Abstract
Background
Although Parkinson’s disease is the second most prevalent neurodegenerative disease worldwide, its cost in Brazil – South America’s largest country – is unknown.
Objective
The goal of this study was to calculate the average annual cost of Parkinson’s disease in the city of São Paulo (Brazil), with a focus on disease-related motor symptoms.
Subjects and methods
This was a retrospective, cross-sectional analysis using a bottom-up approach (ie, from the society’s perspective). Patients (N=260) at two tertiary public health centers, who were residents of the São Paulo metropolitan area, completed standardized questionnaires regarding their disease-related expenses. We used simple and multiple generalized linear models to assess the correlations between total cost and patient-related, as well as disease-related variables.
Results
The total average annual cost of Parkinson’s disease was estimated at US$5,853.50 per person, including US$3,172.00 in direct costs (medical and nonmedical) and US$2,681.50 in indirect costs. Costs were directly correlated with disease severity (including the degree of motor symptoms), patients’ age, and time since disease onset.
Conclusion
In this study, we determined the cost of Parkinson’s disease in Brazil and observed that disease-related motor symptoms are a significant component of the costs incurred on the public health system, patients, and society in general.
Introduction
By 2030, the number of individuals with Parkinson’s disease (PD) will be approximately 9 million worldwide.Citation1 In a survey conducted in 28 European countries, PD was classified as the fourth most expensive disease among the 12 most prevalent and costly neurologic disorders.Citation2 Although methodologies differ among epidemiologic studies, the prevalence of PD in industrialized countries is estimated at ~0%–3% of the entire population, 1% of individuals over 65 years old, and 3%–5% of individuals 85 or older.Citation3,Citation4 The incidence of PD varies between eight and 18/100,000 persons/year, although one study conducted in Argentina reported a rate of 31.2/100,000 persons/year.Citation3,Citation5
Knowing the costs associated with a given disease is critical to formulate, prioritize, and allocate health resources as well as to develop therapies and/or interventions applied by public health managers, insurance companies, as well as patients and their families. Although several variables affect costs, most studies typically focus strictly on disease severity and duration.Citation6–Citation10 Studies about PD-associated costs have been conducted worldwide,Citation6,Citation11,Citation12 but are relatively rare in South America, where notifying PD is not compulsory.
The annual cost of PD is positively correlated with disease severity, such that costs increase with PD progression and may even double with each score on the modified Hoehn and Yahr (H&Y) scale.Citation13,Citation14 Furthermore, the combination of symptoms and the potential side effects of antiparkinsonian medications may adversely affect patients’ quality of life and result in higher costs to the society.Citation15–Citation19
In this study, our goal was to estimate the mean annual cost of PD in São Paulo – Brazil’s most economically developed city – and to assess the impact of motor symptoms specifically, by the societal perspective. We also assessed the indirect and direct (medical and nonmedical) costs and correlated them with several sociodemographic and clinical variables.
Subjects and methods
Participants and study design
A total of 390 patients were contacted between October 2015 and September 2016 during outpatient visits at two tertiary centers in the city of São Paulo: the Hospital at the Universidade Federal de São Paulo (Institution 1) and the Hospital do Servidor Público Estadual (Institution 2). Three patients chose not to participate and the remaining 387 agreed to receive the research material. A total of 268 questionnaires were returned by mail, eight of which were excluded because they were incomplete or the respondents did not properly follow the instructions.
Thus, the final group consisted of 260 patients with PD according to UK Parkinson’s Disease Society Brain Bank criteriaCitation20 (see for patient characteristics). Symptom severity (measured during the “on” phase) and time since disease onset were provided by the physician during the visit. Patients were excluded if their PD costs were covered by private health insurance or if they had undergone surgery for PD. We decided to exclude patients who had undergone deep brain stimulation because Institution 2 did not have this expertise and, in spite of the growing number of patients operated on in Institution 1, we understood this could be addressed separately. Besides, surgery for PD in Brazil is still incipient and does not represent the PD-associated costs.
Table 1 Patient characteristics
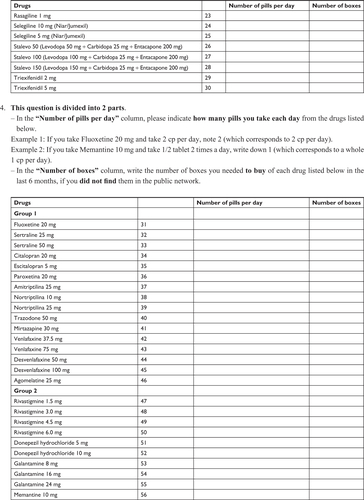
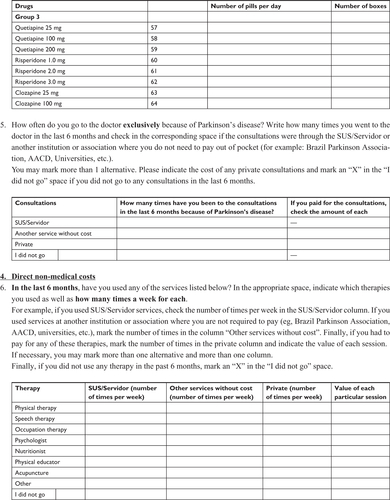
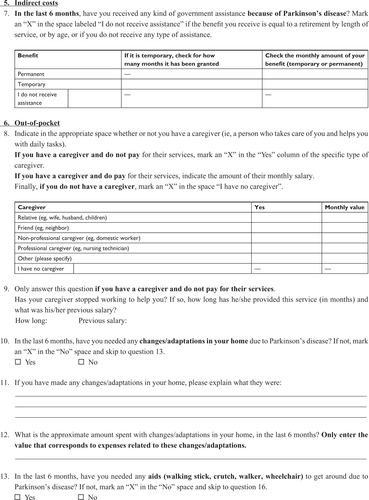
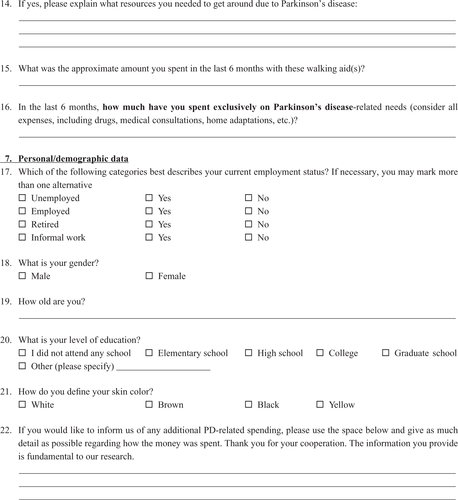
The questionnaire (Supplementary material) contained five parts: 1) direct medical costs (outpatient and/or private visits and antiparkinsonian medications); 2) direct non-medical costs (ambulatory and/or private complementary treatments); 3) indirect costs (benefits given to patients who retired exclusively due to the illness and lost wages of caregivers who stopped working to take care of the patient); 4) out-of-pocket expenses (expenses paid by the patient, including medications and equipment and/or home alterations to facilitate locomotion); and 5) sociodemographic data (age, gender, level of education, and financial situation).
After a face-to-face interview with the patient, explaining the purpose of our research, ethics, and the importance of reliability on data collection, our study questionnaire was sent via mail and patients were instructed to answer this form with the help of a caregiver. Also, with the purpose of minimizing recall bias, information on PD-associated costs was requested covering the previous 6 months and then extrapolated to a full year. Whenever there was data or incorrect/doubtful/incongruent information was filled in, the interviewer got in touch with the respective subject or caregiver.
This was an observational, cross-sectional, retrospective study about disease prevalence that used a bottom-up approach (ie, from the society’s perspective). It was approved by the local Research Ethics Committee (Comitê de Ética em Pesquisa do Hospital Israelita Albert Einstein) under protocol number 45632915.1.0000.0071 on July 6, 2015, and all participants provided informed consent.
Cost calculation
Costs were calculated for the 6-month period prior to the interview and extrapolated to 1 year. Values are described in Real ($) and US Dollar (USD) using the Brazilian Central Bank real-dollar quotation assessed on February 24, 2017.
To calculate the direct medical and nonmedical costs, we obtained data from public and legal sources of the Brazilian public health system, known as the Sistema Único de Saúde (SUS; http://aplicacao.saude.gov.br/bps and http://sigtap.datasus.gov.br). For patients who purchased drugs through private pharmacies or drugstores, we estimated the average market price at the time the research was conducted, and in cases where private therapists were hired, consultation fees were obtained directly from the professionals. Direct medical costs included the cost of medications and consultations purchased privately and through the SUS, while direct nonmedical costs included private and SUS-provided therapies, caregiver services, home adaptations, and the purchase of equipment to facilitate locomotion. We then added all medical and nonmedical costs to obtain the total direct costs. Indirect medical costs included the amount of benefits received (when reported in the questionnaire) as well as the last salary of caregivers who stopped working to assist the patient (reflecting the caregiver’s loss of productivity).
Pilot study
Our initial questionnaire was designed based on previous international studiesCitation9,Citation11,Citation21,Citation22 and contained 31 questions regarding PD-associated costs as well as personal, demographic, and socioeconomic data. To test its validity and improve its content, we randomly selected 21 patients to fill it out as part of a pilot study during the month of August 2015. Thirteen patients returned the questionnaire and based on their answers, we developed a final 22-item questionnaire with improved clarity and data reliability.
Statistical analyses
Categorical variables are described as absolute frequencies and percentages, and numerical variables as summary measures, such as means and SD or medians and quartiles (first and third quartiles), as well as minimum and maximum values.
Cost distribution was plotted in histograms and boxplots using the Shapiro–Wilk normality test, which revealed great variability and an asymmetric distribution.
The total annual cost was estimated by calculating the mean and 95% CI obtained by adjusting a generalized linear model with a gamma probability distribution and log link function.
Generalized linear models were adjusted for the annual total cost with gamma probability distribution and log link function, as well as the following explanatory variables: factors related to the patient, the disease, and the place of recruitment (Institution 1 or 2). The models were constructed for each explanatory variable using a simple approach and later a multiple approach, which also took into account all the first-order interaction effects and applied a step-by-step variable selection process that searched for the variable combination that best explains the total cost.
Model results are presented by the estimated total cost means and 95% CIs and by the means’ ratios and 95% CIs. Multiple comparisons between category variables and the estimated costs were corrected by the Bonferroni method.Citation23
To calculate caregivers’ loss of productivity, we updated the lagged wage values, and current values were estimated by means of a correction model that takes into account the national consumer price index (Índice Nacional de Preços ao Consumidor) and the unemployment rate (used to estimate the probability that an individual will become unemployed at some point between the last salary collected and the current year). Both the Índice Nacional de Preços ao Consumidor and unemployment rates were obtained from government sources. For stability, we generated model simulations and used the obtained means to estimate the updated salary for each caregiver.
All analyses were conducted using SPSSCitation24 and R,Citation25 and significance was set at 5%.
Results
A total of 54.6% of PD patients were from Institution 1 and the remaining 45.4% were from Institution 2. Males made up 55.4% of the sample, 46.2% of all patients had completed elementary school, 223 patients (85.8%) were retired, and 45 of them (17.3%) received some form of government benefits exclusively due to PD. Most patients had an H&Y score of 2.0 or 3.0, and nearly 81% of patients had had the disease for more than 5 years at the time of study inclusion ().
Detailed analyses of the annual average costs associated with PD, including direct, indirect, and total costs, are presented in , while the correlations between H&Y scale scores and treatments, caregivers, and benefits are listed in .
Table 2 Annual costs associated with Parkinson’s disease
Table 3 Correlation between costs and clinical and demographic characteristics according to disease severity
All patients used at least one drug to treat PD, and these drugs were grouped according to therapeutic class: 96.2% used levodopa, 56.9% used dopamine agonists such as pramipexole, 22.3% used antiglutamatergics such as amantadine, 5.4% used anticholinergics such as biperiden, and 1.5% used monoamine oxidase inhibitors such as selegiline.
Another 36.2% of patients took other medications, including antidepressants and/or anxiolytics (30.0%), anti-psychotics (8.8%), and medications for cognitive impairment (4.2%). During the period of the study, 54.2% of patients had to privately pay for part or all of their medications, as some drugs were not available through the public system.
A total of 37.3% and 44.2% of patients, respectively, went to one or two outpatient visits exclusively for PD, and 10.4% paid for private consultations.
Regarding complementary treatments, 48.1% of patients had received at least one type of free therapy during the previous 6 months, including: physiotherapy (34.6%), speech therapy (12.3%), and nutritionist services (3.1%). Another 24.6% paid for at least one other type of therapy.
The monthly amount of government benefits (indirect costs) due to PD ranged from R$800 (US$258) to R$5,250 (US$1,694), with half of the patients receiving up to R$1,500 (US$484) and the interquartile range being R$880 (US$284)–R$2,200 (US$710). The time since retirement was between 6 months and 33 years. Considering the interval between the age of retirement due to illness and the legal age of retirement in Brazil (60 for women and 65 for men in 2017), benefits were anticipated by 0–32 years. Assuming patients accurately reported the monthly amount received, the government’s indirect cost for patients who receive their retirement before the legal age ranged from 0 to R$816,000 (US$263,300).
Of the 168 (64.6%) patients who reported having at least one caregiver, 137 (81.5%) were assisted by an unpaid relative. Of the 43 (16.5%) patients with caregiver costs, four (9.3%) had more than one paid caregiver.
Privately paid home adaptations and the purchase of equipment to improve locomotion were reported by 30.0% and 35.4% of patients, respectively.
Inferential analyses
shows the inferential analyses of the total annual cost of PD and sociodemographic and clinical variables. The patients with the highest costs were those 63 years of age or younger. Also, average costs of patients with >10 years of illness were 2.1 times the average cost of patients with disease duration of <5 years (p<0.001). Patients with bilateral and more severe disease (modified H&Y scores between 2 and 5) had estimated average costs of at least twice the mean cost of patients with a score of 1 (ie, a strictly unilateral disease; p<0.05 for all scores compared to a score of 1).
Table 4 Inferential analyses of the total annual cost of Parkinson’s disease and sociodemographic and clinical variables (generalized linear models using the simple approach)
Average costs of patients who underwent therapy were 1.65 times that of those who did not undergo nonpharmacologic therapy (p<0.001), such as physical therapy, speech therapy, or nutritionist.
Patients who used antidepressants/anxiolytics in addition to PD medications were estimated to cost 1.5 times more than the mean cost of patients who used PD medications alone (p=0.004). Similarly, the mean cost of patients who used antipsychotics and/or medications for cognitive impairment in addition to PD medications was estimated at approximately twice the mean cost of patients who used PD medications alone (p=0.001).
Next, we conducted inferential analyses excluding the amount paid for therapies to assess their impact on the total cost of the disease. Compared to patients who underwent no complementary therapies, those who had undergone at least one session of complementary therapy (physical therapy, speech therapy, occupational therapy, psychologist, nutritionist, physical educator, acupuncture, or others) presented a higher total mean cost (difference of R$6,736.36 [US$2,173]; 95% CI: R$3,260.98 [US$1,052]–R$10,211.75 [US$3,295]; p<0.001). Groups did not otherwise differ from each other when these costs were excluded from the analyses ().
Figure 1 Estimated means and 95% CIs for the total annual cost of Parkinson’s disease considering complementary therapies (A) and drug use (B), when comparison was made between groups of patients.
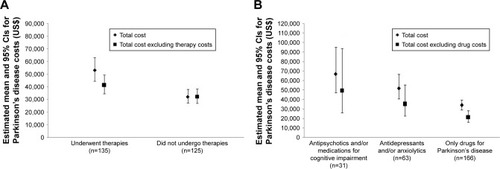
Next, patients were divided according to drug treatment and compared in terms of total PD costs. Patients who used drugs other than those for PD had higher total costs than those who used only PD medications (a difference of R$10,601.48 [US$3,420], 95% CI: R$2,856.51 [US$922]–R$18,346.45 [US$5,920]; p=0.022 compared to the group of patients who used antipsychotics and/or medications for cognitive impairment, and a difference of R$5,809.26 [US$1,874], 95% CI: R$1,359.55 [US$439]–R$10,258.98 [US$3,310]; p=0.032 compared to patients who used antidepressants/anxiolytics). There was no difference in costs between patients who used antipsychotics and/or medications for cognitive impairment and those who used antidepressants/anxiolytics (p=0.827). Excluding these medication-related costs eliminated differences between groups ().
Discussion
This is the first study on the cost of PD conducted in Brazil, South America’s largest country. Our data revealed an average annual cost of PD per patient of R$18,141.64 (US$5,853.50), including 53.4% direct costs and 46.6% indirect costs. We also found that costs are significantly correlated with patient age as well as disease duration and severity. Compared with the use of other drugs and therapies, motor symptoms had a significantly greater impact on PD-associated costs.
Since there is no standardized, validated instrument to study the costs of PD, previous studies vary greatly in methodology as well as results,Citation6,Citation26 with authors independently deciding how to classify the variables studied.
Medications are usually the first aspect studied when researching the costs of a disease. In our study, antiparkinsonian drugs were responsible for 25.0% of the total cost (both drugs provided by the public health system and those purchased privately by patients) and 97.0% of direct medical costs. In one review study,Citation19 drug therapy accounted for 15%–80% of total direct costs. Prescription is undoubtedly an important component of costs: as compared to our study, one study conducted in BrazilCitation27 and another in ItalyCitation28 showed that the use of levodopa varied between 96.9%, 87.5%, and 92.9%, respectively, as did the use of pramipexole (56.9%, 20.8%, and 77.1%) and amantadine (22.3%, 23.6%, and 8.6%), certainly leading to significant variations in final medication costs. While countries such as Germany and Norway differ significantly in how they prescribe medications at different stages of the disease, in both countries, PD drugs accounted for 44% of the total disease cost.Citation29
We observed in our sample that levodopa was prescribed at all stages of the disease, including the early stages (), indicating a reduced belief in the notion that the drug may induce early motor complications in patients, at least in tertiary services. Of the 92 patients 63 years of age or younger (), 56 (61%) also used a dopamine agonist (pramipexole), which certainly raised the costs, considering that dopamine agonists in Brazil are significantly more expensive than levodopa. On the other hand, optimizing treatment by combining levodopa with dopamine agonists may explain why indirect costs were lower than direct costs, since the patient remains independent for a longer period, still working, thus decreasing the costs on the state.
Regarding direct nonmedical costs, 51.9% of our patients did not use any type of complementary therapy, either because free services were unavailable or difficult to access or their physician did not recommend them. In Brazil, several such services are offered to patients at zero or reduced costs by some cities, programs developed for the elderly, associations exclusively dedicated to aid PD patients, or even universities, but these services often fail to reach their target audience. A recent review studyCitation30 on the impact of physical activity in PD showed the role of these therapies in inducing plasticity in several brain regions, especially if performed for at least 45–60 minutes 2–3 times/week. In our sample, 26.9% of patients who practiced some type of therapy did so with this type of frequency and almost 60% of all who underwent some type of treatment had scores between 2.5 and 3.0 on the H&Y scale.
Regular exercise and physical therapy work as a complement to the medical treatment of PD. Increasing evidence suggests that these types of therapies should be encouraged as they can improve motor performance and potentially delay the progression of symptoms; they are especially recommended early on in the disease to maintain physical fitness levels.Citation31 We note that 82.2% of our patients had H&Y scores between 1 and 3 (ie, mild to moderate disability), which makes them ideal candidates for physical activity. Furthermore, we observed that therapies did not significantly add to the total cost of the disease (), which is one more reason to recommend them.
As it is a late-onset, long-term illness (), PD usually manifests when the patient is already close to retirement due to length of service or age. In our sample, 17.3% of patients were retired because of the disease and were still at a productive age (mean =57.3 years; retirement in Brazil usually occurs at 60 and 65 years of age for women and men, respectively). The benefits provided to these patients by the government accounted for 21% of the total cost and 45.8% of the indirect cost, just below that presented in a review study conducted in GermanyCitation19 (30%–60% of the total cost). The mean duration of the disease was 10.3 years at the time of study inclusion, meaning that patients had been receiving retirement benefits (a significant cost to the state) for quite some time. A study conducted in the UKCitation32 revealed that most PD patients continued to work full-time or part-time for up to 10 years before losing their jobs. In Finland,Citation21 the average retirement age of patients with PD is 52.8 years, well below the 58–59 years of the general population in the 1990s. In our study, 7.7% of patients did some type of work (either through regular or informal employment) and none of the 45 patients who retired due to the disease declared having any other form of gainful activity.
As many as 64.6% of our patients had at least one caregiver, and 16.5% of them paid them privately (). Considering that 223 of our patients were retired (by law or illness) and that the average amount of annual benefits received was R$3,811.00 (US$1,230), the amount paid to caregivers (R$2,649.00; US$855) is relatively high. As in Brazil, in Singapore,Citation11 home care is not subsidized by the government or by insurance companies, making up 76.1% of the total cost, while complementary treatments comprise 17.3% and transportation comprises 4.6%. In Germany,Citation33 caregiver costs are considered direct costs (there is no distinction between medical and nonmedical direct costs) and are partly subsidized by the state according to the patient’s degree of disability: R$1,468.00 (US$405; first level), R$3,521.00 (US$973; second level), and R$5,464.00 (US$1,513; third level) per month.
Certain governments such as those of GermanyCitation34 and other European countriesCitation6 subsidize part of the costs associated with walking sticks, wheelchairs, or walkers. Although in Brazil there are lawsCitation35 providing such forms of assistance to individuals with certain diseases or disabilities, these are not adequately enforced, which means that many patients end up privately paying for such resources.Citation36
Naturally, the costs increase as the disease progresses. StudiesCitation37,Citation38 have shown that the total mean cost for patients with an H&Y score of 4 is almost twice that of patients with an H&Y score of 1. In the UK,Citation39 direct costs were most strongly correlated with disease-related disability, with score 5 being associated with a cost six times that of scores 0, 1, or 2. Similarly, in our study, scores 5 and 4 cost 4.8 and 2.9 times more than score 1, respectively. However, we did find that cost among H&Y 3 patients was slightly lower than for H&Y 2.5 patients. We believe this difference was probably related to a sample size bias ().
In summary, we observed that patients with PD presenting the highest costs are male and female patients 63 years of age or younger, who have had PD for more than 10 years, and have high H&Y scores (ie, 5). Similarly, a study conducted in Spain in 2004Citation40 revealed that younger patients with high H&Y scores, longer disease duration, and motor complications had the highest direct costs. By contrast, a study in NorwayCitation41 suggested that higher costs are associated with older patients due to the higher incidence of dementia, which often results in institutionalization (in our study, these would be privately incurred caregiver costs).
Our study had methodological limitations that should be carefully addressed. First, since we tested patients at only two tertiary health centers in the metropolitan region of São Paulo, the results cannot be generalized to Brazil’s entire population of PD patients. Our results do not consider the costs of treating patients in primary and secondary public services, which are likely to be lower. On the other hand, we also did not consider costs in a totally private health care environment or one which included surgical PD patients, which would be more expensive. Second, another limitation of this study was the exclusion of patient/caregiver incomes that could help understand the costs involved in having PD. Actually, at the time of our pilot study, we realized that requesting patient/caregiver incomes would be an embarrassing question to these low-income background subjects, and this could impact our response rate, data reliability, and increase missing data. Third, we used a backdated 6-month period, which may have underestimated some costs. Fourth, we understand that an important drawback of our study was the so-called recall bias. As it is known, the impact of memory can account for 20% of critical details irretrievable after 1 year.Citation42 Next, we understand that disease severity in PD is preferably evaluated during the “off”-state. However, patients in both hospitals were always requested to take their medication as usual and then were clinically evaluated in their “on”-state. Therefore, we understand that we could have found different costs regarding disease severity if we had evaluated patients in the “off”-state. Finally, the rate of non-returned questionnaires was 34%, which is rather high. Nevertheless, we had a considerably large sample (N=260) and were able to correlate costs with in-person medical evaluations.
Another important consideration is that we did not correlate costs with possible motor fluctuations and dyskinesias, complications that generally require more drugs and greatly impact quality of life. We know that most patients with an H&Y score of 3 or higher have a high prevalence of these complications.
As the first study of its kind conducted in Brazil, our work has begun to fill the knowledge gap regarding PD-associated costs in this country. We observed that PD exerts an overload on the public health system, on patients, and on the society in general. In our study sample, patients contributed 32.1% of the total annual cost of the disease (US$1,873.2). Our results may provide public health managers with the necessary tools for better decision making, prioritization, and resource allocation to improve patients’ quality of life. In addition, the current findings could help develop standardized ways of measuring PD-associated costs worldwide.
Acknowledgments
The authors thank all the patients, their relatives, and/or caregivers who participated in the study, as well as the responsible physicians and residents in the two outpatient clinics. They also thank the statistics, ethics, health economics, administrative, and library staff of the Hospital Israelita Albert Einstein for their invaluable support.
Disclosure
The authors report no conflicts of interest in this work.
References
- DorseyERConstantinescuRThompsonJPProjected number of people with Parkinson disease in the most populous nations, 2005 through 2030Neurology200668538438617082464
- Andlin-SobockiPJönssonBWittchenHUOlesenJCost of disorders of the brain in EuropeEur J Neurol200512Suppl 1127
- de LauLMLBretelerMMBEpidemiology of Parkinson’s diseaseLancet Neurology20065652553516713924
- AlvesGForsaaEBPedersenKFGjerstadMDLarsenJPEpidemiology of Parkinson’s diseaseJ Neurol2008255Suppl 5183218787879
- BausoDJTartariJPStefaniCVRojasJIGiuntaDHCristianoEIncidence and prevalence of Parkinson’s disease in Buenos Aires City, ArgentinaEur J Neurol20121981108111322390275
- von CampenhausenSWinterYRodrigues e SilvaACosts of illness and care in Parkinson’s Disease: an evaluation in six countriesEur Neuropsychopharmacol201121218019120888737
- FindleyLJThe economic impact of Parkinson’s diseaseParkinsonism Relat Disord200713SupplS8S1217702630
- McCronePAllcockLMBurnDJPredicting the cost of Parkinson’s diseaseMov Disord200722680481217290462
- ZhaoYJTanLCSAuWLEstimating the lifetime economic burden of Parkinson’s disease in SingaporeEur J Neurol201320236837422978629
- BovolentaTMde Azevedo SilvaSMArb SabaRBorgesVFerrazHBFelicioACSystematic review and critical analysis of cost studies associated with Parkinson’s diseaseParkinson’s Dis20172017341094628357150
- ZhaoYJTanLCSLiSCEconomic burden of Parkinson’s disease in SingaporeEur J Neurol201118351952620840378
- JohnsonSJKaltenboeckADienerMCosts of Parkinson’s disease in a privately insured populationPharmacoeconomics201331979980623907717
- HoehnMMYahrMDParkinsonism: onset, progression, and mortality. 1967Neurology1998502318 and 16 pages following9484345
- DowdingCHShentonCLSalekSSA review of the health-related quality of life and economic impact of Parkinson’s diseaseDrugs Aging200623969372117020395
- BolandDFStacyMThe economic and quality of life burden associated with Parkinson’s disease: a focus on symptomsAm J Manag Care2012187 SupplS168S17523039865
- DuncanGWKhooTKYarnallAJHealth-related quality of life in early Parkinson’s disease: the impact of nonmotor symptomsMov Disord201429219520224123307
- Martinez-MartinPRodriguez-BlazquezCKurtisMMChaudhuriKRGroup NVThe impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s diseaseMov Disord201126339940621264941
- LiHZhangMChenLNonmotor symptoms are independently associated with impaired health-related quality of life in Chinese patients with Parkinson’s diseaseMov Disord201025162740274620945434
- ReeseJPDamsJWinterYBalzer-GeldsetzerMOertelWHDodelRPharmacoeconomic considerations of treating patients with advanced Parkinson’s diseaseExpert Opin Pharmacother201213793995822475391
- GibbWRLeesAJThe relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s diseaseJ Neurol Neurosurg Psychiatry19885167457522841426
- KeranenTKaakkolaSSotaniemiKEconomic burden and quality of life impairment increase with severity of PDParkinsonism Relat Disord20039316316812573872
- TamásGGulácsiLBereczkiDQuality of life and costs in Parkinson’s disease: a cross sectional study in HungaryPLoS One201499e10770425229404
- AltmanDGPractical Statistics for Medical Research1st edLondonChapman & Hall/CRC1991
- IBM CorpISSfW, Version 24.0Armonk, NYIBM Corp2016
- R Core TeamR: a language and environment for statistical computingR Foundation for Statistical Computing VAustria2015 Available from: http://www.R-project.org/Accessed July 25, 2016
- Rodríguez-BlázquezCForjazMJLizáLPazSMartínez-MartínPEstimating the direct and indirect costs associated with Parkinsons diseaseExpert Rev Pharmacoecon Outcomes Res201515688991126511768
- VargasAPCarod-ArtalFJNunesSSMeloMDisability and use of healthcare resources in Brazilian patients with Parkinson’s diseaseDisabil Rehabil200830141055106218953751
- WinterYvon CampenhausenSReeseJPCosts of Parkinson’s disease and antiparkinsonian pharmacotherapy: an Italian cohort studyNeurodegener Dis20107636537220523028
- VossiusCGjerstadMBaasHLarsenJPDrug costs for patients with Parkinson’s disease in two different European countriesActa Neurol Scand2006113422823216542161
- CussoMEDonaldKJKhooTKThe impact of physical activity on non-motor symptoms in Parkinson’s disease: a systematic reviewFront Med (Lausanne)201633527583249
- RedeckerCBilsingACsotiIPhysiotherapy in Parkinson’s disease patients: Recommendations for clinical practiceBasal Ganglia2014413538
- SchragABanksPTime of loss of employment in Parkinson’s diseaseMov Disord200621111839184316941456
- WinterYBalzer-GeldsetzerMSpottkeALongitudinal study of the socioeconomic burden of Parkinson’s disease in GermanyEur J Neurol20101791156116320345926
- SpottkeAEReuterMMachatOCost of illness and its predictors for Parkinson’s disease in GermanyPharmacoeconomics200523881783616097843
- Brazil. [Presidência da República. Lei nº 13.146, de 06 de Julho de 2015. Institui a Lei Brasileira de Inclusão da Pessoa com Deficiência (Estatuto da Pessoa com Deficiência). Diário Oficial da República Federativa do Brasil, Brasília (DF)] Avaiable from: http://www.plan-alto.gov.br/ccivil_03/_ato2015-2018/2015/lei/l13146.htmAccessed Jun 20, 2016
- BovolentaTMFelicioACHow do demographic transitions and public health policies affect patients with Parkinson’s disease in Brazil?Clin Interv Aging20171219720528182156
- WangGChengQZhengREconomic burden of Parkinson’s disease in a developing country: a retrospective cost analysis in Shanghai, ChinaMov Disord20062191439144316773620
- HagellPNordlingSReimerJGrabowskiMPerssonUResource use and costs in a Swedish cohort of patients with Parkinson’s diseaseMov Disord20021761213122012465059
- FindleyLAujlaMBainPGDirect economic impact of Parkinson’s disease: a research survey in the United KingdomMov Disord200318101139114514534917
- CuboEMartínez MartinPGonzálezMFradesBmiembros del grupo ELEPImpact of motor and non-motor symptoms on the direct costs of Parkinson’s diseaseNeurologia2009241152319003550
- VossiusCLarsenJPJanvinCAarslandDThe economic impact of cognitive impairment in Parkinson’s diseaseMov Disord20112681541154421538519
- BradburnNMRipsLJShevellSKAnswering autobiographical questions: the impact of memory and inference on surveysScience198723647981571613563494