Abstract
Alzheimer’s disease (AD) is mainly a late-onset neurodegenerative disorder. Substantial efforts have been made to solve the complex genetic architecture of AD as a means to identify therapeutic targets. Unfortunately, to date, no disease-altering therapeutics have been developed. As therapeutics are likely to be most effective in the early stages of disease (ie, before the onset of symptoms), a recent focus of AD research has been the identification of protective factors that prevent disease. One example is the discovery of a rare variant in the 3′-UTR of RAB10 that is protective for AD. Here, we review the possible genetic, molecular, and functional role of RAB10 in AD and potential therapeutic approaches to target RAB10.
Alzheimer’s disease: overview
Alzheimer’s disease (AD) is mainly a late-onset neurodegenerative disease defined clinically by progressive memory decline.Citation1 AD currently affects nearly 50 million people worldwide, and this number is expected to rapidly increase as the population ages (http://www.alz.co.uk/). Neuropathologically, AD is characterized by neuronal loss and the accumulation of amyloid plaques and neurofibrillary tangles in the brain, much of which occurs many years before the onset of clinical symptoms.Citation2 While clinical diagnosis of AD is challenging because it is dependent primarily on observations of family members and caregivers, changes in protein analytes in plasma and cerebrospinal fluid (CSF) and brain imaging, although not widely used in clinical practice, may facilitate AD diagnosis.Citation2 AD can broadly be defined either as early-onset, occurring prior to age 65, or the much more common late-onset, occurring after age 65 (>96.5% of total cases).
Genetic architecture of AD
The genetics of early-onset AD (EOAD) are relatively well understood. Autosomal dominant mutations occurring in APP, PSEN1, or PSEN2 give rise to a subset of EOAD cases. More than 200 pathogenic mutations have been reported worldwide (http://www.molgen.ua.ac.be/admutations/).Citation3 Clinically, EOAD mutation carriers exhibit disease onset prior to 65 years of age.Citation4 Neuropathologically, EOAD and late-onset AD (LOAD) are indistinguishable.Citation5 Unfortunately, the genetics of LOAD is more complex.
Among individuals with LOAD, there is strong evidence for genetic heritability of disease.Citation6–Citation10 The single largest genetic risk factor for LOAD is APOE.Citation11 APOE plays critical roles in cholesterol transport, neuroplasticity, and inflammation.Citation12 APOE regulates Aβ metabolism directly through binding and clearance of AβCitation13 and indirectly via the LRP1 receptor.Citation14 Moreover, APOE influences tau-mediated neurodegeneration.Citation15 Two different APOE alleles, ε4 and ε2, affect risk for AD. Risk for developing AD is 3-fold higher in individuals carrying one copy of the ε4 allele and 12-fold higher in individuals carrying 2 copies of APOE ε4.Citation16 Conversely, APOE ε2 confers resilience to AD, while the ε3 allele is considered neutral risk.Citation16
Genetic variants that influence risk for AD can be broadly classified into two groups: common and rare. The majority of common variants (minor allele frequency [MAF] >5%) were identified using genome-wide association studies (GWAS).Citation17 With the exception of variants in APOE, all other common variants have small effect sizes on AD risk (ORs =1.08–1.22, 0.73–0.94).Citation17 While GWAS variants provide insights into disease processes, none of the common risk variants for AD that have been identified by GWAS have clear functional effects and efforts to identify functional variants in the regions of GWAS variants have largely been unsuccessful.
The advent of next-generation sequencing has enabled the sequencing of whole exomes and genomes, resulting in progressively larger AD datasets and providing insights into the contribution of low-frequency (MAF 1%–5%) and rare variants (MAF <1%) to the genetic architecture of AD. Using a variety of study designs, multiple rare variants have been identified that affect risk for AD.Citation18–Citation22 Rare variants typically have much larger effect sizes on disease risk than common variants identified by GWAS. Rare variants are also more likely to occur in coding or regulatory regions and, thus, are more likely to be functional. Thus, rare variants represent effective therapeutic targets.
Finally, alternative study designs can provide additional insights into the genetics of AD. For example, the use of quantitative endophenotypes (eg, CSF levels of protein analytes such as Aβ and tau;Citation23–Citation25 mitochondrial copy number;Citation26 metabolic efficiency;Citation27,Citation28 measurements of progressive brain atrophy;Citation29 among others) and the analysis of mitochondrial genomic variationCitation30 have expanded our understanding of the genetic architecture underlying disease onset and disease course.
Using the genetics of AD to identify therapeutic targets
Genes and pathways that regulate AD pathogenesis may represent viable targets for treating disease. For example, therapies targeting the removal of Aβ or inhibition of Aβ production are currently in clinical trials. These strategies are based on our understanding of the mechanisms by which rare mutations in APP, PSEN1, and PSEN2 drive AD pathogenesis in EOAD and focus on inhibition of the deposition of Aβ in the brain. Similarly, targeting APOE function has been investigated for its therapeutic capacity.Citation31–Citation33 AD risk genes implicated in immune function, endocytosis, and lipid biology represent novel avenues of therapeutic development. Unfortunately, to date, more than 90% of all clinical trials have failed. Given the expected increase in disease incidence, developing effective therapeutics is imperative. For the remainder of this review, we discuss the potential role of RAB10 as a therapeutic target.
Protective factors in AD: RAB10
Common and rare variants have been identified that reduce the risk for AD.Citation3 Those with the strongest protective effect include APOE ε2 and APP-A673T.Citation34,Citation35 Thus, genes and pathways involved in increasing AD risk may also confer resilience to disease. To identify additional genetic variants that confer resilience to AD, there is a need to develop novel study designs that focus on those individuals carrying protective factors for AD. In one such case, family pedigrees were identified with a statistical excess of AD mortality (ie, families with a higher number of AD deaths than expected relative to similarly sized pedigrees; termed high-risk pedigrees). Within these high-risk pedigrees, elderly individuals with genetic risk for AD (ie, APOE ε4 carriers) but who had escaped AD were evaluated using whole-genome sequencing. Ridge et al identified a rare variant (rs142787485) in the 3′-UTR of RAB10 that confers resilience to AD.Citation10 In a cell model of APP metabolism, silencing of Rab10 expression leads to a significant decrease in Aβ42 and the Aβ42/40 ratio, consistent with prior reports.Citation10,Citation36 Interestingly, independent of the RAB10 SNP, individuals with a neuropathologic diagnosis of AD exhibited significantly higher levels of RAB10 compared to neuropathology-free controls.Citation10 Thus, genetic and molecular evidences support a role for RAB10 in AD pathogenesis.
RAB10 in AD pathology
RAB10 is expressed in all cell types in the brain.Citation37 RAB10 is activated by phosphorylation at Thr73, which is mediated by LRRK2, a protein kinase associated with Parkinson’s disease.Citation38,Citation39 This phosphorylation event may represent a pathologic feature in the brains of AD patients.Citation38 pRAB10-Thr73 was observed to colocalize with neurofibrillary tangles in AD brains as well as with granulovascular degeneration, neuropil threads, and dystrophic neurons.Citation38 Double immunofluorescence staining of these structures further revealed positive staining for phosphorylated tau. However, this colocalization of pRAB10-Thr73 and phosphorylated tau was not complete, with some instances showing neuropil threads or dystrophic neurons containing only phosphorylated tau or only pRAB10-Thr73. Interestingly, dystrophic neurons near amyloid plaques stained positive for pRAB10-Thr73, while the amyloid plaques themselves were negative for pRAB10.Citation38 Total RAB10 staining, however, did not differ between AD and control brains. Thus, while pRAB10-Thr73 is associated with AD pathology, it remains unclear whether this represents excess activation or aberrant function of RAB10.
RAB10: function in development
RAB10 is a member of the RAB family of small GTPases, which are key regulators of vesicular trafficking. RAB proteins are cyclically controlled, requiring a GDP–GTP exchange that is facilitated by RAB guanine nucleotide exchange factors.Citation40 However, unlike most of the other RAB proteins, which have relatively specific roles within intracellular transport, RAB10 is relatively unique because it carries out a wide variety of functions and has multiple subcellular localizations.Citation41
Rab10 plays essential functional roles in development: mice are embryonic lethal when both alleles are deleted.Citation42 In addition, RAB10 plays a role in maintenance and regulation of endoplasmic reticulum (ER) morphology.Citation43–Citation45 When RAB10 is mutated or constitutively inactive (eg, GDP-locked), the ER contains more cisternae, suggesting that RAB10 plays a critical role in maintaining and/or generating tubule extension and fusion in the ER.Citation43 RAB10 function has also been linked to neuronal morphology and polarization, playing a critical role in axonal development and dendrite arborization.Citation46,Citation47 During neuronal development, RAB10 associates with plasmalemmal precursor vesicles, which are linked to kinesin 1 via c-Jun N-terminal protein kinase-interacting protein 1.Citation48 Together this complex mediates anterograde transport of RAB10-positive vesicles to axonal tips, promoting axonal growth.Citation48 Thus, RAB10 function is critical for proper neuronal function.
A role for RAB10 and APP in the retromer pathway
RAB10 has been linked to many roles involved in intracellular transport (), including but not limited to a role in endocytic recycling. In Caenorhabditis elegans, RAB10 is required for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor recycling in postsynaptic membranes.Citation49 RAB10 is likely functional upstream of RME-1/EHD, a protein involved in recycling endosome tubulization and endosome function.Citation50–Citation52 RAB10 is expressed at the interface of early endosomes and recycling endosomes. These structures are morphologically defective in rab-10 mutant C. elegans models.Citation50,Citation51 RAB10 is required for endosomal recruitment of CNT-1.Citation53 This likely contributes to biogenesis and/or maintenance of recycling endosome compartments. RAB10 could also play a role in promoting the maturation of early endosomes to recycling endosome. RAB10 is also involved in protein degradation. RAB10 and RAB3A are essential for lysosomal exocytosis and plasma membrane repair.Citation54,Citation55 In addition, RAB10 associates with lipid droplets.Citation56 Complexes of RAB10–EHBP1–EHD2 promote autophagic engulfment and degradation of lipid droplets.Citation57 Thus, these functional roles for RAB10 in recycling and degradation make it an interesting candidate protein in AD pathogenesis.
Figure 1 Diagram of APP trafficking and the role of RAB10 in these intracellular transport pathways.
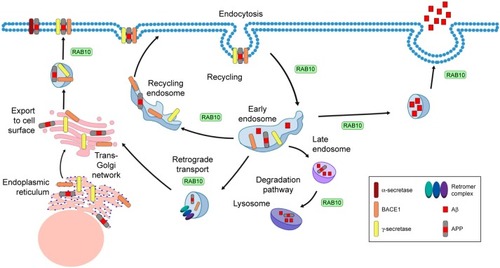
RAB10 is involved in transport of proteins from the early endosome to the trans-Golgi network (TGN), where it may also function in the retromer pathway.Citation58 The retromer complex consists of Vps26, Vps35, Vps29, SNX1, SNX2, SNX5, and SNX6.Citation59 The retromer complex regulates transport of protein cargo from endosomes to the TGN by the retrograde pathway or to the cell surface through the recycling pathway.Citation60
To form the amyloid plaques that define a primary component of AD pathology, the transmembrane APP must first be processed into Aβ peptides. APP is thought to be retrieved from the cell surface and trafficked to the early endosomes where it is initially cleaved by β-site APP-cleaving enzyme 1 (BACE1) to generate a C-terminal fragment (β-CTF).Citation61,Citation62 The β-CTF fragments are then trafficked via retromer-dependent transport to the TGN, which facilitates further cleavage by γ-secretase to produce Aβ40.Citation63 The retromer complex and its receptors can transport APP and BACE1 from endosomes to the TGN, ultimately regulating production of Aβ.Citation60 The retromer complex sorts APP and BACE, either through direct or indirect binding to the transmembrane adaptor protein SORLA.Citation64 Interestingly, common variants in the gene that encodes SORLA have been associated with AD risk.Citation65 Thus, genes that are involved in retromer function may influence AD pathogenesis and represent promising therapeutic targets.
Retromer complex dysfunction promotes APP accumulation in neurons and Aβ production. In addition, the retromer also appears to have a differential effect on Aβ isoforms, with evidence that retromer dysfunction alters Aβ40 secretion, which results in increasing the Aβ42/40 ratio.Citation66 In mouse models, reduction of a single component within the retromer complex, Vps26, is sufficient to increase soluble Aβ and APP.Citation67 Stabilization of the retromer in human-induced pluripotent stem cell-derived neurons through the use of the pharmacologic chaperone R33 has been shown to significantly reduce the phosphorylation of Tau in a manner that correlated with, but ultimately independent of, Aβ production.Citation68 An RNAi screen of RABs that modify APP processing revealed that the silencing of RAB10 reduces Aβ without altering sAPPβ levels.Citation36 The LRRK2-RAB10 signaling pathway also causes an overproduction of Aβ.Citation38
Therapeutic implications for RAB10
Novel genes and pathways that contribute to the pathogenesis of AD provide insights into strategies to prevent or treat the disease. The amyloid hypothesis, which proposes that the accumulation of Aβ triggers the cascade of events that lead to AD, has been the focus of AD therapeutic development for decades. The development of anti-Aβ therapeutics remains the primary approach to treating AD based on our current understanding of the earliest features of this disease.Citation69 In this review, we emphasize the importance of RAB10 in the processing of APP and the production of Aβ. The function of RAB10 in intracellular trafficking and evidence that a reduction of Rab10 results in a reduction of the Aβ42/40 ratio makes inhibition of RAB10 a potential therapeutic avenue.
There are several existing strategies for therapeutic inhibition of a gene product. Potential technologies for targeting of RAB10 are small molecules, antibodies, and antisense oligodeoxynucleotide technologies. For each of these approaches the challenge of delivery across the blood–brain barrier (BBB) is a significant one.Citation70 A molecule with high lipid solubility and molecular mass <400 Da generally can diffuse across the BBB. These criteria limit the classes of small molecules that can be considered as potential therapeutic molecules for AD. Antibodies do not cross the BBB at therapeutic concentrations. Several approaches have been developed to deliver antibodies to the brain. Bispecific antibodies have been developed that leverage the transferrin receptor to successfully increase BBB penetration.Citation71,Citation72 In addition, antibody analogs that can cross the BBB are in development (Patent #EP3309171). Antisense oligonucleotides do not readily cross the BBB.Citation73 However, at present, intrathecal bolus injection is an effective method for ensuring distribution in neurons and glial cells in the brain.Citation74
Approaches to the development and delivery of small-molecule therapeutics are mature and the path and pitfalls are well established. Small molecules are effective enzyme inhibitors, receptor ligands, or allosteric modulators. The nature of small-molecule activity can make it difficult to find the balance between efficacy and side effects when treating humans.Citation70 Despite these challenges, small-molecule drug development has seen many successes and >90% of existing therapeutics use small molecules. Small GTPases similar to RAB10 have been successfully targeted using small molecules in the past. One important use is the development of molecules to block oncogenic properties of the RAS protein to treat cancer.Citation75 Recent discoveries about the structure of small GTPases and advances in targeting strategies have further increased efforts to use these important proteins as therapeutic targets.Citation76 Targeting approaches, including interference with nucleotide binding, inactivation by irreversible covalent modification, inhibition of GTPase–GEF interactions, inhibition of GTPase–effector interactions, and stabilization of GTPase–protein complexes have been successful and are reviewed by Cromm et al.Citation76 While challenges regarding specificity and crossing the BBB remain, these advances create some optimism about the use of small molecules to inhibit RAB10 and impact AD pathology.
Antibodies function in extra- and intracellular immunity to recognize foreign or abnormal agents and target them for destruction with very high specificity. The production of monoclonal antibodies (mAbs) has resulted in their use for the treatment of a wide range of human diseases.Citation71 There are currently >60 approved mAbs for human therapy and >50 in late-stage clinical trials.Citation77
RNase H-dependent antisense oligonucleotides technology is a popular method for knockdown in cell culture. It offers specific and efficient knockdown and is a powerful tool for functional studies of genes with unknown function.Citation78 Antisense technology has challenges such as site specificity, toxicity at high concentrations, and the difficulty of targeting specific cell types. However, it is a mature technology and antisense oligonucleotides offer a means to manipulate specific steps in mRNA processing, for example, splicing.Citation69 As discussed previously, in vitro reduction of Rab10/RAB10 expression using shRNA and RNAi has been successful.Citation36 Expansion of antisense oligodeoxynucleotide from in vitro to in vivo represents a promising therapeutic avenue given recent successes with spinal muscular atrophy and Huntington’s disease.Citation79,Citation80
Conclusion
Here we have outlined the potential role of RAB10 in AD pathology and the case for RAB10 inhibition as a therapeutic intervention for AD. While small GTPases like RAB10 present significant challenges for therapeutic development, recent work in small molecules, antibodies, and antisense oligonucleotides suggests that success can be achieved.
Acknowledgments
This work was supported by grants from the National Institutes of Health (K01 AG046374, U01 AG052411, R01 AG062359, and RF1 AG054052).
Disclosure
The authors report no conflicts of interest in this work.
References
- HoltzmanDMMorrisJCGoateAMAlzheimer’s disease: the challenge of the second centurySci Transl Med20113777777sr1
- JackCRHoltzmanDMBiomarker modeling of Alzheimer’s diseaseNeuron20138061347135824360540
- KarchCMGoateAMAlzheimer’s disease risk genes and mechanisms of disease pathogenesisBiol Psychiatry2015771435124951455
- RymanDCAcosta-BaenaNAisenPSSymptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysisNeurology201483325326024928124
- CairnsNJPerrinRJFranklinEENeuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN)Neuropathology201535439040025964057
- RidgePGHoytKBBoehmeKAssessment of the genetic variance of late-onset Alzheimer’s diseaseNeurobiol Aging201641200.e13200.e20
- RidgePGMukherjeeSCranePKKauweJSAlzheimer’s disease genetics C. Alzheimer’s disease: analyzing the missing heritabilityPLoS One2013811e7977124244562
- KauweJSRidgePGFosterNLCannon-AlbrightLAStrong evidence for a genetic contribution to late-onset Alzheimer’s disease mortality: a population-based studyPLoS One2013810e7708724116205
- Cannon-AlbrightLADintelmanSManessTPopulation genealogy resource shows evidence of familial clustering for Alzheimer diseaseNeurol Genet201844e24930109265
- RidgePGKarchCMHsuSLinkage, whole genome sequence, and biological data implicate variants in RAB10 in Alzheimer’s disease resilienceGenome Med20179110029183403
- StrittmatterWJWeisgraberKHHuangDYBinding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer diseaseProc Natl Acad Sci U S A19939017809881028367470
- HoltzmanDMHerzJBuGApolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer diseaseCold Spring Harb Perspect Med201223a00631222393530
- CastellanoJMKimJStewartFRHuman apoE isoforms differentially regulate brain amyloid-peptide clearanceSci Transl Med201138989ra57
- VerghesePBCastellanoJMGaraiKApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditionsProc Natl Acad Sci U S A201311019E1807E181623620513
- ShiYYamadaKLiddelowSAApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathyNature2017549767352352728959956
- CorderEHSaundersAMStrittmatterWJGene dose of apo-lipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset familiesScience199326151239219238346443
- LambertJCIbrahim-VerbaasCAHaroldDMeta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s diseaseNat Genet201345121452145824162737
- CruchagaCKarchCMJinSCRare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s diseaseNature2014505748455055424336208
- Wetzel-SmithMKHunkapillerJBhangaleTRA rare mutation in UNC5C predisposes to late-onset Alzheimer’s disease and increases neuronal cell deathNat Med201420121452145725419706
- GuerreiroRWojtasABrasJTREM2 variants in Alzheimer’s diseaseN Engl J Med2013368211712723150934
- JinSCBenitezBAKarchCMCoding variants in TREM2 increase risk for Alzheimer’s diseaseHum Mol Genet201423215838584624899047
- JinSCCarrasquilloMMBenitezBATREM2 is associated with increased risk for Alzheimer’s disease in African AmericansMol Neurodegener20151011925886450
- MaxwellTJCorcoranCdel-AguilaJLGenome-wide association study for variants that modulate relationships between cerebrospinal fluid amyloid-beta 42, tau, and p-tau levelsAlzheimer’s Res Ther20181018630153862
- KauweJSBaileyMHRidgePGGenome-wide association study of CSF levels of 59 Alzheimer’s disease candidate proteins: significant associations with proteins involved in amyloid processing and inflammationPLoS Genet20141010e100475825340798
- DemingYLiZKapoorMGenome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiersActa Neuropathol2017133583985628247064
- RidgePGMaxwellTJFoutzSJMitochondrial genomic variation associated with higher mitochondrial copy number: the Cache County Study on Memory Health and AgingBMC Bioinformatics201415Suppl 7S6
- KringSIBrummettBHBarefootJImpact of psychological stress on the associations between apolipoprotein E variants and metabolic traits: findings in an American sample of caregivers and controlsPsychosom Med201072542743320467002
- OstergaardSDMukherjeeSSharpSJAssociations between potentially modifiable risk factors and alzheimer disease: a mendelian randomization studyPLoS Med2015126e1001841 discussion e100184126079503
- LorenziMAltmannAGutmanBSusceptibility of brain atrophy to TRIB3 in Alzheimer’s disease, evidence from functional prioritization in imaging geneticsProc Natl Acad Sci U S A2018115123162316729511103
- RidgePGKauweJSKMitochondriaKJSKMitochondria and Alzheimer’s disease: the role of mitochondrial genetic variationCurr Genet Med Rep20186111029564191
- KimJBasakJMHoltzmanDMThe role of apolipoprotein E in Alzheimer’s diseaseNeuron200963328730319679070
- KimJEltoraiAEJiangHAnti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Aβ amyloidosisJ Exp Med2012209122149215623129750
- HuynhTVDavisAAUlrichJDHoltzmanDMApolipoprotein E and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-β and other amyloidogenic proteinsJ Lipid Res201758582483628246336
- CorderEHSaundersAMRischNJProtective effect of apoli-poprotein E type 2 allele for late onset Alzheimer diseaseNat Genet1994721801847920638
- JonssonTAtwalJKSteinbergSA mutation in APP protects against Alzheimer’s disease and age-related cognitive declineNature20124887409969922801501
- UdayarVBuggia-PrévotVGuerreiroRLA paired RNAi and RabGAP overexpression screen identifies Rab11 as a regulator of β-amyloid productionCell Rep2013561536155124373285
- ZhangYSloanSAClarkeLEPurification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouseNeuron2016891375326687838
- YanTWangLGaoJRab10 phosphorylation is a prominent pathological feature in Alzheimer’s diseaseJ Alzheimers Dis201863115716529562525
- EguchiTKuwaharaTSakuraiMLRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasisProc Natl Acad Sci U S A201811539E9115E912430209220
- BarrFLambrightDGGefsRRab GEFs and GAPsCurr Opin Cell Biol201022446147020466531
- ChuaCELTangBLRab 10-a traffic controller in multiple cellular pathways and locationsJ Cell Physiol201823396483649429377137
- LvPShengYZhaoZTargeted disruption of Rab10 causes early embryonic lethalityProtein Cell20156646346725860786
- EnglishARVoeltzGKRab10 GTPase regulates ER dynamics and morphologyNat Cell Biol201315216917823263280
- ChangJBlackstoneCRab10 joins the ER social networkNat Cell Biol201315213513623377026
- SchuldtAMembrane dynamics: ER trailblazing by RAB10Nat Rev Mol Cell Biol20131426323271010
- LiuYXhXChenQMyosin Vb controls biogenesis of post-Golgi Rab10 carriers during axon developmentNat Commun200520134
- ZouWYadavSDevaultLJanYNSherwoodDRRAB-10-dependent membrane transport is required for dendrite arborizationPLoS Genet2015119e100548426394140
- DengCYLeiWLXuXHJuXCLiuYLuoZGJIP1 mediates anterograde transport of Rab10 cargos during neuronal polarizationJ Neurosci20143451710172324478353
- GlodowskiDRChenCCHSchaeferHGrantBDRongoCRAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathwayMol Biol Cell200718114387439617761527
- ChenCCHSchweinsbergPJVashistSMareinissDPLambieEJGrantBDRAB-10 is required for endocytic recycling in the Cae-norhabditis elegans intestineMol Biol Cell20061731286129716394106
- ShiAChenCCBanerjeeREHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegansMol Biol Cell201021162930294320573983
- PantSSharmaMPatelKCaplanSCarrCMGrantBDAMPH-1/Amphiphysin/Bin1 functions with RME-1/Ehd1 in endocytic recyclingNat Cell Biol200911121399141019915558
- ShiALiuOKoenigSRAB-10-GTPase-mediated regulation of endosomal phosphatidylinositol-4,5-bisphosphateProc Natl Acad Sci U S A201210935E2306E231522869721
- EncarnaçãoMEspadaLEscreventeCA Rab3a-dependent complex essential for lysosome positioning and plasma membrane repairJ Cell Biol2016213663164027325790
- ReddyACalerEVAndrewsNWPlasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomesCell2001106215716911511344
- SatoSFukasawaMYamakawaYProteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core proteinJ Biochem2006139592193016751600
- LiZSchulzeRJWellerSGA novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid dropletsSci Adv2016212e160147028028537
- BabbeyCMAhktarNWangEChenCCHGrantBDDunnKWRab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cellsMol Biol Cell20061773156317516641372
- BonifacinoJSHurleyJHRetromerCurr Opin Cell Biol200820442743618472259
- ZhangQYTanMSYuJTTanLThe role of retromer in Alzheimer’s diseaseMol Neurobiol20165364201420926215837
- HuseJTPijakDSLeslieGJLeeVMDomsRWMaturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretaseJ Biol Chem200027543337293373710924510
- KinoshitaAFukumotoHShahTWhelanCMIrizarryMCHymanBTDemonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomesJ Cell Sci2003116163339334612829747
- ChoyRWYChengZSchekmanRAmyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid β (Aβ) production in the trans-Golgi networkProc Natl Acad Sci U S A201210930E2077E208222711829
- GandySOdceSEdceSSuzukiTEhrlichMSmallSAmyloid precursor protein sorting and processing: transmitters, hormones, and protein phosphorylation mechanismsIntracellular Traffic and Neu-rodegenerative DisordersGeorge-HyslopPeter H StMobleyWilliam CSpringerBerlin Heidelberg200819
- LambertJCIbrahim-VerbaasCAHaroldDMeta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s diseaseNat Genet201345121452145824162737
- SullivanCPJayAGStackECRetromer disruption promotes amyloidogenic APP processingNeurobiol Dis201143233834521515373
- MuhammadAFloresIZhangHRetromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and a accumulationProc Natl Acad Sci U S A2008105207327733218480253
- YoungJEFongLKFrankowskiHPetskoGASmallSAGoldsteinLSBStabilizing the retromer complex in a human stem cell model of Alzheimer’s disease reduces TAU phosphorylation independently of amyloid precursor proteinStem Cell Reports20181031046105829503090
- HardyJSelkoeDJThe amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeuticsScience2002297558035335612130773
- PardridgeWMThe blood-brain barrier: bottleneck in brain drug developmentNeuroRx20052131415717053
- AtwalJKChenYChiuCA therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivoSci Transl Med201138484ra43
- ZhangYj YKenrickYMBoosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis targetSci Transl Med201138484ra44
- PhillipsJACraigSJBayleyDChristianRAGearyRNicklinPLPharmacokinetics, metabolism, and elimination of a 20-mer phosphoro-thioate oligodeoxynucleotide (CGP 69846A) after intravenous and subcutaneous administrationBiochem Pharmacol19975466576689310342
- RigoFChunSJNorrisDAPharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primatesJ Pharmacol Exp Ther20143501465524784568
- GibbsJBOliffAKohlNEFarnesyltransferase inhibitors: Ras research yields a potential cancer therapeuticCell19947721751788168127
- CrommPMSpiegelJGrossmannTNWaldmannHDirect modulation of small GTPase activity and functionAngew Chem Int Ed Engl20155446135161353726470842
- ReichertJMAntibodies to watch in 2017MAbs20179216718127960628
- DevosSLMillerTMAntisense oligonucleotides: treating neurodegeneration at the level of RNANeurotherapeutics201310348649723686823
- SouthwellALKordasiewiczHBLangbehnDHuntingtin suppression restores cognitive function in a mouse model of Huntington’s diseaseSci Transl Med201810461eaar395930282695
- PassiniMABuJRichardsAMAntisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophySci Transl Med201137272ra18
