Abstract
Evaluation of behavior and cognition in rodent models underpins mechanistic and interventional studies of brain aging and neurodegenerative diseases, especially dementia. Commonly used tests include Morris water maze, Barnes maze, object recognition, fear conditioning, radial arm water maze, and Y maze. Each of these tests reflects some aspects of human memory including episodic memory, recognition memory, semantic memory, spatial memory, and emotional memory. Although most interventional studies in rodent models of dementia have focused on pharmacological agents, there are an increasing number of studies that have evaluated nutritional interventions including caloric restriction, intermittent fasting, and manipulation of macronutrients. Dietary interventions have been shown to influence various cognitive and behavioral tests in rodents indicating that nutrition can influence brain aging and possibly neurodegeneration.
Introduction
The major translational goals in aging research are to extend healthy lifespan and to reduce chronic diseases of aging. Old age is the greatest risk factor for all major causes of mortality and morbidity: cardiovascular diseases, cancers, and in particular the neurodegenerative diseases including movement disorders and dementia.Citation1 Most research into dementia has focused on developing pharmacological interventions to treat and prevent cognitive impairment, but despite an extensive international effort, the only medications that are available are cholinesterase inhibitors and memantine, which have modest effects on symptoms but not disease progression.Citation2
An alternative approach to prevent the onset of age-related neurodegenerative disease, while simultaneously improving overall health, is nutrition. Nutritional interventions such as caloric restriction, intermittent fasting (IF), and manipulation of macronutrients have often been reported to improve healthspan and lifespan in a variety of organisms and laboratory settings, with increasing evidence that they are effective in humans.Citation3–Citation5 Nutritional interventions are readily translatable because they are available to entire populations and have less economic burden and adverse effects associated with prescription medicines.Citation6 Yet, the study of nutritional interventions in the context of neurodegenerative disease remains undeveloped.
Rodent models of cognition and behavior have various strengths and weaknesses, particularly in how they reflect human brain aging and cognitive deficits. Here, we review the effects of manipulations of nutrient intake on cognition and behavior in rodent models of aging, brain aging and dementia.
Nutritional interventions in aging research
Three nutritional interventions have been at the forefront of aging research: 1) calorie restriction (CR) – a total reduction by 10%–50% in daily caloric intake without malnutrition; 2) IF – extended periods of food deprivation (typically 16–24 hours) with intervening periods of food intake; and 3) varying macronutrient ratios in ad libitum diets.Citation3,Citation7,Citation8
The molecular mechanisms by which those interventions counteract aging are complex, and recent findings implicate nutrient-sensing pathways (eg, sirtuins [SIRT-1], insulin-like growth factor-1 [IGF1], peroxisome proliferator-activated receptor gamma coactivator 1-alpha [PGC-1α], mechanistic target of rapamycin [mTOR], and fibroblast growth factor 21 [FGF21]).Citation9,Citation10 The plasticity of these pathways in response to nutritional changes result in reduced levels of inflammation, increased insulin sensitivity, efficient autophagy, and increased cellular stress resistance, all leading to lifespan extension and health benefits in a variety of species.Citation11,Citation12
While CR can extend lifespan and counteract many age-related diseases in a wide range of species, it is difficult to implement in humans with essentially unlimited access to calorie-rich food. Another criticism of CR is that health benefits may not be due to a reduction in total calories, but rather a reduction in one or several dietary macronutrients. This line of reasoning grew from the field of nutritional ecology, in which the ratios and amounts of macronutrients in foods, meals, and diets have been shown to exert a driving influence on animal physiology across numerous species.Citation13 We recently confirmed this in rodents using a systematic experimental design, where an ad libitum low-protein, high-carbohydrate (LPHC) diet was shown to be more effective than CR (by dilution with fiber) in improving lifespan and several markers of cardiometabolic health.Citation14 Conversely, high-protein, low-carbohydrate diets resulted in reduced lifespan and poorer cardiometabolic health. We hypothesized that reducing protein while simultaneously increasing carbohydrate in the diets functioned in a similar way to CR and IF by changing the underlying molecular pathways. Specifically, we suggested that high-protein intake coupled with low carbohydrate was detrimental to health possibly due to branched-chain amino acids (valine, leucine, and isoleucine) activating mTOR which subsequently mediated the response between healthspan and lifespan. The mTOR and other pathways, including pathways involving insulin, growth hormone and IGF-1, have been implicated in lifespan in a variety of organisms.Citation15 IF can extend lifespan and forestall many age-related disease processes in rodents with minimal overall reduction in caloric intake.Citation7,Citation16
Studies have investigated the roles of specific amino acids on healthspan and lifespan, with a focus on branched-chain amino acids, methionine (Met), and tryptophan (Trp). Met restriction has been shown to improve lifespan of yeast and mammalian cells through a mechanism involving reduced amino acid intake and suppression of the mTOR pathway, resistance to stress, and attenuated levels of inflammation.Citation17 In rodents, Met restriction reduces mitochondrial oxidative stress and improves insulin sensitivity, with subsequent studies showing lifespan benefits.Citation18 There has been limited research on dietary Trp reduction, with results pointing toward reduced inflammation and constraint of cell growth.Citation19
While the role of LPHC diets in extending healthspan has begun to be elucidated, there is limited information on potential roles of LPHC diets, Met, and Trp on brain health during aging. Such animal studies manipulated one dietary macronutrient for a short period and then assessed performance in a cognitive task shortly thereafter. A recent study demonstrated that a large increase in only one macronutrient, while keeping the other two at low percentages, exacerbates cognitive decline in a mouse model of Alzheimer’s disease (AD).Citation20 Similar studies have concluded that each macronutrient plays a critical role in brain health, but it is not known what ratios of macronutrients may be optimal for memory and cognition. However, such “one-nutrient-at-a-time” manipulations do not consider the interactive effects of single nutrient manipulations on balance with respect to other dietary components.Citation21 There is evidence that the brain, like other physiological systems, requires a target ratio of macronutrients to develop and function optimally, with this ratio changing across the life course and with aging.Citation22 The developing brain may be particularly sensitive to macronutrient intake, such that restriction of certain macronutrients during embryonic and early postnatal development can adversely affect cognitive outcomes.Citation23
A key concept that has emerged from studies of macronutrient intake is that nutrient sensing pathways responsible for metabolic adaptations also affect brain functionality and the pathogenesis of many different neurological disorders.Citation24 In the case of energy restriction, we reviewed the effects of CR and IF on cognitive health during aging in rodents.Citation22 In brief, CR and IF can increase dendritic spine density in hippocampal neurons, improve mitochondrial respiration, promote neuron viability/survival, enhance synaptic plasticity, and regulate genes involved in memory and cognitive function.Citation25 CR and IF have consistently been reliable and robust interventions to hinder neurodegeneration in mouse and rat models of Alzheimer’s, Parkinson’s, and Huntington’s diseases and stroke.Citation26,Citation27 A limited number of studies have also demonstrated neuroprotective effects of CR in non-human primates.Citation28–Citation30
Although there is strong evidence that CR and IF are two of the most effective non-pharmacological or non-genetic treatments to prevent neurodegeneration, there are no studies on the potential benefits of protein restriction (particularly LPHC diets) on brain health during the aging process. Given the robust evidence that long-term LPHC nutritional interventions are beneficial for improving lifespan and metabolic health in rodents, coupled with the fact that those treatments appear to activate similar nutrient response pathways as CR and IF,Citation14 it is plausible that LPHC diets may also be beneficial for preventing the onset of neurodegenerative disease.
Many tools and methods have been used to evaluate the effects of macronutrient intake on the aging brain. Here, we review studies in which the impact of CR, IF, and restriction of dietary protein and specific amino acids on cognition have been evaluated in rodents. A better understanding of how nutritional interventions affect aging brain biology might enable the development of specific macronutrient-based interventions to optimize brain health during aging.
Overview of the impact of aging on cognition in rodents
Extensive research about age-related changes in learning and memory, and underlying cellular and molecular mechanisms has accrued during the past three decades.Citation31–Citation34 Memory tests in rodents ideally will parallel those tests commonly used in humans; however, this is not possible because of major species differences in memory and cognition. There are limited animal tests that serve as models for many complex manifestations of dementia such as emotional and language impairment, and executive function.Citation35 There are, however, a variety of behavioral assessments () that have been developed which are meant to target the same declining memory processes as seen in humans during aging and with dementia diagnoses.Citation36
Table 1 A selection of different types of memory common to both rodents and humans
The first types of memory to deteriorate during AD in humans are episodic and semantic memory, while working spatial memory closely follows. These forms of memory are usually affected because of early involvement of the hippocampus and associated long-term potentiation processes.Citation37,Citation38 Episodic and semantic memory loss often coincides with lapses in spatial and navigational learning and memory. Spatial learning is a process that involves multiple cognitive and perceptual processes and refers to attaining and maintaining a certain trajectory from one place (or object) to another and is learned over time. Therefore, cognitive–behavioral tasks assessing spatial memory are particularly important in rodent research and are widely used in nutritional interventions. There are a variety of well-established memory-assessing cognitive–behavioral tasks, either at a specific point or over a time course in rodents (). It is important to assess rodent cognition using behavioral tests that are relatively simple and with low levels of inter-animal variability within a laboratory and high reproducibility between laboratories. However, some learning and memory deficits are subtle; therefore, cognitive tests that are more demanding are often required to discriminate differences between ages and experimental groups of animals.Citation39 Testing animals in both negative- and positive-reinforcement paradigms can reduce the effects of confounding factors unrelated to memory, such as sensory or motor function, anxiety, or depression.
Table 2 Commonly used tasks to assess rodent memory acquisition
Behavioral tests of learning and memory in rodents
Several experimental tests have been utilized to study the effects of nutrients on cognition and synaptic plasticity ().
Figure 1 The most commonly used memory behavioral tests in rodents.
Abbreviations: CR, calorie restriction; IF, intermittent fasting; LPHC, low-protein, high-carbohydrate.
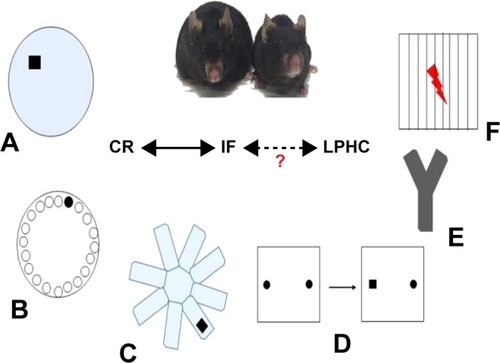
Morris water maze (MWM)
The MWM () tests spatial learning and memory retention in rodents and involves an acquisition phase where the animal learns the location of a “goal or escape platform” target over a period of multiple days.Citation40,Citation41 In this test, rodents swim in a circular pool and learn to find a platform which is hidden 1–2 cm below the surface of opaque water. The rodents use cues placed around the room to orient and learn the location of the platform. Memory acquisition is typically measured by how quickly the animals learn to find the platform over designated number of training trials (memory acquisition), and then probe trials are performed (with increasing lag times) in which the platform is removed from the pool and the amount of time the animal spends close to the former location of the platform is determined (memory retention). MWM probe trial results are most typically represented as the percentage of time the animal spends in the target quadrant and the number of entries to the previous location of the platform.
Barnes maze (BM)
The BM () was developed based on the same principles as the MWMCitation42,Citation43 but, in contrast to the MWM, imposes little or no stress on the animal because there is no swimming.Citation44,Citation45 The test consists of a circular platform comprising 20 equally spaced concentric open holes. Under one of the holes lies a black escape box. Memory acquisition is measured by how quickly it takes the rodent to find the escape box over a 4-day period, and a probe trial is performed 1 day later to assess if the rodent actually learned the task. It is known that high-stress situations increase corticosterone levels in mice, which are inversely correlated with spatial memory acquisition.Citation46 For those reasons, the BM is recommended for the study of spatial memory acquisition.
Radial arm water maze (RAWM)
The RAWM () test was primarily developed as a spatial and reference memory task, although it may also be used to evaluate working memory.Citation47 The RAWM is a popular test because it does not require extensive training and minimizes olfactory cues.Citation48 The basic premise of RAWM is that rodents learn to locate a hidden platform in one of the eight arms. After a brief training period, results are quantified by the number of errors (ie, incorrect arm entries) and the number of seconds the mouse takes to reach the escape platform.Citation49
Novel object recognition (NOR)
One of the most popular memory tests in rodents is NOR () because it parallels similar tests in humans. NOR in mice was predicated upon the primate and human preference to explore a novel stimulus rather than a known one.Citation50 This highlights the translational characteristics of the test and takes advantage of the natural tendency of rodents to explore a novel object rather than the one previously present.Citation51 The NOR testing protocol is relatively simple, cheap, and quick, thus widely utilized.Citation52 After a short period of habituation to the study arena (typically a box with opaque walls), the rodent is exposed to two similar objects. There is an inter-trial interval (24 hours) and one of the objects is replaced with a novel object. A “recognition index” is thus calculated as a percentage of time the rodent explores the new object compared with the familiar one.Citation53
Y-maze
The Y-maze is a relatively simple behavioral test meant to quantify working memory and anxiety in rodents. Therefore, this assay might have an advantage in that dementia and anxiety are often comorbid.Citation54 Similar to the NOR task, the Y-maze test utilizes rodents’ natural exploratory tendency and there is minimal human interaction with the animal. In keeping with its name, the Y-maze contains three identical arms with high walls (). In trial number 1, one arm is blocked (the novel arm) and the rodent explores for 5 minutes. In trial 2, the rodent freely moves among all the three arms. The general premise of the second trial is that mice will remember the previously explored arms while entering the novel arm a higher number of times, commonly termed “spontaneous alteration.”Citation55
Fear conditioning (FC)
FC () is a widely used behavioral memory test in rodents because of its Pavlovian nature and its relative ease of performance. FC is in a general sense similar to the “eye-blink” associative learning paradigm seen in humans and primates, where adverse events are learned quickly and anticipated.Citation56 Studies have indicated that classical conditioning and amygdala-dependent FC are impaired in AD patients.Citation57 The basic FC protocol is to place rodents in an enclosed box on an electrified wire floor. After a brief exploration period, a series of audible tones/bright lights are presented and followed by mild foot shocks (pain). The rodents are then removed from the box for a certain time interval (usually 24 hours) and then placed back into the box. The same tone is presented and the measure of learning is quantified by how quickly, and to what extent, the rodent freezes after exposure.Citation58
IntelliCage and touch screen
Novel behavioral assessing technologies are under development and therefore not currently used in the context of nutritional interventions. However, those technologies may be beneficial to further investigate the roles of nutrition in rodent memory. One tool is the rodent touch screen which is a useful tool for assessing working memory.Citation59 Another powerful tool is the IntelliCage which can be used for a variety of learning and memory paradigms.Citation60 After a time of extensive training, the touch screen and IntelliCage paradigms are heavily automated and therefore greatly reduce experimenter interference.
The impact of CR and IF on memory acquisition during aging in rodents
CR and IF positively affect MWM, BM, and RAWM performance under a variety of conditions and ages in rodents.Citation61 Recent studies have demonstrated that CR improved proximity and time to reach the escape platform in the MWM task highlighting improved spatial discrimination.Citation62 In another study, short-term CR (28 days) significantly decreased the time necessary to reach the escape platform, and animals spent significantly more time in the target quadrant during the probe trial.Citation63 Furthermore, it was demonstrated that exercise training plus CR enhanced rat spatial memory in the MWM, possibly due to upregulation of brain-derived neurotrophic factor (BDNF).Citation64 In a transgenic mouse model of AD, 1 year of 40% CR or every other day IF ameliorated spatial memory acquisition and retention in the MWM.Citation65
In the BM, 11 months of IF significantly improved spatial acquisition and the time necessary to reach the escape hole when compared with those mice fed the same diet ad libitum.Citation66 In another study, short-term (6 weeks) IF did not improve RAWM memory acquisition in mice and did not significantly change hippocampal BDNF levels.Citation67 The results were similar to another study where starting CR at 6 months of age did not improve spatial memory in old rats.Citation68 Conversely, another study showed that CR significantly improved RAWM performance in rats possibly by the upregulation of BDNF.Citation69 Approximately, 40% CR for 3 months significantly improved RAWM performance in a rat model of cerebral ischemia.Citation70
Spatial memory is one of the most widely studied forms of memory in animal models, although there are other forms of memory that must be taken into consideration to capture the full scope of memory loss that occurs during aging and neurodegenerative states. Associative and recognition memory, for example, are important to study as those forms of memory loss often occur either in conjunction with, or prior to, the loss of spatial memory in AD.Citation71 Familiar object detection, a form of recognition memory, is known to deteriorate in early stages of AD.Citation72 Patients may not recognize physical objects that they recently encountered, and in very late stages of the disease those “objects” include those experienced regularly throughout life, including the faces of family members and friends. This is often termed “associative memory,” and it has been suggested that this effectively paired with working memory.Citation73 One form of associative memory has led to the paired associates learning task in humans, which tests the ability to remember the location of objects in the environment.Citation74 Those types of associative and working memory, often called “executive function,” decline progressively in dementia.Citation75
The Y-maze has been used to evaluate effects of nutritional interventions on working memory. One study demonstrated that 20% and 35% CR for 3 months significantly improved learning in male Kunmin mice.Citation76 In another study, a periodic diet designed to mimic fasting improved spontaneous alternation behavior in 23-month-old mice.Citation77 One study that investigated a 40% lifelong CR treatment in rats showed a decrease in NOR in the CR group, suggesting that CR treatment worsened NOR performance.Citation62 Another study demonstrated that long-term CR improved NOR memory in old LOU/C/Jall (LOU) rats, which are considered a “gold-standard” for aging research because they live much longer than other strains.Citation78
There have been a variety of nutritional studies which utilized FC for assessing memory. One recent study suggested that hunger may play a critical role in the formation of fear-related contextual and cued memory by strengthening amygdala connections – more specifically the basolateral nucleus and central amygdala, both of which are involved in fear extinction learning. One recent study confirmed these results, where the deletion of the hunger gene Y4 reduced appetite and impaired the FC response in mice. Interestingly, this result was reversed by fasting, as quantified by FC.Citation79 Other studies have confirmed those results, for example, CR significantly improved contextual freezing behavior in a mouse model of AD.Citation80 Another genetic study showed that CR ameliorated freezing behavior seen in presenilin-1 and -2 double knockout mice.Citation81 With regard to amino acids, one study looked at the effects of limiting dietary Trp intake for at least 1 month in young mice and assessing FC responses, concluding that Trp is essential in the formation of hippocampal contextual and cued memory formation.Citation82
Influence of individual macronutrients on learning and memory in rodents
The limited number of studies looking at macronutrient ratios in mouse memory acquisition highlights the need for further investigation in that area. While there have been limited interventional studies in this regard, there is information on the general roles of single macronutrients on cognition.Citation83
Protein and amino acids
Dietary protein restriction can extend lifespan in a range of organisms, including rodents.Citation84 Cycles of dietary protein restriction ameliorated working memory deficits evaluated in the Y-maze and lessened hyperphosphorylated Tau levels without affecting Aβ levels.Citation85 Conversely, a high-protein diet worsened memory acquisition in male Wistar rats in the MWM.Citation86 Most of the studies on dietary amino acids and cognition have focused on Trp because it is a precursor to serotonin, which is a neurotransmitter that regulates learning and memory, and mood.Citation87 When rats were maintained on a Trp-deficient diet during the first 2 postnatal months, they exhibited impaired memory acquisition in the MWM.Citation88 BDNF likely plays a role in the beneficial effects of dietary Trp on cognition because serotonergic synaptic activity increases BDNF expression.Citation89 Indeed, it was recently reported that dietary Trp supplementation increases BDNF expression in the hippocampus and frontal cortex of aged rats.Citation90
Fat
It is a well-established fact that excessive and chronic high-fat diet (HFD) hinders rodent memory performance and injures hippocampal neurons in many strains of mice and rats.Citation91,Citation92 It was recently shown that an energy-dense diet worsens cognitive decline in a rat model of AD by increasing the levels of amyloid precursor protein and dysregulation of the sirtuin pathways.Citation93 In another study, a 3-month HFD period significantly increased the proinflammatory cytokine profile in mice and impaired NOR test performance.Citation94 A chronic HFD results in impaired short- and long-term memory in mice as measured by NOR and MWM, possibly due in part to impaired hippocampal insulin signaling. CR can attenuate the adverse effects of a HFD on cognition.Citation95
Carbohydrate
Chronically excessive glucose or fructose intake impairs synaptic plasticity and cognition by mechanisms involving the production of proinflammatory factors, insulin resistance, and glucose metabolism dysregulation, possibly contributing to the development of AD and other neurodegenerative diseases.Citation96,Citation97 Poor glycemic control and type-2 diabetes may accelerate the onset of dementia during aging.Citation98 IF is believed to enhance hippocampal synaptic plasticity and cognitive performance, in part, by elevation of the circulating ketone beta-hydroxybutyrate which is not only an energy substrate for neurons but also induces the expression of BDNF.Citation99,Citation100 It was recently reported that a ketone ester diet can ameliorate learning and memory deficits and lessen anxiety in a mouse model of AD.Citation101
Conclusion and future research
Nutrition influences brain aging. CR and IF are two robust interventions that positively impact the aging brain. Studies to date have not addressed the issue of dietary nutrient balance on the aging brain. There is mounting evidence that the ratios and amounts of individual macronutrients influence late-life cardiometabolic health and lifespan and do so via similar metabolic molecular pathways as CR and IF. Nutritional geometry offers an integrative framework for designing, interpreting, and implementing dietary studies that explore the main and interactive effects of different nutrients, and offers a powerful tool for future research into brain health.Citation102–Citation104
Regarding experimental tools, rodent behavioral testing provides a useful model for assessing the role of nutrition in brain health. There are a variety of behavioral assays available to assess those changes, each with their strengths and limitations. We suggest that the establishment of cooperative efforts and agreement among scientists with regard to rodent behavioral testing will help to “bridge the gap” in nutritional cognitive research. Those collaborative efforts will help establish similarities and differences in determining the effects of various nutritional interventions on rodent memory.
Acknowledgments
We thank the coauthors in studies cited in this review. We also would like to thank Dr Rahul Gokarn for his valuable contributions to the manuscript.
This work was supported by the Aging and Alzheimer’s Research Institute, National Health and Research Council of Australia (NHMRC) (grant numbers 571328 and 1084267); the Intramural Program of the National Institute on Aging, NIH (RdC and MPM); the American Australian Association (AAA) Education Fund (DW); and the NHMRC Peter Doherty Early Career Fellowship (grant number 1110098) (SMS-B).
Disclosure
The authors report no conflicts of interest in this work.
References
- KaeberleinMRabinovitchPSMartinGMHealthy aging: the ultimate preventative medicineScience201535062651191119326785476
- WaiteLMTreatment for Alzheimer’s disease: has anything changed?Aust Prescr2015382606326648618
- de CaboRCarmona-GutierrezDBernierMHallMNMadeoFThe search for antiaging interventions: from elixirs to fasting regimensCell201415771515152624949965
- Le CouteurDGSolon-BietSCoggerVCThe impact of low-protein high-carbohydrate diets on aging and lifespanCell Mol Life Sci20167361237125226718486
- Le CouteurDGSolon-BietSWahlDNew horizons: dietary protein, ageing and the Okinawan ratioAge Ageing201645444344727130207
- SiroisCLarocheMLGuenetteLKrogerECooperDEmondVPolypharmacy in multimorbid older adults: protocol for a systematic reviewSyst Rev2017610428526062
- MattsonMPLongoVDHarvieMImpact of intermittent fasting on health and disease processesAgeing Res Rev201631163025130253
- Solon-BietSMMitchellSJde CaboRRaubenheimerDLe CouteurDGSimpsonSJMacronutrients and caloric intake in health and longevityJ Endocrinol20152261R17R2826021555
- VaisermanAMLushchakOVKoliadaAKAnti-aging pharmacology: promises and pitfallsAgeing Res Rev20163193527524412
- Solon-BietSMWaltersKASimanainenUKMacronutrient balance, reproductive function, and lifespan in aging miceProc Natl Acad Sci U S A2015112113481348625733862
- IngramDKAnsonRMde CaboRDevelopment of calorie restriction mimetics as a prolongevity strategyAnn N Y Acad Sci2004412423
- MerckenEMCarboneauBAKrzysik-WalkerSMde CaboROf mice and men: the benefits of caloric restriction, exercise, and mimeticsAgeing Res Rev201211339039822210414
- RaubenheimerDSimpsonSJLe CouteurDGSolon-BietSMCooganSCNutritional ecology and the evolution of agingExp Gerontol201686506127094469
- Solon-BietSMMcMahonACBallardJWThe ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed miceCell Metab201419341843024606899
- PanHFinkelTKey proteins and pathways that regulate lifespanJ Biol Chem2017292166452646028264931
- AnsonRMGuoZde CaboRIntermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intakeProc Natl Acad Sci U S A2003100106216622012724520
- JohnsonJEJohnsonFBMethionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cellsPLoS One201495e9772924830393
- AblesGPJohnsonJEPleiotropic responses to methionine restrictionExp Gerontol201717178388
- Brown-BorgHMBuffensteinRCutting back on the essentials: can manipulating intake of specific amino acids modulate health and lifespan?Ageing Res Rev201626163020430205
- KadishIKumarABeitnereUJenningsEMcGilberryWvan GroenTDietary composition affects the development of cognitive deficits in WT and Tg AD model miceExp Gerontol201686394927167583
- SimpsonSJLe CouteurDGRaubenheimerDDietary protein, aging and nutritional geometryAgeing Res Rev201739788628274839
- WahlDCoggerVCSolon-BietSMNutritional strategies to optimise cognitive function in the aging brainAgeing Res Rev201631809227355990
- HallRDIs hippocampal function in the adult rat impaired by early protein or protein-calorie deficiencies?Dev Psychobiol19831653954116413285
- MattsonMPEnergy intake and exercise as determinants of brain health and vulnerability to injury and diseaseCell Metab201216670672223168220
- MattsonMPThe impact of dietary energy intake on cognitive agingFront Aging Neurosci201025
- StranahanAMMattsonMPRecruiting adaptive cellular stress responses for successful brain ageingNat Rev Neurosci201213320921622251954
- RaefskySMMattsonMPAdaptive responses of neuronal mitochondria to bioenergetic challenges: roles in neuroplasticity and disease resistanceFree Radic Biol Med201710220321627908782
- MaswoodNYoungJTilmontECaloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s diseaseProc Natl Acad Sci U S A200410152181711817615604149
- MattisonJAColmanRJBeasleyTMCaloric restriction improves health and survival of rhesus monkeysNat Commun201781406328094793
- WilletteAACoeCLBirdsillACInterleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaquesAge20133562215222723463321
- BurkeSNRyanLBarnesCACharacterizing cognitive aging of recognition memory and related processes in animal models and in humansFront Aging Neurosci201241522988437
- EngleJRBarnesCACharacterizing cognitive aging of associative memory in animal modelsFront Aging Neurosci201241022988435
- FosterTCDefazioRABizonJLCharacterizing cognitive aging of spatial and contextual memory in animal modelsFront Aging Neurosci201241222988436
- KosikKSRappPRRazNSmallSASweattJDTsaiLHMechanisms of age-related cognitive change and targets for intervention: epigeneticsJ Gerontol A Biol Sci Med Sci201267774174622522509
- WelshKButtersNHughesJMohsRHeymanADetection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measuresArch Neurol19914832782812001185
- WebsterSJBachstetterADNelsonPTSchmittFAVan EldikLJUsing mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse modelsFront Genet2014588
- KlencklenGDespresODufourAWhat do we know about aging and spatial cognition? Reviews and perspectivesAgeing Res Rev201211112313522085884
- LithfousSDufourADespresOSpatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: insights from imaging and behavioral studiesAgeing Res Rev201312120121322771718
- HodgesHMaze procedures: the radial-arm and water maze comparedBrain Res Cogn Brain Res199633–41671818806020
- VorheesCVWilliamsMTMorris water maze: procedures for assessing spatial and related forms of learning and memoryNat Protoc20061284885817406317
- MorrisRDevelopments of a water-maze procedure for studying spatial learning in the ratJ Neurosci Methods198411147606471907
- RosenfeldCSFergusonSABarnes maze testing strategies with small and large rodent modelsJ Vis Exp20148451194
- BarnesCAMemory deficits associated with senescence: a neurophysiological and behavioral study in the ratJ Comp Physiol Psychol197993174104221551
- AkiravISandiCRichter-LevinGDifferential activation of hippocampus and amygdala following spatial learning under stressEur J Neurosci200114471972511556896
- VorheesCVWilliamsMTAssessing spatial learning and memory in rodentsILAR J201455231033225225309
- HarrisonFEHosseiniAHMcDonaldMPEndogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasksBehav Brain Res2009198124725118996418
- Shukitt-HaleBMcEwenJJSzprengielAJosephJAEffect of age on the radial arm water maze-a test of spatial learning and memoryNeurobiol Aging200425222322914749140
- PenleySCGaudetCMThrelkeldSWUse of an eight-arm radial water maze to assess working and reference memory following neonatal brain injuryJ Vis Exp2013825094024335781
- WolfABauerBAbnerELAshkenazy-FrolingerTHartzAMA comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 micePLoS One2016111e014773326808326
- LegerMQuiedevilleABouetVObject recognition test in miceNat Protoc20138122531253724263092
- BevinsRABesheerJObject recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory”Nat Protoc2006131306131117406415
- BengoetxeaXRodriguez-PerdigonMRamirezMJObject recognition test for studying cognitive impairments in animal models of Alzheimer’s diseaseFront Biosci201571029
- WangKLuJMXingZHEffect of 1.8 GHz radiofrequency electromagnetic radiation on novel object associative recognition memory in miceSci Rep2017744521
- GoodarziZMeleBGuoSGuidelines for dementia or Parkinson’s disease with depression or anxiety: a systematic reviewBMC Neurol201616124427887589
- ConradCDLupienSJThanasoulisLCMcEwenBSThe effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-mazeBrain Res1997759176839219865
- CurzonPRustayNRBrowmanKEFrontiers in neuroscience cued and contextual fear conditioning for rodentsBuccafuscoJJMethods of Behavior Analysis in NeuroscienceBoca RatonCRC Press/Taylor & Francis Group, LLC2009
- GoldCABudsonAEMemory loss in Alzheimer’s disease: implications for development of therapeuticsExpert Rev Neurother20088121879189119086882
- LugoJNSmithGDHolleyAJTrace fear conditioning in mice. J Vis Exp20148551180
- KwakCLimCSKaangBKDevelopment of a touch-screen-based paradigm for assessing working memory in the mouseExp Neurobiol2015241848925792872
- HolterSMGarrettLEinickeJAssessing cognition in miceCurr Protoc Mouse Biol20155433135826629775
- StewartJMitchellJKalantNThe effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazesNeurobiol Aging19891066696752628778
- CarterCSLeeuwenburghCDanielsMFosterTCInfluence of calorie restriction on measures of age-related cognitive decline: role of increased physical activityJ Gerontol A Biol Sci Med Sci200964885085919420296
- KishiTHirookaYNagayamaTCalorie restriction improves cognitive decline via up-regulation of brain-derived neurotrophic factor: tropomyosin-related kinase B in hippocampus of obesity-induced hypertensive ratsInt Heart J201556111011525503654
- KishiTSunagawaKExercise training plus calorie restriction causes synergistic protection against cognitive decline via up-regulation of BDNF in hippocampus of stroke-prone hypertensive ratsConf Proc IEEE Eng Med Biol Soc20127106347547
- HalagappaVKGuoZPearsonMIntermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s diseaseNeurobiol Dis200726121222017306982
- LiLWangZZuoZChronic intermittent fasting improves cognitive functions and brain structures in micePLoS One201386e6606923755298
- KhabourOFAlzoubiKHAlomariMAAlzubiMAChanges in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exerciseHippocampus201020563764519530223
- BondNWEverittAVWaltonJEffects of dietary restriction on radial-arm maze performance and flavor memory in aged ratsNeurobiol Aging198910127302755555
- AlomariMAKhabourOFAlzoubiKHAlzubiMACombining restricted diet with forced or voluntary exercises improves hippocampal BDNF and cognitive function in ratsInt J Neurosci2016126436637326000806
- RobergeMCHotte-BernardJMessierCPlamondonHFood restriction attenuates ischemia-induced spatial learning and memory deficits despite extensive CA1 ischemic injuryBehav Brain Res2008187112313217949826
- Parra-DamasAChenMEnriquez-BarretoLCRTC1 function during memory encoding is disrupted in neurodegenerationBiol Psychiatry201781211112327587263
- LaatuSRevonsuoAJaykkaHPortinRRinneJOVisual object recognition in early Alzheimer’s disease: deficits in semantic processingActa Neurol Scand20031082828912859283
- PolcherAFrommannIKopparaAWolfsgruberSJessenFWagnerMFace-name associative recognition deficits in subjective cognitive decline and mild cognitive impairmentJ Alzheimers Dis20175631185119628106560
- BarnettJHBlackwellADSahakianBJRobbinsTWThe paired associates learning (PAL) test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia researchCurr Top Behav Neurosci20162844947427646012
- BaddeleyADBressiSDella SalaSLogieRSpinnlerHThe decline of working memory in Alzheimer’s disease. A longitudinal studyBrain1991114Pt 6252125421782529
- WuAWanFSunXLiuYEffects of dietary restriction on growth, neurobehavior, and reproduction in developing Kunmin miceToxicol Sci200270223824412441368
- BrandhorstSChoiIYWeiMA periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance and healthspanCell Metab2015221869926094889
- MenardCQuirionRBouchardSFerlandGGaudreauPGlutamatergic signaling and low prodynorphin expression are associated with intact memory and reduced anxiety in rat models of healthy agingFront Aging Neurosci2014681
- VermaDWoodJLachGHerzogHSperkGTasanRHunger promotes fear extinction by activation of an amygdala microcircuitNeuropsychopharmacology201641243143926062787
- BrownlowMLJoly-AmadoAAzamSPartial rescue of memory deficits induced by calorie restriction in a mouse model of tau depositionBehav Brain Res2014271798824925454
- WuPShenQDongSXuZTsienJZHuYCalorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout miceNeurobiol Aging200829101502151117499883
- UchidaSUmeedaHKitamotoAMasushigeSKidaSChronic reduction in dietary tryptophan leads to a selective impairment of contextual fear memory in miceBrain Res200729149156
- BourreJMEffects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2: macronutrientsJ Nutr Health Aging200610538639917066210
- MirzaeiHSuarezJALongoVDProtein and amino acid restriction, aging and disease: from yeast to humansTrends Endocrinol Metab2014251155856625153840
- ParrellaEMaximTMaialettiFProtein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse modelAging cell201312225726823362919
- Mendez-LopezMMendezMAriasJAriasJLEffects of a high protein diet on cognition and brain metabolism in cirrhotic ratsPhysiol Behav201514922022826048304
- RobertsAJHedlundPBThe 5-HT(7) receptor in learning and memoryHippocampus201222476277121484935
- Olvera-CortesEPerez-VegaMIBarajas-LopezGDel Angel-MezaARGonzalez-BurgosIFeria-VelascoAPlace learning impairment in chronically tryptophan-restricted ratsNutr Neurosci19981322323527406201
- MattsonMPMaudsleySMartinBA neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotoninAgeing Res Rev20043444546415541711
- MusumeciGCastrogiovanniPSzychlinskaMAProtective effects of high tryptophan diet on aging-induced passive avoidance impairment and hippocampal apoptosisBrain Res Bull2017128768227889579
- StranahanAMNormanEDLeeKDiet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged ratsHippocampus200818111085108818651634
- LedreuxAWangXSchultzbergMGranholmACFreemanLRDetrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged ratsBehav Brain Res201631229430427343935
- Martino AdamiPVGaleanoPWallingerMLWorsening of memory deficit induced by energy-dense diet in a rat model of early-Alzheimer’s disease is associated to neurotoxic Abeta species and independent of neuroinflammationBiochim Biophys Acta20173731743
- BarronAMTokunagaMZhangMRJiBSuharaTHiguchiMAssessment of neuroinflammation in a mouse model of obesity and beta-amyloidosis using PETJ Neuroinflammation201613122127578213
- Sims-RobinsonCBakemanABrunoEDietary reversal ameliorates short- and long-term memory deficits induced by high-fat diet early in lifePLoS One2016119e016388327676071
- HsuTMKonanurVRTaingLEffects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent ratsHippocampus201525222723925242636
- Arrieta-CruzIGutierrez-JuarezRThe role of insulin resistance and glucose metabolism dysregulation in the development of Alzheimer’s diseaseRev Invest Clin2016682535827103040
- SheenYJSheuWHAssociation between hypoglycemia and dementia in patients with type 2 diabetesDiabetes Res Clin Pract201611627928727321346
- MarosiKKimSWMoehlK3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neuronsJ Neurochem2016139576978127739595
- SleimanSFHenryJAl-HaddadRExercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrateElife20165 pii: e15092
- KashiwayaYBergmanCLeeJHA ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s diseaseNeurobiol Aging20133461530153923276384
- RaubenheimerDSimpsonSJNutritional ecology and human healthAnnu Rev Nutr20163660362627296501
- SimpsonSJRaubenheimerDMacronutrient balance and lifespanAging200911087588020157561
- SimpsonSJRaubenheimerDCaloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of agingJ Gerontol A, Biol Sci Med Sci200762770771317634316
- BurgessNMaguireEAO’KeefeJThe human hippocampus and spatial and episodic memoryNeuron200235462564112194864
- PattwellSSBathKGEmotional learning, stress, and development: an ever-changing landscape shaped by early-life experienceNeurobiol Learn Mem201727173648
- DaldrupTRemmesJLestingJExpression of freezing and fear-potentiated startle during sustained fear in miceGenes Brain Behav201514328129125761115
- AlamedJWilcockDMDiamondDMGordonMNMorganDTwo-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic miceNat Protoc2006141671167917487150
