause end-stage renal disease.Citation1 The key manifestations of diabetic nephropathy are albuminuria and high blood pressure, eventually lead to renal failure which is one of the most common causes of death in patients with diabetes.Citation2 Seaquist et al showed the involvement of genetic susceptibility in diabetes-induced renal injury.Citation3,Citation4 Hur et alCitation5 found the involvement of polymorphisms of poly (ADP-ribose) polymerase (single nucleotide polymorphisms [SNPs] 1963AG and þ28077GA) in development of nephritis among Korean patients.
It has been reported that poly (ADP-ribose) polymerase-1 (PARP-1)-mediated signaling can trigger an outburst of proinflammatory regulators and the cell death cascade is also well known.Citation6 The PARP-1 catalyzes the process of PARylation by attaching the polymers of ADP-ribose to target protein motifs via ester linkage and triggers an array of vital cell functions including chromatin structure, DNA repair, transcriptional regulation, apoptosis, necrosis, cell separation and differentiation.Citation7 PARP-1 maintains the genomic integrity by PARylation of histones and other key enzymes, the DNA repair mechanism, interprotein interactions and gene expression to ensure optimum cellular homeostasis.Citation8–Citation13 However, through cellular and/or genotoxic stress, prolonged activation of PARP-1 leads to a decrease in β-nicotinamide adenine dinucleotide and adenosine triphosphate and causes release of apoptosis-inducing factor, a mitochondrial proapoptotic protein.Citation13 Such stress-induced dysregulation in cellular homeostasis mediated by PARP-1 triggers the downstream necrosis cascade of cell death.Citation12
Hur et alCitation5 suggested the involvement of PARP-1 polymorphisms in the development of nephritis among Korean patients and identified the involvement of two SNPs (1963AG and þ28077GA). However, there was no study evaluating the involvement of PARP-1 polymorphisms in development of acute renal injury in diabetic patients. The primary objective of this study was to evaluate whether the polymorphism of PARP-1 is involved as a potential risk factor in the development of acute renal injury in elderly Chinese patients with diabetes mellitus. Our pilot study was designed to assess involvement of PARP-1 alleles and SNPs in development of acute renal injury in diabetic patients. We also investigated the cytokine levels among Chinese patients with acute kidney injury. Our study results can serve the basis for conducting large multicentric randomized clinical study.
Subjects and methods
In this pilot study, the diabetic patients of both genders (age ≥65 years) with a confirmed diagnosis of acute renal injury and individuals with no acute renal injury (control group) were enrolled at Nanxishan Hospital of Guangxi Zhuang Autonomous Region, China. The study was approved by the institutional ethics committee of Nanxishan Hospital of Guangxi Zhuang Autonomous Region, and written consent was obtained from each study participant. All participants were informed about the study procedures and the potential benefits to society. All participants underwent laboratory tests to confirm their eligibility.
Plasma samples were collected from each participant, and DNA from leukocytes was extracted by the high salt DNA extraction method. All isolated DNA samples were kept at −80°C for further assessment. Polymorphisms of the PARP-1, NF-κB1 and NF-κBIA genes were studied in DNA samples by polymerase chain reaction-restriction fragment length polymorphism analysis. For the PARP-1 C410T and G1672A promoter regions, 297 and 187 base pair polymerase chain reaction (PCR) fragments were amplified in a 25 μL reaction volume at pH 8.3 consisting of 50 mM KCl, 100 ng genomic DNA, 200 pM deoxynucleotide, 200 pM of 10 mM Tris-HCl, 1 U Taq polymerase (Sigma) and 2 mM MgCl2 (Fermentas). The upstream sequences of PARP-1 promoter regions C410T and G1672A were 5′-TCCAGTGGCACTATCAT-3′ and 5′-GCGAGACCCTGTCCCTAA-3′, respectively. The downstream sequences of PARP-1 promoter regions C410T and G1672A were 5′-GTTGTGAGACATAGGCCGAAT-3′ and 5′-TCCCCCTTTTATTTTTGAGACTG-3′, respectively. The polymerase chain reaction-restriction fragment length polymorphism was performed using an optimized sequence of cycles: a denaturation phase at 95°C (2 min) followed by 30 cycles at 95°C (30 s), 60°C (30 s), 72°C (1 min) and a final incubation phase at 72°C (5 min). The PCR products were treated with 5 U of HpyF3I (DdeI; Fermentas) and Bsh1236I (Fermentas) at 37°C overnight for digestion. The resulting fragments were run on ethidium bromide-stained 3% agarose gel for 45 min at 90 V and quantified through direct detection under ultraviolet light ( and ). Likewise, the NF-κB1 and NF-κBIA genes were amplified by 285 and 424 base pair PCR fragments using the same reagents. The PCR running sequence included a denaturing step at 95°C (1 min) followed by 35 cycles at 95°C (30 s), 61°C (30 s), 72°C (1 min) and a final incubation at 72°C (5 min). The PCR products were treated with 5 U PflMI (Van91I) and HaeIII (BsuRI; Fermentas) at 37°C overnight for digestion and the digested products were run on ethidium bromide-stained 3% agarose gel for 45 min at 90 V and quantified directly under ultraviolet light. Cytokine levels (interleukin [IL]-6, IL-1β and tumor necrosis factor alpha [TNF-α]) were measured in the serum samples by a specific sandwich enzyme-linked immunosorbent assay technique using commercial kits (Assaypro Human IL-6, IL-1β and TNF-α) coated with antibodies specific to human leptin and adiponectin antibodies, according to the manufacturer’s protocols (St Charles).
Figure 1 PARP-1 C410T polymorphism (A) and G1672A polymorphism (B).
Notes: (A) Sample 1 shows TT genotype, samples 2 and 3 show CC genotype and samples 4 and 5 show CT genotype of PARP-1 C410T. (B) Samples 1 and 4 show GA genotype, samples 2 and 3 show GG genotype and sample 5 shows AA genotype of PARP-1 G1672A. Patient-related data were masked in the figure.
Abbreviation: PARP-1, poly (ADP-ribose) polymerase-1.
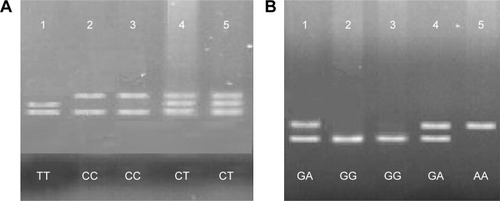
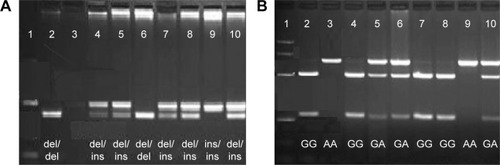
Figure 2 NF-κB1 polymorphism (A) and NF-κBIA polymorphism (B).
Statistical analysis
The study was designed as a preliminary pilot study to assess the polymorphism association of PARP-1 with acute renal injury in Chinese diabetic patients. Hence, no formal sample size calculation was performed. We planned to enroll at least 100 individuals in each group (patients with acute renal injury and patients without acute renal injury). Our study can serve as a basis for large genetic clinical studies to assess PARP-1 polymorphism in patients with acute renal injury. Quantitative variables were presented as mean (±SD) and analyzed by parametric and nonparametric statistical tests depending on the number of groups for comparison and the distribution of data, using two-sided statistical tests. Categorical variables were presented as absolute numbers and/or percentages of subjects in each category and analyzed by the chi-square or Fisher’s exact test depending on the size of the data, using two-sided statistical test. Demographic characteristics were presented by descriptive statistics, using mean (SD) (for numerical data) and as absolute values (for categorical data). Genotype involved in gene polymorphism in patients with acute renal injury was analyzed using univariate analysis. Odds ratios (ORs) were calculated based on univariate analysis using the chi-square or Fisher’s exact test depending on the size of the data. Plasma levels of cytokines in individuals with acute renal injury and without acute renal injury by different genotypes were analyzed either using unpaired t-test or Mann–Whitney test, based on normality of data. Kolmogorov–Smirnov test or Shapiro–Wilks test was used to check the distribution of numerical data. Unpaired t-test was applied if the data followed normal distribution (parametric data), whereas Mann–Whitney test was applied if data followed non-normal distribution (nonparametric data). All the statistical tests were two-sided statistical tests. The data of each study participant were coded, and analyzed using the GraphPad Prism statistical software (version 6.0).
Results
A total of 130 Chinese patients with acute renal injury and 130 individuals without acute renal injury (healthy groups) were enrolled and completed the study. The data of all the enrolled individuals were analyzed. Demographic characteristics were found to be similar in both the groups ().
Table 1 Demographic characteristics of healthy individuals and acute renal injury patients
For the G1672A genotype, we found that the individuals with a G allele in PARP-1 were also at very high risk of developing acute renal injury (OR 3.96, 95% CI 2.33–6.74, p<0.0001). Also, we observed that the individuals with a GG genotype in the PARP-1 gene were at very high risk of developing acute renal injury (OR 4.09, 95% CI 2.42–6.90, p<0.001). There was no involvement of other genotypes such as GA or AA among patients with acute renal injury. None of the other genotypes of NF-κB1, NF-κBIA and PARP-1 were found to be involved (). Moreover, no evidence of PARP-1 polymorphisms was observed in individuals with any acute renal injury.
Table 2 Genotypes involved in gene polymorphism in patients with acute renal injury
For the C410T genotype, we found that the individuals with a C allele in the PARP-1 gene were at high risk of developing acute renal injury (OR 3.77, 95% CI 1.99–7.12, p<0.0001). Among patients with acute renal injury, del/ins of the NF-κB1 gene was also noted (OR 3.32, 95% CI 1.96–5.61, p<0.001). This suggests the involvement of del/ins of NF-κB1 in the development of acute renal injury. No statistically significant association of other NF-κB1 genotypes with acute renal injury was observed (). We also studied the role of cytokines in acute renal injury in relation to different genes. The results showed a significantly higher level of cytokines (IL-6, IL-1β and TNF-α) in acute renal injury patients as compared to patients without acute kidney injury ().
Table 3 Plasma level of cytokines in individuals with acute renal injury and in healthy individuals by different genotypes
Discussion
This was the first study to assess involvement of PARP-1 alleles and SNPs in development of acute renal injury among Chinese diabetic patients. We found that individuals with the GG genotype were at very high risk of developing acute renal injury. Our finding in Chinese patients was consistent with the findings of Hur et alCitation5 in Korean patients in terms of involvement of polymorphisms of PARP-1 in development of kidney-related diseases. Hur et al have identified two SNPs (1963AG and þ28077GA) of PARP-1 in development of nephritis among Korean patients. Our study identified the involvement of two new SNPs of PARP-1 (C410T and G1672A) in development of acute renal injury in Chinese diabetic patients. In this Chinese study, we also observed that individuals carrying C and G alleles of PARP-1 were at high risk of developing acute renal injury. Additionally, we observed the involvement of a del/ins polymorphism in NF-κB1 in acute renal injury development among Chinese individuals.
In our study, the individuals with a GG genotype and G allele were at very high risk of developing acute renal injury. The possible reason for involvement of the GG genotype as a risk factor for acute renal injury could be explained by molecular heterosis, which is observed iñ50% of cases of gene associations.Citation14 We also observed the involvement of an NF-κB1 polymorphism in developing acute renal injury.
It has been reported that cytokines that are released by renal tubular cells into the injured kidney play an important role in the initiation and extension of inflammation in acute kidney injury.Citation15,Citation16 Among the cytokines, interleukin IL-6, IL-1β and TNF-α play an important role in development of kidney injury.Citation15,Citation16 Studies have shown a close correlation between IL-6 expression and acute kidney injury.Citation17 Also, IL-6 contributes to the progression of renal disease and associated complications.Citation18 IL-1β is one of the cytokines most commonly present in development of kidney diseases.Citation15,Citation16 Also, IL-1β is associated with the development and progression of acute kidney injury.Citation19 TNF-α is a potent proinflammatory cytokine and an important mediator of inflammatory tissue damage,Citation16 and has been implicated in the pathobiology of acute kidney injury.Citation20 A role for TNF-α in mediating the inflammatory injury in cisplatin-induced acute renal failure has recently been established.Citation21 Based on the above facts, we measured the cytokine levels in Chinese subjects with and without acute kidney injury. In our study, we noted high levels of cytokines among Chinese patients with kidney injury; our finding was consistent with the previous reports that showed the involvement of cytokines in acute kidney injury.Citation15–Citation21
This was the first study to suggest the association of two SNPs of PAPR-1 (C410T and G1672A) with acute renal injury in Chinese patients. We also identified the association of C and G alleles of PARP-1 in acute kidney injury. Since the study was designed as pilot study and conducted at single study center in China (limitation of study). Therefore, the present findings cannot be generalized to the overall Chinese population. Based on the study results, we suggest for conducting large multi-centric randomized clinical study in future to generalize our findings. We also suggest investigating ethnic difference in involvement of PAPR-1 polymorphism in development of acute renal injury among Chinese diabetic patients, which was not possible in our study. Our study is the first pilot study which identified the involvement of SNPs and allele of PAPR-1 in diabetic patient with acute renal injury. The involvement of identified SNPs in other kidney disorders subtypes has not yet been established. Our finding encourages the researchers to focus on these two SNPs (C410T and G1672A) in other disease conditions associated with chronic diabetes mellitus. Since the objective of our pilot study was to assess the involvement of PARP-1 alleles and SNPs in development of acute renal diseases in diabetic patients; we therefore have not measured the sirtuin and PARP activity in relation with development of diabetes and associated kidney problems. We also encourage measuring the sirtuin and PARP activity in relation with the development of diabetes and associated kidney problems in future studies.
Conclusion
Our findings showed the involvement of PARP-1 polymorphisms in the development of acute renal injury in Chinese individuals. Our study identified the involvement of two SNPs of PARP-1 (C410T and G1672A) in development of acute renal injury among Chinese diabetic patients. Also, increased expression of C and G alleles of PARP-1 can be considered as one of the potential risk factors for developing acute renal injury. We also observed the involvement of an NF-κB1 polymorphism in the increased risk of acute renal injury. Moreover, the increased serum cytokine levels can be considered as one of the potential risk factors for developing acute renal injury. Our study results can serve as a basis for conducting large multicenter, multicountry, genetic clinical studies to assess the involvement of PARP-1 and NF-κB1 polymorphism in acute renal injury in elderly Chinese patients with diabetes mellitus.
Acknowledgments
This study was supported by a grant from the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region Self-financing Funds in Clinical Application Plan of Scientific Research Subject (NO Z2016261). The authors thank all patients for their participation in the study. They would also like to thank Dr Rakesh Ojha (senior consultant medical writer) for writing the manuscript and for the editorial support provided in the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- DronavalliSDukaIBakrisGLThe pathogenesis of diabetic nephropathyNat Clin Pract Endocrinol Metab20084844445218607402
- NakaiSIsekiKItamiNAn overview of regular dialysis treatment in Japan (as of 31 December 2010)Ther Apher Dial201216648352123190510
- SeaquistERGoetzFCRichSBarbosaJFamilial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathyN Engl J Med198932018116111652710189
- FreedmanBIBostromMDaeihaghPBowdenDWGenetic factors in diabetic nephropathyClin J Am Soc Nephrol2007261306131617942768
- HurJWSungYKShinHDParkBLCheongHSBaeSCPoly(ADP-ribose) polymerase (PARP) polymorphisms associated with nephritis and arthritis in systemic lupus erythematosusRheumatology (Oxford)200645671171716461442
- SmulsonMESimbulan-RosenthalCMBoularesAHRoles of poly(ADP-ribosyl)ation and PARP in apoptosis, DNA repair, genomic stability and functions of p53 and E2F-1Adv Enzyme Regul200040118321510828352
- CorcoranNMClarksonMJStuchberyRHovensCMMolecular pathways: targeting DNA repair pathway defects enriched in metastasisClin Cancer Res201622133132313727169997
- HassaPOHottigerMOThe diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerasesFront Biosci2008133046308217981777
- PhulwaniNKKielianTPoly (ADP-ribose) polymerases (PARPs) 13 regulate astrocyte activationJ Neurochem2008106257859018410506
- BaXGargNJSignaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseasesAm J Pathol2011178394695521356345
- ChiarugiAMoskowitzMAPoly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disordersJ Neurochem200385230631712675907
- YuSWWangHPoitrasMFMediation of poly(ADP-ri-bose) polymerase-1-dependent cell death by apoptosis-inducing factorScience2002297557925926312114629
- AlbulescuRCodriciEPopescuIDCytokine patterns in brain tumour progressionMediators Inflamm2013201397974823864770
- ComingsDEMacMurrayJPMolecular heterosis: a reviewMol Genet Metab2000711–2193111001792
- JohnDIMichaelJRImmune and inflammatory role in renal diseaseCompr Physiol20133295797623720336
- AliAQuocanNCharlesLEdelstein mediators of inflammation in acute kidney injuryMediators of Inflamm2009200912
- GrigoryevDNLiuMHassounHTCheadleCBarnesKCRabbHThe local and systemic inflammatory transcriptome after acute kidney injuryJ Am Soc Nephrol200819354755818235097
- JonesSAFraserDJFieldingCAJonesGWInterleukin-6 in renal disease and therapyNephrol Dial Transplant201530456457425011387
- TadayoshiKReiNRyoMExpression and function of interleukin-1β-induced neutrophil gelatinase-associated lipocalin in renal tubular cellsPLoS One20161111e016670727851800
- SusantitaphongPPerianayagamMCTighiouartHLiangosOBonventreJVJaberBLTumor necrosis factor alpha promoter polymorphism and severity of acute kidney injuryNephron Clin Pract20131231–2677323796916
- RameshGReevesWBInflammatory cytokines in acute renal failureKidney Int Suppl200491S56S61
