Abstract
Objective
This study aimed to explore whether a computerized cognitive stimulation program (CCS) induced differential effects in older adults with mild cognitive impairment (MCI) according to the severity of white matter hyperintensities (WMH), which are associated with cognitive impairment and increased risk of progression to Alzheimer’s disease because of the damage they cause to cortical and subcortical networks.
Patients and methods
Twenty-nine MCI patients with no or little WMH (MCI-non-WMH) and 22 MCI patients with moderate or severe WMH (MCI-WMH) attended a 24-session CCS program (two sessions per week for a duration of 3 months) focused on executive functions, attention, and processing speed. Cognitive and psychosocial assessments were performed at baseline, postintervention, and 3 months after the intervention.
Results
Both groups improved on several cognitive measures after the intervention. However, the MCI-non-WMH group improved on a higher number of cognitive measures than the MCI-WMH group. At postintervention assessment, CCS had a more beneficial effect on the MCI-non-WMH group than on the MCI-WMH group with regard to improving categorical fluency (4.6±6.8 vs 0.4±6.4; effect size=0.37; p=0.002). During the 3-month follow-up assessment, significantly higher score improvements were observed in the MCI-non-WMH group for the paired-associate learning test (6.4±3 vs 4.7±3.5 points; effect size=0.43; p=0.005) as well as categorical fluency (3.8±7.8 vs −0.7±6 points; effect size=0.55; p=0.0003).
Conclusions
These findings suggest that WMH severity was related to cognitive improvement induced by a CCS program and highlight the importance of considering WMH in interventional studies on subjects with MCI.
Introduction
Mild cognitive impairment (MCI) is commonly regarded as being a transitional phase between normal aging and early Alzheimer’s disease (AD), the most common cause of dementia. Older adults with MCI have a higher risk of developing dementia (10%–15% per year),Citation1 compared to the general older population (1%–2% per year).Citation2 In the absence of effective treatment for dementia, MCI is becoming of interest for pharmacologic and nonpharmacologic interventions, in order to prevent further cognitive decline.
It is now well established that cognitive interventions such as cognitive training, cognitive rehabilitation, and cognitive stimulationCitation3 could help maintain or enhance cognitive and functional abilities in MCI subjects.Citation4,Citation5 The last decade has seen the emergence of various computerized cognitive intervention programs, which seem promising for these patients.Citation6–Citation9 Computerized programs involve both cognitive training and novel learning experiences. On one hand, participants are required to focus on tasks tapping into either multiple or specific cognitive domains. On the other hand, older adults who are not familiar with digital devices need to acquire new skills, which in turn stimulates several cognitive functions.Citation10 However, there is a lack of consensus regarding their efficacy, in part due to the heterogeneity of MCI, a factor that may affect intervention outcome.Citation4,Citation5,Citation11,Citation12 MCI is a heterogeneous clinical entity, encapsulating different cognitive profiles, underlying brain pathologies and follow-up outcomes. In most of the studies on cognitive interventions in MCI, underlying brain lesions are rarely taken into consideration. However, it has been suggested that MCI patients with more severe brain lesions might benefit less or differently from cognitive interventions than those with less severe brain lesions.Citation9
In biologic terms, AD is commonly defined as the accumulation of beta-amyloid protein and the deposition of neurofibrillary tangles. However, increasing evidence shows that white matter hyperintensities (WMH) also contribute to the development of MCI and AD.Citation13,Citation14 WMH are visualized as increased signal on T2-weighted fluid-attenuated inversion recovery magnetic resonance imaging (MRI) sequences. WMH are often considered to be markers of cerebral small-vessel disease and are frequently observed in older adults with MCI and AD.Citation15,Citation16 It is hypothesized that WMH might precipitate the clinical manifestation of MCI and AD.Citation17,Citation18 Neuroimaging studies have shown that WMH may cause a frontal-subcortical pathway disruption, leading to executive, attentional, and processing speed impairmentCitation19–Citation21 as well as memory deficits, due to retrieval impairment.Citation17,Citation22,Citation23 WMH have been observed in amnestic and nonamnestic subtypes of MCI.Citation15,Citation17,Citation24,Citation25
To address the lack of literature regarding the effects of cognitive intervention programs according to brain lesions, this study aimed to compare effects of a computerized cognitive stimulation (CCS) program in MCI patients according to the severity of WMH.
Patients and methods
Design
This is a prospective, single-blind, parallel-group, quasi-experimental study. The flow chart of the study is reported in .
Figure 1 The flow chart of the study.
Abbreviations: MCI, mild cognitive impairment; MCI-WMH, MCI with moderate or severe white matter hyperintensities; MCI-non-WMH, MCI with no or little white matter hyperintensities.
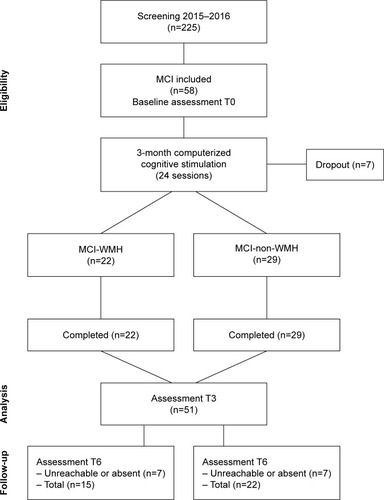
Participants
Participants were recruited in Broca Hospital’s memory clinic between 2015 and 2016.
To be included in the study, participants must have had 1) a diagnosis of MCI:Citation26 subjective memory complaints, preferably corroborated by an informant, objective impairment on more than one of the neuropsychological tests used in the memory clinic (French version of the Free and Cued Selective Reminding Test,Citation27 Frontal Assessment Battery,Citation28 Digit Span,Citation29 verbal fluency,Citation30 Trail Making Test parts A and B [TMT-B],Citation31 80-item naming test,Citation32 Praxic Ability Scale;Citation33 1.5 SD below the mean for age and education or below the cutoff score), preserved global intellectual function, preserved or minimal impairment in daily living activities, and the absence of dementia; 2) MRI evidence indicating the severity of WMH. The severity of WMH was estimated by an experienced neurologist using the Fazekas Scale,Citation34 providing two different scores for periventricular WMH and deep WMH, rated on four points (0–3). Periventricular WMH were rated as 0=no lesions; 1=“caps” or pencil-thin lining; 2=smooth “halo”; and 3=irregular lesions extending into the deep white matter. Deep WMH were rated as 0=no lesions; 1=punctuate foci; 2=beginning confluence of foci; and 3=large confluent areas. We classified participants into two groups according to the severity of WMH, taking into account both periventricular and deep WMH: MCI subjects with no or little WMH (MCI-non-WMH; grade 0 and 1) vs MCI subjects with moderate to severe WMH (MCI-WMH; grades 2 and 3).
The exclusion criteria were 1) psychiatric and neurologic disorders (eg, bipolar disorder, Parkinson’s disease, epilepsy); 2) history of alcohol or other substance abuse; 3) sensory and/or motor deficits that could interfere with the use of a digital device; 4) participation in another intervention. Two hundred and twenty-five patients were screened. Of the 58 participants eligible for the study, seven subjects dropped out (three participants without WMH and four with WMH), yielding 51 patients who completed the intervention program (39 women and 12 men; age=75.3±6.2 years) as well as pre-and postintervention assessments. Fourteen participants did not complete the follow-up assessment (seven MCI-WMH and seven MCI-non-WMH). Written informed consent was obtained from all participants. This study was approved by the ethical committee from Paris Descartes University’s Institutional Review Board (N°IRB: 20161300001072) and was registered at ClinicalTrials.gov as NCT3195803.
Intervention
All participants benefited from the same CCS program, developed using the concept of cognitive stimulation,Citation35–Citation37 an ecologic approach based on engaging participants in a range of cognitive exercises in a group setting, thus aiming to stimulate cognitive functions and promote social interactions. The participants attended a 1 and a half hour session twice a week, during a period of 3 months (24 sessions in total) in Broca hospital. Three experienced neuropsychologists conducted group sessions with five to seven participants per group.
The cognitive exercises were selected from the “KODRO” software (Altera-Group, Paris, France), a web-based platform, developed for older adults. We chose KODRO for its large content of playful cognitive exercises and for the ecologic nature of some of the exercises, which can be applied in everyday life. These cognitive exercises targeted several cognitive processes, such as mental flexibility, processing speed, working memory, planning/organization, categorization, attention, reaction time, inhibition, and visual tracking. An example of one of these exercises is “Instruction” (tapping into mental flexibility, divided attention, and processing speed), which consists in touching specific stimuli (with different shapes and colors), among multiple distractors, as quickly as possible within the time allocated (indicated by a countdown timer). Within this series of exercises, participants must pay attention to the countdown timer while touching target stimuli. At the end of each countdown timer, instructions change and participants are then required to touch stimuli of another color and shape. Neuropsychologists managed the sessions and the difficulty level of the exercises, thanks to an iPad linked to a TV screen and to each participant’s tablet-PC. The neuro psychologist’s iPad displayed the success and failure rate for each exercise, and participants’ tablet-PCs display feedback (good, very good, and excellent) at the end of each step of the exercise, in order to encourage them. The TV screen displayed the date at the beginning of the session and the instructions for each exercise, allowing the participants to refer to them when necessary. The content of the exercises was scheduled to change every 2 weeks.
During the first session, the participants introduced themselves. Explanations about the outline of the program were given. They were trained to use some basic functionalities of a tablet-PC.
The remaining sessions were structured as follows:
Greeting and discussion about personal events and the news, to foster exchanges before cognitive exercises (5 minutes).
Cognitive exercises with a tablet-PC: four 15-minute cognitive exercises (60 minutes).
Brief conclusion/debriefing with feedback about the session (5 minutes).
Outcomes
Participants were assessed at baseline (T0), immediately after the 3-month intervention (T3) and during a 3-month follow-up postintervention (T6). Neuropsychologists involved in data collection at any point of the assessment process were blinded to which group participants were part of. Outcomes were composed of neuropsychological and psychosocial measures.
At baseline, we collected sociodemographic data such as age and sex as well as education level and premorbid intelligence, as assessed by the French adaptation of the National Adult Reading Test.Citation38 The presence or absence of hippocampal atrophy was also recorded.
Neuropsychological measures
Mini Mental State Evaluation (MMSE)Citation39 assessing global cognitive efficiency (score range: 0–30).
Rey Auditory Verbal Learning Test (RAVLT)Citation40 for the assessment of episodic memory. Two scores were used: the sum of all correct immediate recall scores across five consecutive trials (score range: 0–75) and the delayed recall score (score range: 0–15).
Paired-associate learning test (associative memory; score range: 0–12) and logical memory (trial 1 and trial 2; score range: 0–12) subtests from the Cognitive Efficiency Profile.Citation41
TMT-A and TMT-BCitation31 assessing processing speed, attention, and executive functions. The completion time was recorded. A completion time of 300 seconds was assigned if a subject failed to complete the TMT-B.
Symbol Digit Modalities Test (SDMT) of the Wechsler Adult Intelligent Scale-Fourth Edition (WAIS-IV)Citation29 measuring processing speed/attentional control (score range: 0–133).
Backward Digit Span from the WAIS-IVCitation29 assessing working memory (score range: 0–14).
Verbal fluency (“letter P” for phonemic fluency and “animals” for categorical fluency, 2 minutes) measuring verbal ability and executive control.Citation30
Rey–Osterrieth Complex Figure (ROCF) assessing planning and organization, visual memory, and visuospatial processing.Citation42,Citation43 Copy completion time, copy score (score range: 0–36), and 3-minute immediate recall score (score range: 0–36) were recorded.
To reduce the risk of test performances improving following CCS because of the learning effect of repeated assessments, parallel versions were used when available.
Psychosocial measures
French versions of the Cognitive Difficulties ScaleCitation44 assessing subjective memory complaints.
Geriatric Depression Scale (30-item GDS)Citation45 assessing symptoms of depression.
Goldberg Anxiety ScaleCitation46 assessing symptoms of anxiety.
Quality of life was assessed using the Quality of Life Scale for older French People (Echelle de Qualité de Vie adaptée aux Personnes Agées; EQVPA).Citation47
French version of the Rosenberg Self-esteem Scale assessing global self-worth.Citation48
Level of motivation, with regard to interest in participating in this study and in future interventions like this, was assessed using a 20-point Likert Scale (not at all=0 to very motivated=20).
Statistical analysis
Data were summarized in the form of mean and SD for quantitative variables and count and percentage for categorical variables. Baseline characteristics and assessments were compared between the two groups (MCI-non-WMH vs MCI-WMH) using Fisher’s exact test. The main analysis focused on postintervention assessment and secondary analysis on follow-up assessment. Differences between postintervention (T3) and baseline (T0) and between follow-up (T6) and baseline (T0) were compared within each group using paired t-tests. Intervention effects at T3 and T6 were compared between the two groups, using analysis of covariance, with the baseline score being the covariate and groups being the factor. All models were adjusted for age, sex, education, and hippocampal atrophy. To estimate the magnitude of difference between the two groups with regard to change over time, effect size index was calculated using Cohen’s f2. Effect size is categorized as small (f2=0.10), medium (f2=0.25), or large (f2=0.40).Citation49
All tests were two-sided, with a significance level of 0.05. No corrections were made for multiple comparisons because of the exploratory nature of the analyses. Analyses were carried out using R statistical software version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The MCI-non-WMH group included 29 MCI subjects who presented no or minimal WMH (grades 0 and 1), and the MCI-WMH group was composed of 22 MCI subjects who presented moderate-to-severe WMH (grades 2 and 3). Demographics and clinical data for the two groups are summarized in . There were no significant differences in sex, education, premorbid intelligence, or presence of hippocampal atrophy between the two groups. However, MCI-WMH subjects were significantly older than those in the MCI-non-WMH group.
Table 1 Baseline characteristics and demographics of the subjects
Baseline assessments
Neuropsychological and psychosocial scores are summarized in . The MCI-non-WMH group outperformed the MCI-WMH group on the MMSE (p=0.039), two measures of the RAVLT (p=0.039 and 0.029 for immediate recall and delayed recall, respectively) and ROCF copy completion time (p=0.033). No significant difference was found on other measures.
Table 2 Comparison between two groups on cognitive and psychosocial measures at baseline assessment
Cognitive and psychosocial changes after the intervention within each group
Changes in scores for cognitive and psychosocial measures after the intervention are presented in and .
Table 3 Score changes on cognitive measures from baseline (T0) to postintervention (T3) and 3-month follow-up (T6) in two groups and comparison between groups for score changes
Table 4 Score changes on mood and psychosocial measures from baseline (T0) to postintervention (T3) and 3-month follow-up (T6) in two groups and comparison between groups for score changes
From baseline to postintervention, the MCI-WMH group showed significant improvement on the MMSE (p=0.027), the paired-associate learning test (p=0.0003), TMT-A (p=0.041), TMT-B (p=0.035), and motivation (p=0.001). The MCI-non-WMH group improved on a higher number of outcome measures, namely the paired-associate learning test (p<0.0001), TMT-A (p=0.054), Backward Digit Span (p=0.023), digit symbol test (p=0.037), ROCF copy (p=0.007), ROCF recall (p=0.001), phonemic fluency (p=0.007), categorical fluency (p=0.001), and motivation (p=0.01).
Between the baseline and the 3-month follow-up, improvement in the MCI-WMH group was found for the immediate and the delayed recall of the RAVLT (p=0.0005 and 0.0008, respectively), the paired-associate learning test (p=0.0001), TMT-A (0.016), and ROCF copy score (p=0.015). Once again, improvement on a higher number of outcome measures was found in the MCI-non-WMH group, namely on the paired-associate learning test (p<0.0001), TMT-A (p=0.01), ROCF copy (p=0.021), ROCF recall (p<0.0001), categorical fluency (p=0.033), and motivation (p=0.009).
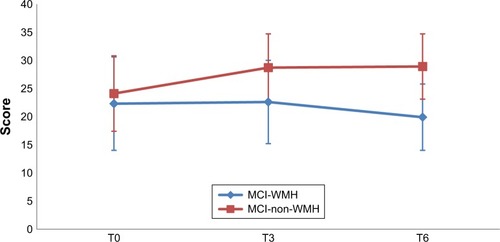
and depict the performances for the immediate recall and the delayed recall of the RAVLT in the two groups, at the three assessment points. The performances for the paired-associate memory test and categorical fluency are depicted in and , respectively.
Figure 2 RAVLT the sum of five immediate recall scores in the MCI-WMH vs MCI-non-WMH at baseline (T0), postintervention (T3), and 3-month follow-up (T6).
Abbreviations: MCI, mild cognitive impairment; MCI-WMH, MCI with moderate or severe white matter hyperintensities; MCI-non-WMH, MCI with no or little white matter hyperintensities; RAVLT, Rey Auditory Verbal Learning Test.
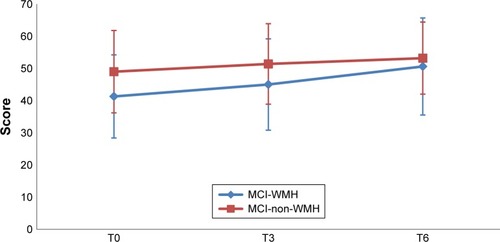
Figure 3 RAVLT delayed recall scores in the MCI-WMH vs MCI-non-WMH at baseline (T0), postintervention (T3), and 3-month follow-up (T6).
Abbreviations: MCI, mild cognitive impairment; MCI-WMH, MCI with moderate or severe white matter hyperintensities; MCI-non-WMH, MCI with no or little white matter hyperintensities; RAVLT, Rey Auditory Verbal Learning Test.
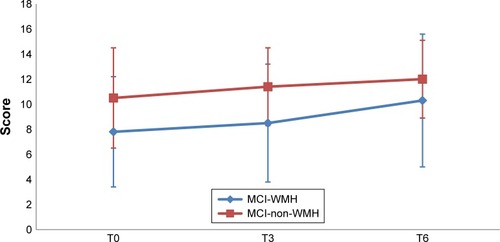
Figure 4 Paired-associate memory test scores in the MCI-WMH vs MCI-non-WMH at baseline (T0), postintervention (T3), and 3-month follow-up (T6).
Abbreviations: MCI, mild cognitive impairment; MCI-WMH, MCI with moderate or severe white matter hyperintensities; MCI-non-WMH, MCI with no or little white matter hyperintensities.
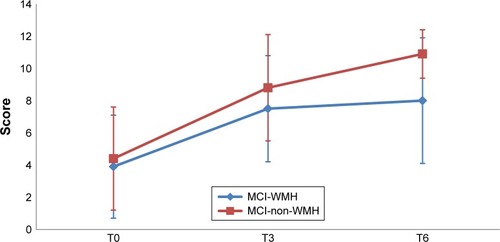
Figure 5 Categorical verbal fluency scores in the MCI-WMH vs MCI-non-WMH at baseline (T0), postintervention (T3), and 3-month follow-up (T6).
Differences between groups in changes on outcome measures
At postintervention assessment, CCS had a more beneficial effect on the MCI-non-WMH group than on the MCI-WMH group with regard to improving categorical fluency (4.6±6.8 vs 0.4±6.4 points; effect size=0.37; p=0.002).
With regard to the 3-month follow-up assessment, significantly higher score gains were observed in the MCI-non-WMH group on the paired-associate learning test (6.4±3 vs 4.7±3.5 points; effect size=0.43; p=0.005) and categorical fluency (3.8±7.8 vs −0.7±6 points; effect size=0.55; p=0.0003).
Discussion
In this study, we investigated whether a CCS program induced differential effects in older adults diagnosed with MCI, according to the severity of WMH. All participants attended 24 sessions of the CCS program over a period of 3 months. The main findings were that 1) at baseline, in comparison with the MCI-non-WMH group, the subjects in MCI-WMH group were older and presented lower global cognitive efficiency and worse episodic memory, and needed more time to copy a complex figure; 2) the two groups improved on several cognitive measures after the intervention; 3) the MCI-non-WMH group improved on a higher number of cognitive measure than the MCI-WMH group, suggesting that the CCS program seemed to be more beneficial for MCI subjects without WMH; 4) although two cognitive measures were improved in both groups, the differential improvements for the other cognitive measures depend on the group status.
The baseline differences between the two groups in our study are not surprising. It is reported that WMH is associated not only with older age,Citation50 but also with impairment of perceptual/processing speed,Citation51,Citation52 global cognition, executive functions, working memory, episodic memory, and semantic memory.Citation13,Citation53–Citation55
Both groups improved on the paired-associate learning test and the TMT-A at postintervention. The improvement persisted for another 3 months after the intervention had been discontinued. The paired-associate learning test requires several memory strategies, such as semantic processing, visual imagery categorization, association, and organization.Citation56 A significant time reduction on the TMT-A suggests an improvement of processing speed. These improvements could be attributed to several cognitive exercises used within the CCS program, which were focused on stimulating these cognitive processes.
Aside from the two measures mentioned above, the MCI-WMH group improved on global cognitive efficiency and motivation at postintervention. However, the improvement was not observed during the 3-month follow-up. It is worth noting that these subjects improved their performance on the immediate and delayed recall of the RAVLT as well as the ROCF copy score during the 3-month follow-up. Compared with MCI-non-WMH subjects, MCI-WMH subjects had impaired retrieval and long-term consolidation processes at baseline. However, they could still improve these cognitive functions, thanks to the CCS intervention, suggesting potential cognitive and brain plasticity in these patients. Previous studies have shown that in both younger and older adulthood, cognitive training can modify white matter microstructureCitation57 and that there is a significant link between memory improvement and changes in white matter.Citation58 Our findings are in concordance with a recent study on the effects of a training program focusing on attentional processes (weekly 2-hour sessions for 20 weeks) in MCI patients with moderate-to-severe WMH. The authors also found improvements on the immediate recall of the RAVLT and ROCF copy in MCI patients receiving the intervention, compared with the no-treatment group.Citation59
MCI patients with no or little WMH improved their performance on a higher number of cognitive measures than those with moderate-to-severe WMH at postintervention and during the follow-up assessments. At postintervention, they improved on the Backward Digit Span, the SDMT, and phonemic fluency, although these improvements did not last. However, improvements on copy and recall of the ROCF and categorical fluency were observed both at postintervention and during the 3-month follow-up assessments. Moreover, significantly higher score gains with medium-to-large effect sizes, favoring the MCI-non-WMH group, were observed on the paired-associate memory test during the 3-month follow-up and on categorical fluency both at postintervention and during follow-up assessments. Overall, these findings suggest that MCI patients with WMH did not benefit from the CCS program as much as those with no or little WMH, with regard to enhancing specific cognitive functions. Taken together, our findings suggest that severity of WMH was related to baseline cognitive performance and cognitive improvement induced by a CCS program. These findings conflict with those of a study exploring the effects of working memory training on cognitive functions in MCI subjects and healthy older adults, in which authors found that hippocampal atrophy, instead of WMH, may be a predictor of cognitive training outcome.Citation9
Finally, the two groups did not improve on psychosocial measures, except for motivation, which was initially high, being enhanced after the intervention for both groups. The enhancement persisted through to the follow-up assessment only for the MCI-non-WMH group. This finding suggests that this short-term cognitive intervention was not effective with regard to enhancing psychosocial functioning in MCI subjects.
Limitations
One of the limitations of the study is the lack of a control group, which did not receive the CCS intervention. However, our principal objective was to compare the effects of the CCS program on two clinical samples in order to understand if WMH affect intervention outcome. A randomized controlled trial is required to determine the efficacy of the CCS as a clinical intervention. In addition, the small sample size keeps us from analyzing the intervention effects according to the periventricular or deep nature of the WMH, as some studies reported that these two types of lesions may differentially impact the clinical presentation.Citation52,Citation60–Citation63
Conclusions
This study constitutes a preliminary exploration, which aims to highlight the importance of considering WMH in interventions in MCI patients. Our findings suggest that both MCI subjects with and without WMH could benefit from this type of CCS program, with improvements being observed within several cognitive domains, and some improvements being maintained through to a short-term follow-up. However, the differential effects in the two groups suggest that brain lesions could influence intervention outcomes. MCI-non-WMH subjects improved on a higher number of cognitive measures than MCI-WMH subjects, for whom no improvement was observed for certain cognitive functions. Interestingly, our findings did show that MCI-WMH subjects could improve on some components of executive functions, processing speed, and memory, suggesting potential cognitive plasticity in these patients, who are at higher risk of worsening cognitive decline with the progression of lesions, and of developing AD and dementia. These findings are encouraging and should be confirmed in a randomized controlled trial. We also suggest that in future cognitive intervention studies, brain lesions, such as hippocampal atrophy and WMH, should be taken into consideration when analyzing intervention effects on patients with MCI.
Acknowledgments
We would like to thank the Fondation des Gueules Cassées (FGC) for their financial support in the form of a doctoral scholarship awarded to Leila Djabelkhir-Jemmi. Thanks to KODRO for supporting this thesis through the use of Kodro software, and to all participants for their contribution to this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- FariasSTMungasDReedBRThe measurement of everyday cognition (ECog): scale development and psychometric propertiesNeuropsychology200822453154418590364
- PetersenRCSmithGEWaringSCIvnikRJTangalosEGKokmenEMild cognitive impairment: clinical characterization and outcomeArch Neurol199956330330810190820
- ClareLWoodsRTCognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a reviewNeuropsychol Rehabil2004144385401
- LiHLiJLiNLiBWangPZhouTCognitive intervention for persons with mild cognitive impairment: a meta-analysisAgeing Res Rev201110228529621130185
- ReijndersJvan HeugtenCvan BoxtelMCognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic reviewAgeing Res Rev201312126327522841936
- GagnonLGBellevilleSTraining of attentional control in mild cognitive impairment with executive deficits: results from a double-blind randomised controlled studyNeuropsychol Rehabil201222680983522712452
- HerreraCChambonCMichelBFPabanVAlescio-LautierBPositive effects of computer-based cognitive training in adults with mild cognitive impairmentNeuropsychologia20125081871188122525705
- OskoeiASNejatiVAjilchiBThe effectiveness of cognitive rehabilitation on improving the selective attention in patients with mild cognitive impairmentJ Behav Brain Sci201336474478
- VermeijAClaassenJADautzenbergPLKesselsRPTransfer and maintenance effects of online working-memory training in normal ageing and mild cognitive impairmentNeuropsychol Rehabil2016265–678380926010573
- VaportzisEMartinMGowAJA tablet for healthy ageing: the effect of a tablet computer training intervention on cognitive abilities in older adultsAm J Geriatr Psychiatry201725884185128082016
- RodakowskiJSaghafiEButtersMASkidmoreERNon-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: an updated scoping reviewMol Aspects Med2015443853
- SimonSSYokomizoJEBottinoCMCognitive intervention in amnestic mild cognitive impairment: a systematic reviewNeurosci Biobehav Rev20123641163117822322184
- BoylePAYuLFleischmanDAWhite matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old ageAnn Clin Transl Neurol201631079180027752514
- BrickmanAMProvenzanoFAMuraskinJRegional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the communityArch Neurol201269121621162722945686
- RossiRGeroldiCBrescianiLClinical and neuropsychological features associated with structural imaging patterns in patients with mild cognitive impairmentDement Geriatr Cogn Disord200723317518317220628
- LeeJMMarkusHSDoes the white matter matter in Alzheimer disease and cerebral amyloid angiopathy?Neurology20066616716401835
- LuchsingerJABrickmanAMReitzCSubclinical cerebrovascular disease in mild cognitive impairmentNeurology200973645045619667320
- ProvenzanoFAMuraskinJTostoGAlzheimer’s disease neuroimaging initiativeWhite matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease?JAMA Neurol201370445546123420027
- PughKGLipsitzLAThe microvascular frontal-subcortical syndrome of agingNeurobiol Aging200223342143111959405
- TullbergMFletcherEDeCarliCWhite matter lesions impair frontal lobe function regardless of their locationNeurology200463224625315277616
- GrambaiteRSelnesPReinvangIExecutive dysfunction in mild cognitive impairment is associated with changes in frontal and cingulate white matter tractsJ Alzheimers Dis201127245346221841261
- HabesMErusGToledoJBWhite matter hyperintensities and imaging patterns of brain ageing in the general populationBrain201613941164117926912649
- Gunning-DixonFMRazNAmerican Psychological AssociationThe cognitive correlates of white matter abnormalities in normal aging: a quantitative reviewNeuropsychology200014222423210791862
- LamCLMYiendJLeeTMCImaging and neuropsychological correlates of white matter lesions in different subtypes of mild cognitive impairment: a systematic reviewNeuro Rehabilitation201741118920428527230
- YoshitaMFletcherEHarveyDExtent and distribution of white matter hyperintensities in normal aging, MCI, and ADNeurology200667122192219817190943
- PetersenRCMild cognitive impairment as a diagnostic entityJ Intern Med2004256318319415324362
- Van der LindenMCoyetteFPoitrenaudJet les membres du GRE-MEML’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16)Van der LindenMAdamSAgnielAet les membres du GREMEML’Evaluation Des Troubles De La Mémoire Présentation De Quatre Tests De Mémoire Episodique (avec leur étalonnage)MarseilleSolal20042547
- DuboisBSlachevskyALitvanIPillonBThe FAB: a Frontal Assessment Battery at bedsideNeurology200055111621162611113214
- WechslerDWechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV)San Antonio, TX, USAPearson2008
- LezakMDHowiesonDBLoringDWNeuropsychological AssessmentNew York, NY, USAOxford University Press2004
- ReitanRMTrail Making Test: Manual for administration and scoringTucson, AZ, USAReitan Neuropsychology Laboratory1992
- DelocheGHannequinDDO 80: Test De Dénomination Orale d’Images [DO 80: Oral Denomination Test Images]ParisLes Editions du Centre de Psychologie Appliquée (ECPA)1997 French
- Mahieux-LaurentFFabreCGalbrunEDubrulleAMoroniCValidation d’une batterie brève d’évaluation des praxies gestuelles pour consultation Mémoire. Évaluation chez 419 témoins, 127 patients atteints de troubles cognitifs légers et 320 patients atteints d’une démence. [Validation of a brief screening scale evaluating praxic abilities for use in memory clinics. Evaluation in 419 controls, 127 mild cognitive impairment and 320 demented patients]Rev Neurol20091656560567 French19150097
- FazekasFChawlukJBAlaviAHurtigHIZimmermanRAMR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal agingAm J Roentgenol198714923513563496763
- De RotrouJMéthodologie pour une stimulation psychologique des fonctions cognitives. [Methodology for a psychological stimulation of cognitive functions]Paper presented at: Démences du sujet âgé et environnement: actes du 2e colloque, les 1985 [Dementia of the elderly subject and environment: Proceedings of the 2nd Symposium, 1985]Paris, France French
- De RotrouJCognitive stimulation and educationGérontol Soc20012497175192
- BreuilVDe RotrouJForetteFCognitive stimulation of patients with dementia: preliminary resultsInt J Geriatr Psychiatry199493211217
- MackinnonAMulliganRThe estimation of premorbid intelligence levels in French speakersEncephale2005311 Pt 13143 French15971638
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state”. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res19751231891981202204
- ReyAL’examen clinique en psychologie [Psychological Clinical Examination]ParisPresses Universitaires de France1964 French
- De RotrouJForetteFHervyMPThe cognitive efficiency profile: description and validation in patients with Alzheimer’s diseaseInt J Geriatr Psychiatry199167501509
- ReyAL’examen psychologique dans les cas d’encéphalopathie traumatique. [Psychological examination in cases of traumatic encephalopathy]Arch Psychol194128286340 French
- OsterriethPAThe test de copie d’une figure complexe: contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory]Arch Psychol194430286356 French
- PoitrenaudJIsraelLBarrereHLe Roc’hKVersion française de l’échelle de difficulties cognitive de McNair et Khan [French version of McNair and Khan’s Cognitive Difficulty Scale]MichelBFDerouesneCGely-NargeotMCDe la Plainte Mnésique à la Maladie d’Alzheimer [From the Mnemic Complaint to Alzheimer’s disease]MarseilleSolal1997159177 French
- YesavageJABrinkTLRoseTLDevelopment and validation of a geriatric depression screening scale: a preliminary reportJ Psychiatr Res198217137497183759
- GoldbergDBridgesKDuncan-JonesPGraysonDDetecting anxiety and depression in general medical settingsBMJ198829766538978993140969
- PetitSBerguaVPeresKBouissonJKoleckMÉlaboration et validation d’une échelle de qualité de vie adaptée aux personnes âgées (EQVPA). [Development and validation of a quality of life scale adapted to the elderly]Gériatr Psychol Neuropsychiatr Vieil2014124379386 French25515902
- RosenbergMBlackSRSelf-EsteemWThe Urban School ChildWashington, DC, USAAmerican Sociological Association1971
- CohenJStatistical Power Analysis for the Behavioral Sciences2nd edHillsdale, NJ, USALawrence Erlbaum Associates1988
- BasileAMPantoniLPracucciGLADIS Study GroupAge, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) StudyCerebrovasc Dis2006215–631532216490940
- ArvanitakisZFleischmanDAArfanakisKLeurgansSEBarnesLLBennettDAAssociation of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairmentBrain Struct Funct201622142135214625833685
- van den HeuvelDMten DamVHde CraenAJIncrease in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly populationJ Neurol Neurosurg Psychiatry200677214915316421114
- AuRMassaroJMWolfPAAssociation of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart StudyArch Neurol200663224625016476813
- HeddenTMorminoECAmariglioRECognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adultsJ Neurosci20123246162331624223152607
- MoonSYde Souto BarretoPChupinMAssociations between white matter hyperintensities and cognitive decline over three years in non-dementia older adults with memory complaintsJ Neurol Sci201737926627028716257
- WenischECantegreil-KallenIDe RotrouJCognitive stimulation intervention for elders with mild cognitive impairment compared to normal aged subjects: preliminary resultsAging Clin Exp Res200719431632217726363
- LovdenMBodammerNCKuhnSExperience-dependent plasticity of white-matter microstructure extends into old ageNeuropsychologia201048133878388320816877
- EngvigAFjellAMWestlyeLTMemory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging studyHum Brain Mapp201233102390240621823209
- PantoniLPoggesiADiciottiSEffect of attention training in mild cognitive impairment patients with subcortical vascular changes: the RehAtt StudyJ Alzheimers Dis201760261562428869475
- MakinoTUmegakiHSuzukiYRelationship between small cerebral white matter lesions and cognitive function in patients with Alzheimer’s disease and amnestic mild cognitive impairmentGeriatr Gerontol Int201414481982624215176
- Delano-WoodLAbelesNSaccoJMWierengaCEHorneNRBozokiARegional white matter pathology in mild cognitive impairmentStroke200839379479918258826
- De GrootJCDe LeeuwFEOudkerkMPeriventricular cerebral white matter lesions predict rate of cognitive declineAnn Neurol200252333534112205646
- SmithEESalatDHJengJCorrelations between MRI white matter lesion location and executive function and episodic memoryNeurology201176171492149921518999
