Abstract
Objective
To describe a practical method for family practitioners to stage chronic obstructive pulmonary disease (COPD) by the use of office spirometry.
Methods
This is a review of the lessons learned from evaluations of the use of office spirometry in the primary care setting to identify best practices using the most recent published evaluations of office spirometry and the analysis of preliminary data from a recent spirometry mass screening project. A mass screening study by the American Association for Respiratory Care and the COPD Foundation was used to identify the most effective way for general practitioners to implement office spirometry in order to stage COPD.
Results
A simple three-step method is described to identify people with a high pre-test probability in an attempt to detect moderate to severe COPD: COPD questionnaire, measurement of peak expiratory flow, and office spirometry. Clinical practice guidelines exist for office spirometry basics for safety, use of electronic peak flow devices, and portable spirometers.
Conclusion
Spirometry can be undertaken in primary care offices with acceptable levels of technical expertise. Using office spirometry, primary care physicians can diagnose the presence and severity of COPD. Spirometry can guide therapies for COPD and predict outcomes when used in general practice.
Introduction
Spirometry is the most reproducible, standardized, and objective way of measuring airflow obstruction for the diagnosis and management of chronic obstructive pulmonary disease (COPD).Citation1 The spirogram, first described by John Hutchinson in 1846, is the oldest clinical test still in use today to measure patients’ maximum exhalations from total lung capacity.Citation2 In obstructive pulmonary disease, the changes seen in spirometry are a reduction in forced expiratory volume in the first second (FEV1) – commonly referred to as “airflow” in primary practice – with respect to forced vital capacity (FVC). Using these measurements, the primary care physician can diagnose the presence and severity of airway obstructionCitation3,Citation4 and assess the risk of COPD, lung cancer, coronary artery disease, and stroke.Citation5 Spirometry can be incorporated into family practice with acceptable levels of technical adequacy and accurate interpretations,Citation6 the results of which have been shown to influence general practitioner management of patients with previously diagnosed asthma and COPD.Citation3 A recent evidence-based review projectCitation7 concluded that spirometry by primary care physicians should be reserved for high-risk subjects, eg, smokers aged >40 years with symptoms, who would benefit from in-depth evaluation and management.Citation4,Citation8,Citation9 All smokers should be encouraged to stop smoking, regardless of whether or not they have COPD. Although it has been argued that office spirometry should not be used to encourage smokers to stop, because a normal spirogram in an active smoker may encourage continuation of the behavior and give a patient a false sense of being disease free,Citation10–Citation12 it has been demonstrated that abnormal spirometry and informing a patient of their “lung age” are effective strategies in encouraging smoking cessation.Citation7,Citation13
The exact definition of COPD can be complex but should be considered in a patient who has dyspnea, chronic cough, or sputum production and/or a history of exposure to risk factors (eg, smoking/second-hand smoke, or biomass fuels) with airflow obstruction that is partially reversible with acute use of bronchodilators.Citation1 Typically, patients with asthma respond more fully to bronchodilators than those with COPD, but there is no clear-cut presentation; for example, patients with well established COPD have been shown to have better responsiveness to acute use of bronchodilators than previously expected,Citation14 and asthmatics may have very little airway dilation, especially if they have used their bronchodilator therapy before arriving for spirometry. Some asthma patients will not exhibit substantial airway responsiveness to bronchodilators until their therapy has the asthma under better control.Citation15 Basing clinical decisions and plans on airway response to bronchodilators is therefore often controversial.
The severity of COPD should be assessed once the diagnosis has been made using the 2009 updated guidelines from the Global Initiative for Chronic Lung Disease (GOLD) to determine the degree of spirometric abnormality and by the presence of complications, such as respiratory failure and/or right-sided heart failure.Citation1,Citation16,Citation17 The GOLD guidelines propose four stages of COPD based on spirometry: FEV1/FVC ratio < 0.70 and FEV1 > 80% predicted (Stage 1 Mild), FEV1 of 50%–80% predicted (Stage 2 Moderate), FEV1 of 30%–50% predicted (Stage 3 Severe), and FEV1 < 30% predicted or FEV1 < 50% predicted plus chronic respiratory failure (Stage 4 Very Severe). Patients with symptoms of GOLD Stage 1 COPD can have significant abnormalities of ventilatory mechanics with greater exertional symptoms and exercise limitation than age-matched healthy subjects.Citation18
Is screening spirometry a waste of resources?
Spirometry should be used to stage COPD and to guide therapy. Diagnosing airflow obstruction is important because there are effective therapeutic interventions in asthma and COPD that improve outcomes.Citation1,Citation16 Staging COPD using the GOLD guidelines for FEV1/FVC ratio and FEV1 in general practice with office spirometry provides a logical framework to guide therapy. Examples of interventions include smoking cessation,Citation19 drugs,Citation20,Citation21 oxygen,Citation22 rehabilitation,Citation23 and surgical options.Citation24 Treatment goals should include improving quality of life and exercise tolerance and decreasing the number of exacerbations. If exacerbations can be reduced, then health care costs will be reduced by fewer hospitalizations and visits to the emergency department. It is important to confirm that respiratory symptoms suggest COPD and are the result of airflow obstruction. A decision will need to be made regarding whether a bronchodilator via an inhaler should be administered to improve airflow.
The prevalence of COPD in a primary care setting in patients with a smoking history and self-reported bronchitis has been studied.Citation25,Citation26 A recent multicenter, cross-sectional study by Yawn et al determined the percentage of patients with airway obstruction (post-bronchodilator FEV1/FVC ratio ≤ 0.70) compared with those without airway obstruction (post-bronchodilator FEV1/FVC ratio > 0.70) and confirmed 26% patients with airflow obstruction consistent with COPD. This latter group had a mean age of 52.9 years, an FEV1 of 81.4% predicted, a smoking history of 39.8 pack-years, and reported chronic bronchitis symptoms.Citation26 Airflow obstruction has been observed to increase with age and smoking history, and Yawn et al noted that slight or moderate dyspnea was reported by 68% of the patients with a post-bronchodilator FEV1/FVC ratio ≤ 0.70.Citation26 The majority of the patients with newly diagnosed COPD had not discussed their coughing with their doctor and continued to smoke.Citation26 Several studies around the globe have found that many patients with COPD remain undiagnosed in the primary care setting.Citation8,Citation25,Citation26 Use of spirometry in patients with a smoking history and chronic bronchitis symptoms can aid in the diagnosis of COPD, allowing early treatment;Citation8,Citation25,Citation26 however, screening patients at risk of COPD remains controversial,Citation1 and the US Preventive Services Task Force (USPSTF) recommends against screening adults for COPD using spirometry following a systematic review of evidence of the benefits and harms and an assessment of the net benefit.Citation27 Each patient needs to be evaluated individually, according to their smoking history, lifestyle, and comorbidities, prior to a decision to perform spirometry.
Lessons learned from mass screening experience
The lessons learned from mass screening of people outside of primary care offices have led to the following recommendations by the American Association for Respiratory Care (AARC) and the COPD Foundation:Citation28
Use a COPD questionnaire to identify those at risk.
Use an electronic device to detect those likely to have a low peak flow.
Only perform spirometry on those at higher risk of COPD (smoking, biomass fuel exposure).
Take the time to perform good spirometry.
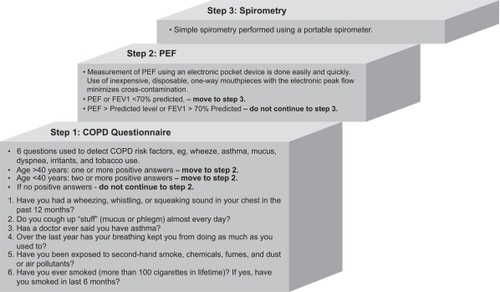
The AARC, the COPD Foundation, and Columbia University have completed a pilot study,Citation29 which screened 4901 people for COPD using a shortened version of the Martinez et al population screening questionnaire,Citation30 peak expiratory flow measurement (PEF), and spirometry. The study used a three-step method ()Citation29 to identify people with a high pre-test probability in an attempt to detect moderate to severe COPD.Citation28,Citation29
Figure 1 Three-step method to identify people with a high pre-test probability for moderate to severe COPD.Citation29
Spirometry basics
Spirometry is the gold standard for the diagnosis and assessment of COPD, as it is the most reproducible, standardized, and objective way of measuring airflow obstruction.Citation1 It also detects airway obstruction in people with poorly controlled asthma. If PEF is <70% predicted, simple spirometry should be performed on a portable spirometer.
Spirometry can be undertaken in primary care offices with acceptable levels of technical adequacy and accurate interpretations, and can alter clinical decisions in the management of asthma and COPD.Citation6,Citation31 In April 2010, the Office Spirometry Certificate (OSC) was presented to the AARC state representatives meetings; it was presented again at the American Thoracic Association meeting (ATS) in May 2010. The OSC is a national initiative whose aim is to “teach practitioners to obtain good quality spirometry >90% of the time, standardize testing performed in the office setting, assure test accuracy and validity and to ensure staff competency” (http://www.aarc.org/osc/). The process consists of an Internet-based online exam and additional direct competency-based testing, and is intended to provide a way for people outside the traditional pulmonary function laboratory setting to demonstrate understanding and receive quality feedback on performance. Having two trained and certified spirometer experts as part of a practice team is the ideal situation in which to provide the most readily available and reliable spirometry.
The National Lung Health Education Program (NLHEP) has recommended that all smokers aged 45 years and older have a screening lung function measured by simple spirometry;Citation32 however, PEF is quicker to measure than spirometry,Citation33 although this would require two devices and would not be supported by all health authorities. Older patients often have multiple chronic health problems that can magnify the impact of COPD on a patient’s health status and can complicate the management of COPD. Confirming the diagnosis of COPD is an important first step. The prevalence of airway obstruction in the US increases in smokers aged >40 years and reaches a peak of 31% in all men aged 65–75 years who are currently smoking.Citation34 In 2004, the NLHEP implemented a spirometer review process to encourage the development of simple office-based spirometers for use in the primary care setting.Citation35 The goal of this process is to clarify the elements required to make a device appropriate for use in the primary care physician’s office and to provide a checklist of these elements for use by companies and reviewers to validate the device. As of January 2010, the NLHEP has approved four outpatient spirometers, which are reliable, accurate, and affordable; these are listed on the NLHEP website with links to the manufacturers of the devices.Citation35 They comprise the NDD EasyOne Frontline 2000–2, the EasyOne Diagnostic 2001–2 (www.nddmed.com), the CP200 models (CP2AS-1E1 and CP2S-1E1 [Welch Allyn; www.welchallyn.com]), and the Astra300 (SDI Diagnostics; www.sdidiagnostics.com).
Safety
Members of the practice office staff trained to carry out spirometry should take the precautions described in .
Table 1 Precautions to be taken during spirometry
Pocket spirometry for PEF
An inexpensive (US$50) electronic device with disposable mouthpieces should be used.Citation36 The first step is for the practice office staff to write down the predicted PEF using age and height from gender- and race-specific tables. The maneuver should be demonstrated with the mouthpiece (after a deep breath, coaching the patient to “blast” out the air). The practice office staff should attach a clean mouthpiece without touching it, and encourage a deep breath from the patient and then a 1-second blast. The maneuver should be repeated in cases of low or poor effort by the patient.
Spirometry to measure only FEV1 and FEV1/FVC ratio
In the US, Medicare payments for office spirometry averaged about US$40 during 2009 and allowed the cost to be covered after 25 tests. The office staff administering spirometry should dramatically demonstrate the three steps for the FVC maneuver: 1) “deep” inhalation, 2) “blast” out, and 3) “keep blowing” for 6 seconds. A procedure for spirometry is described in .
Table 2 A procedure for spirometry
Common mistakes or problems encountered during office spirometry and the appropriate solutions can be found in .
Table 3 Common problems during office spirometry and the appropriate solutions
Overall test session quality goals for spirometry technique should include those described in .Citation37 Modern portable spirometers are programmed to apply the ATS recommendations for good maneuvers, and this, together with certification (OSC) of well trained spirometry experts, will help to improve the quality of office spirometry.
Table 4 Overall spirometry test session quality goals
Quality spirometry is best achieved by gaining rapport with the patient and by dramatically demonstrating the maneuver, closely observing the patient’s body language during the maneuver, repeating the demonstration as needed, and repeating the maneuver after a rest. Submaximal maneuvers will frequently cause falsely high and falsely low results. There can be a wide range of quality among staff who administer the spirometry tests; however, with a quality assurance program, spirometry can be performed and interpreted for asthma and COPD patients, and the spirometry results used to modify care.Citation35 Even the elderly can perform good spirometry.Citation37
Conclusion
Staging of COPD is driven by percent predicted FEV1 and FEV1/FVC ratio (providing the spirometry is carried out correctly); however, this takes only one piece of information into account. The authors advise that the reality of concordance of symptoms (according to the validated St Georges Respiratory QuestionnaireCitation38 and the newly developed short Clinical COPD Questionnaire)Citation39 and the impact of COPD on a patient’s daily living according to their individual needs/occupation should be taken into account. Although it has been suggested that PEF can be used rather than spirometry,Citation33 this is not the case in primary care, as the gold standard in diagnosing and tracking the path of COPD is spirometry, FEV1, and the FEV1/FVC ratio. Using spirometry, primary care physicians can diagnose the presence and severity of COPD. In summary, spirometry can guide therapies for COPD and predict outcomes when used in a primary care setting.
Acknowledgements
This article was developed on the basis of the authors’ presentations and discussions at “Overcoming Barriers to COPD Identification and Management”, held in New York, NY, USA, 23–24 March 2009. This meeting, the authors’ participation, and manuscript preparation were supported by Boehringer Ingelheim Pharmaceuticals Inc. and Pfizer Inc. Medical writing assistance was provided by Gill Sperrin CBiol MSB of Envision Scientific Solutions. The article reflects the concepts of the authors and is their sole responsibility. It was not reviewed by Boehringer Ingelheim Pharmaceuticals Inc. or Pfizer Inc., except to ensure medical and safety accuracy.
Disclosures
Tom Barnes has received consultation fees from Boehringer Ingelheim Pharmaceuticals Inc. and Pfizer. Len Fromer has received honoraria for speakers’ bureau from Boehringer Ingelheim Pharmaceuticals Inc. and Pfizer.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, 2009. Available from: http://www.goldcopd.org. Accessed Jan15 2010
- PettyTLJohn Hutchinson’s mysterious machine revisitedChest2002121Suppl 5S219S223
- ChavannesNSchermerTAkkermansRImpact of spirometry on GPs’ diagnostic differentiation and decision-makingRespir Med200498111124113015526814
- DalesREVandemheenKLClinchJSpirometry in the primary care setting: influence on clinical diagnosis and management of airflow obstructionChest200512842443244716236907
- YoungRPHopkinsREatonTEForced expiratory volume in one second: not just a lung function test but a marker of premature death from all causesEur Respir J200730461662217906084
- YawnBPEnrightPLLemanskeRFJrSpirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPDChest200713241162116817550939
- LinKWatkinsBJohnsonTScreening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the US Preventive Services Task ForceAnn Intern Med2008148753554318316746
- BednarekMMaciejewskiJWozniakMPrevalence, severity and underdiagnosis of COPD in the primary care settingThorax200863540240718234906
- LusuardiMde BenedettoFPaggiaroPA randomized controlled trial on office spirometry in asthma and COPD in standard general practice: data from spirometry in asthma and COPD: a comparative evaluation Italian studyChest2006129484485216608929
- BuffelsJDegryseJDecramerMSpirometry and smoking cessation advice in general practice: a randomised clinical trialRespir Med2006100112012201716580189
- EnrightPDoes screening for COPD by primary care physicians have the potential to cause more harm than good?Chest2006129483383516608923
- StratelisGMolstadSJakobssonPThe impact of repeated spirometry and smoking cessation advice on smokers with mild COPDScand J Prim Health Care200624313313916923621
- TashkinDPMurrayRPSmoking cessation in chronic obstructive pulmonary diseaseRespir Med2009103796397419285850
- TashkinDPCelliBDecramerMBronchodilator responsiveness in patients with COPDEur Respir J200831474275018256071
- McCormackMCEnrightPLMaking the diagnosis of asthmaRespir Care2008535583590 discussion 590–59218426612
- GoldPMThe 2007 GOLD Guidelines: a comprehensive care frameworkRespir Care20095481040104919650945
- MacintyreNRSpirometry for the diagnosis and management of chronic obstructive pulmonary diseaseRespir Care20095481050105719650946
- O’DonnellDELavenezianaPOraJEvaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPDThorax200964321622319052054
- GoodfellowLTWaughJBTobacco treatment and prevention: what works and whyRespir Care20095481082109019650948
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med2008178433233818511702
- RestrepoRDA stepwise approach to management of stable COPD with inhaled pharmacotherapy: a reviewRespir Care20095481058108119650947
- TarpySPCelliBRLong-term oxygen therapyN Engl J Med1995333117107147637750
- LacasseYGoldsteinRLassersonTJPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20064CD00379317054186
- FishmanAMartinezFNaunheimKNational Emphysema Treatment Trial Research GroupA randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med2003348212059207312759479
- LeuppiJDMiedingerDChhajedPNQuality of spirometry in primary care for case finding of airway obstruction in smokersRespiration201079646947419786731
- YawnBManninoDLittlejohnTPrevalence of COPD among symptomatic patients in a primary care settingCurr Med Res Opin200925112671267719757984
- US Preventive Services Task ForceScreening for chronic obstructive pulmonary disease using spirometry: US Preventive Services Task Force recommendation statementAnn Intern Med2008148752953418316747
- COPD Foundation Mobile spirometry unit, 2010. Available from: http://www.copdfoundation.org/Programs/MobileSpirometryUnit/tabid/104/language/en-US/Default.aspx. Accessed Nov 24 2010
- NelsonSBThomashawBMEnrightPA tiered, economical approach for COPD case-finding in the general populationAm J Respir Crit Care Med2010181A5949
- MartinezFJRaczekAESeiferFDDevelopment and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS)COPD200852859518415807
- LamprechtBSchirnhoferLTiefenbacherFSix-second spirometry for detection of airway obstruction: a population-based study in AustriaAm J Respir Crit Care Med2007176546046417556719
- The National Lung Health Education Program (NLHEP)Strategies in preserving lung health and preventing COPD and associated diseasesChest1998113Suppl 2S123S163
- WhitePSpirometry and peak expiratory flow in the primary care management of COPDPrim Care Respir J20041315816701628
- ManninoDMCOPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneityChest2002121Suppl 5S121S126
- American Association for Respiratory Care Dallas TexasNational Lung Health Education Program – Spirometer Review Process, 2007 Available from: http://www.nlhep.org/spirometer-review-process.html. Accessed 2010 Nov 24
- Vitalograph Ltd Buckingham EnglandElectronic peak flow meters, 2009 Available from: http://www.vitalograph.co.uk/products/asma-1.php. Accessed Nov 24 2010.
- EnrightPLHow to make sure your spirometry tests are of good qualityRespir Care200348877377612890297
- EngstromCPPerssonLOLarssonSReliability and validity of a Swedish version of the St. George’s Respiratory QuestionnaireEur Respir J199811161669543271
- StallbergBNokelaMEhrsPOValidation of the Clinical COPD Questionnaire (CCQ) in primary careHealth Qual Life Outcomes200972619320988