Abstract
Background
The Beers Criteria were developed with the aim of improving the safety of medicines among older persons. While the association between the Beers’ list of potentially inappropriate medicines (PIMs) and adverse drug reactions (ADRs) among older Caucasians is contentious, the ability of the Criteria to predict ADRs among older persons in Africa remains unexplored.
Objectives
This study aimed to compare the prevalence of PIMs and ADRs among hospitalized older persons in Nigeria and South Africa, and to determine the association between the 2015 American Geriatrics Society-Beers (AGS-Beers) PIMs and ADRs.
Methods
The medical records of older persons aged ≥60 years who were hospitalized in teaching hospitals in Nigeria and South Africa were randomly selected, and retrospectively evaluated for ADRs by two clinical pharmacists using the Naranjo algorithm. The PIMs were assessed using the 2015 AGS-Beers Criteria. A multivariate logistic regression was used to determine the associated factors for ADRs among the hospitalized older persons, with P<0.05 being considered significant.
Results
The samples which comprised 268 and 339 hospitalized older persons (mean age 70.53±8.22; 95% CI −0.21 to 2.32 vs mean age 69.49±7.64; 95% CI −0.25 to 2.34, P=0.11) were evaluated in Nigeria and South Africa, respectively. The PIMs among the older persons in Nigeria were 32.1% (86/268) and 30.1% (102/339, OR=0.91, 95% CI 0.64–1.29, P=0.6) for South Africa; 13.8% (37/268) of the hospitalized older persons in Nigeria experienced 43 cases of ADRs compared to 9.1% (31/339) in South Africa (95% CI 0.38–1.04, P=0.07). The multivariate analysis showed no association between PIMs and ADRs among the hospitalized older persons in Nigeria (OR=1.48 95% CI 0.70–3.17, P=0.31) and South Africa (OR=1.09, 95% CI 0.48–2.49, P=0.83).
Conclusion
The 2015 AGS-Beers PIMs were not associated with ADRs among the hospitalized older persons in Nigeria and South Africa. However, physicians should be cautious when prescribing certain medications in the AGS-Beers list.
Introduction
Adverse drug reactions (ADRs) are a public health issue that contributes to increased morbidity and mortality among older persons worldwide.Citation1 According to the World Health Organization (WHO), an ADR is defined as “a noxious, unintended effect of a medication that occurs at doses normally used in man for prophylaxis diagnosis, therapy of diseases, or for the manipulation of physiological functions”.Citation2 Many explicit criteria including the Beers Criteria, European Union-Potential Inappropriate Medicines, and Screening Tool of Older People’s Prescriptions and Screening Tool to Alert to Right Treatments (STOPP/START Criteria) are currently being applied in clinical practice to guide prescriptions among older persons.Citation3–Citation5 These criteria provide lists of potentially inappropriate medicines (PIMs), which are believed to contribute to ADRs among older persons. Potential inappropriate medicines are those that are regarded as being high risk or less effective in older persons when used in the presence of safer and more effective alternatives.Citation3 The nexus between the PIMs and ADRs among older persons has however remained controversial in the medical literature.
A review of studies that evaluated the associations between the earlier versions of the Beers Criteria and ADRs concluded that there was evidence to support the association.Citation6 This is supported by another study that found a significant association between the 2012 sanctioned American Geriatrics Society-Beers (AGS-Beers) Criteria and adverse outcomes among older persons in Switzerland.Citation7 Other studies, however, found no association between the 2012 AGS-Beers PIMs and ADRs.Citation8–Citation10 Another study that compared the Beers and STOPP Criteria reported a moderate prognostic predictive ability of ADRs for both the 2003, 2012 AGS-Beers Criteria and the STOPP/START Criteria among older persons in the United States.Citation11 However, many clinicians and researchers in Europe appear to identify with the STOPP/START Criteria which have been reported to predict more ADRs among the continent older persons than the Beers Criteria.Citation12–Citation14 However, the literature suggests that in view of the release of the 2015 AGS-Beers Criteria, further studies will be needed to determine the risk of ADRs associated with the updated PIM list.Citation10
There is a dearth of studies that have explored the associations between the 2015 AGS-Beers Criteria and ADRs among older persons. However, a recent study in India that applied the 2015 AGS-Beers Criteria to older persons aged ≥60 years reported no association between the updated Beers PIMs and ADRs.Citation15 Another study in the United States associated falls among a Parkinson’s disease population with the PIMs that are recommended to be avoided in patients with a history of falls in the 2015 AGS-Beers Criteria.Citation16
Many ADR causality assessment tools are currently available to assist clinicians to evaluate ADRs, especially among older persons in whom identification of ADRs usually poses a challenge.Citation17 The Naranjo algorithm is among the most commonly applied tools in clinical practice,Citation18 being easier to use than many other tools, and adjudged to have a high specificity and sensitivity.Citation17,Citation18 A recent study that evaluated how well the Naranjo algorithm categorized ADRs among Japanese reported that the algorithm had a specificity of 0.95 and sensitivity of 0.59.Citation19 It is a questionnaire-based algorithm that determines the probability of ADR occurrence based on defined scores, and classifies them into “definite”, “probable”, “possible”, and “doubtful”.Citation20 In addition, the use of triggers, such as the Institute for Healthcare Improvement (IHI) global trigger tool has been found useful in retrospective evaluations of ADRs among inpatients in many health care settings as they provide clues that assist in identifying ADRs.Citation21
In Africa, including Nigeria and South Africa, the Beers Criteria have been the most applied explicit criteria for evaluating the quality of prescribing among older persons. A review of interventions to reduce ADRs among older persons in Africa showed that more than three-quarters of the studies applied the Beers Criteria.Citation22 However, to the best of the authors’ knowledge, no study has evaluated associations between the Beers Criteria and ADRs among older persons in Africa. There are conflicting reports on the associations between the earlier itinerary of the Beers Criteria and ADRs among older Caucasians, but only little information is available about the Criteria PIMs and ADRs among older Africans. Therefore, this study aimed to compare the prevalence of PIMs and ADRs among hospitalized older persons in Nigeria and South Africa, and to determine the associations between the 2015 AGS-Beers PIMs and ADRs.
Methods
Study design, sites, and setting
This was an epidemiological cross-sectional study that utilized a medical chart review among hospitalized older persons. Patients’ medical records were randomly selected, and the charts were retrospectively reviewed for ADRs. The study was carried out in two internal medicine wards, each in a university public teaching hospital in a cosmopolitan city, in both Nigeria and South Africa. The two hospitals provided tertiary health care services and were referral centers for many other health care facilities in the regions in which they are located. The hospitals had no standard protocol to detect or monitor ADRs, and no dedicated geriatric wards at the time of this study between 13th April and 25th November, 2017. However, there were specialists in geriatrics consulting in the medical wards of the two hospitals.
Study population/eligibility criteria
This study eligibility included older persons aged ≥60 years who were hospitalized in the internal medicine wards of the two hospitals between 1st January and 31st December, 2016. The age is in line with the United Nations definition of older persons for developing countries,Citation23 and is consistent with previous studies on ADRs in low and middle-income countries.Citation8,Citation10,Citation15,Citation24 Hospitalized older persons without complete demographic information and missing charts, or who were discharged, transferred to intensive care, readmitted, or who died within 24 hours of hospitalization were excluded.
Sample size estimation
A total of 846 eligible older persons’ medical records were available for selection in Nigeria, based on the inclusion criteria. The sample size was calculated using a formula previously described.Citation25 Using a 50% response distribution, a margin of error of 5% and a power of 95%, a minimum sample size of 265 was calculated. With an additional 10% included for attrition, a maximum sample size of 292 was calculated. The number of records required in South Africa was calculated using the same formula as in Nigeria, but with a population of 20,000 and the same response distribution, margin of error and power, resulting in a minimum sample size of 377, with 10% attrition being added to give a maximum sample size of 415.
Selection of study populations
The study populations in Nigeria and South Africa were identified from the hospital admission records office. The patients’ file numbers were listed, and eligible records selected using a simple randomization technique with the aid of computer-generated random numbers. No blinding was applied in selecting the patients’ medical chart records. The same sampling procedure used in Nigeria was repeated on the South African sample for internal consistency.
Data collection
A checklist that had been pretested was used to capture patients’ information: sociodemographics, sociomedical, and medication histories, medicine prescriptions during hospitalization, and dates of admission and discharge/death. It also captured patients’ specific baseline laboratory parameters obtained at hospitalization and during the hospital stay. The laboratory data/parameters of interest included estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) or serum creatinine (mg/dL), serum electrolytes (potassium [mmol/L] and sodium [mmol/L]), and platelet counts (cells/mm3), where available. The attending physicians’ notes on ADR occurrence in patients as documented in the patients’ records, and the clinical decisions that were taken to mitigate them, including a reduction in dosage, stoppage of the medication, and the use of other medications to counteract the effect of the offending medications were also extracted.
Study procedure
Evaluation of ADRs among the older persons
ADRs among the study population were assessed using a multifaceted approach including a comprehensive review of the physicians’ and nursing charts. Attention was paid to patients verbalized complaints documented in the charts. Changes in patients’ baseline-laboratory data and other clinical parameters during the inpatient care were evaluated. Radio imaging data, such as echocardiography, ultrasound, and electrocardiogram parameters were however not considered for review. Although the radio imaging parameters were part of the baseline data for the admitted patients, the procedures were rarely ordered by the physicians during the inpatients’ care, making it impracticable to evaluate ADRs due to certain medications. ADRs due to neoplastic agents or infusions were not evaluated in this study.
The medical charts were independently reviewed by two clinical pharmacists using the IHI global tool for measuring ADRs.Citation21 The triggers found during the review were documented irrespective of whether they detected the actual ADRs or not. The suspected ADRs were evaluated for causality using the Naranjo algorithms which comprises of ten weighted questions with “yes”, “no”, or “do not know” as responses.Citation20 Each of the responses is assigned different point values (−1 to +2), the total scores for an assessment ranging from −4 to −13. The total score for each suspected ADR encounter was calculated and the causal relationship determined. The suspected ADR was considered as definite when the total score is ≥9, probable when it is between 5 and 8, possible when it is from 1 to 4, and doubtful when it is ≤0. Where disagreements occurred between the assessors, the cases were referred to an internal physician and consensus reached. In cases where the attending physicians documented specific ADRs (not inferred from the treatment), only one of the assessors had to agree with the attending physicians.
In this study, the PIMs listed in the 2015 AGS-Beers Criteria were evaluated for both predictable and unpredictable ADRs, though the criteria were meant to mitigate predictable/preventable ADRs. However, associations were determined between the PIMs and predictable ADRs with only “possible” and “probable” categories of ADRs being considered for further analysis.
The independent variables assessed in this study included age group (<80 years and ≥80 years), gender, comorbidity index (<4.0 and ≥4.0), length of hospital stay (<12 days and ≥12 days), exposure to the 2015 AGS-Beers PIMs, presence of musculoskeletal and urogenital disorders, exposure to psychoanaleptics (N06CA01) and anti-inflammatory, antirheumatic NSAIDs (M01A). The patients’ ages, as documented by the physicians, were used while the length of hospital stay was determined from the date of admission and discharge/death, as recorded in the patients’ charts. The age-adjusted Charlson’s comorbidity index for patients’ preexisting diseases was calculated using the formula previously described.Citation26 A comorbidity index of ≥4 and age ≥80 years were used as references in line with a study that found associations between these reference values and ADRs among older persons.Citation27 The reference of ≥12 days was used to define the duration of hospital stay in conformity with previous studies.Citation10,Citation28
Evaluation of PIMs
The PIMs were evaluated using the full application of the 2015 AGS-Beers Criteria. The patients’ latest eGFR/serum creatinine data were considered in evaluating PIMs due to kidney functions. Medication utilization during hospitalization was extracted with generic names and presented using the Anatomical Therapeutic Chemical (ATC) classification system. Patients’ current diagnosis for which they received medications was classified using the ICD10, version 2016.
Ethical consideration
This study was conducted after ethics approval was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BE 591/16) and the hospital research ethics committees of both study sites. Consent was also obtained from the heads of the medical departments. The study was granted an exemption from patients’ consents as it entailed a chart review. The identities of the participants were anonymized, with unique codes being used for patients’ identities, and access to the codes and data being secured through a password. Only authorized personnel stated in the ethics document have access to the code and the data.
Data management analysis
The data were manually cleaned and analyzed using the SPSS, version 25 software, primarily using descriptive statistics. The Student’s independent t-test was used to compare the means of normally distributed continuous variables. The results were presented in means and SDs with 95% CI. Records with a missing value were excluded before analysis. A comparison was made between patients’ variables in Nigeria and South Africa using a Pearson chi-squared test (or Fisher’s exact as appropriate).
A univariate analysis was carried out to identify factors associated with ADRs in each geo-facility using Pearson chi-squared test. A multivariate logistic regression model was built, with confounding variables including comorbidity, use of NSAIDs, and duration of hospital stay being tested. Only variables with P<0.05 in the univariate analysis were retained in the final model. Exposure to PIM was however forced into the model. The Hosmer-Lemeshow goodness of fit was used to assess the adequacy of the final models. The results were presented using OR with 95% CI with P<0.05 being considered significant.
Results
The outcome of the selection of the study population
describes the outcome of the selection process of the patients’ medical records. A total of 846 and 1,236 records of eligible hospitalized older persons were available for sampling in Nigeria and South Africa, respectively.
Figure 1 Flowchart depicting the selection process of the included older persons.
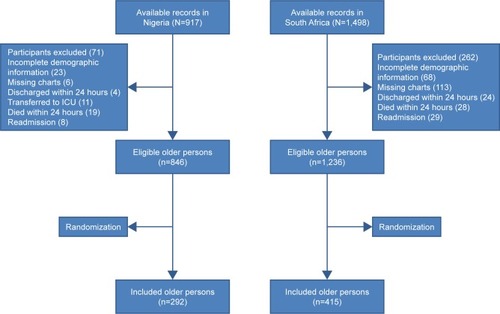
presents the clinical and sociodemographic characteristics of the study populations in Nigeria and South Africa. A total of 292 records were selected in Nigeria, of which 268 (91.8%) were evaluated, and 415 records in South Africa, of which 339 (81.7%) were evaluated. The hospitalized older persons in Nigeria and South Africa had mean ages of 70.53±8.22 years (95% CI −0.21 to 2.32) and 69.49±7.64 years, (95% CI −0.25 to 2.34, P=0.11), respectively. The study population in South Africa included the indigenous Africans (239, 70.5%), Asian descent (38, 11.2%), Khoisan descent (23, 6.8%), and European descent (39, 11.5%) populations. Musculoskeletal disorders (23/268, 8.6% vs 32/339, 9.4%) and urinary diseases (22/268, 8.2% vs 28/339, 8.3%) were almost of equal distribution among the cohorts in Nigeria and South Africa, respectively.
Table 1 Characteristics of the older persons investigated in the study
presents the PIMs in Nigeria and South Africa according to the ATC classification. Almost one-third of the older persons evaluated in Nigeria (86/268, 32.1%) and South Africa (102/339, 30.1%) received at least one PIM during hospitalization, as per the 2015 AGS-Beers Criteria (OR=0.91, 95% CI 0.64–1.29, P=0.6; Table S1). There were no significant differences in PIM exposure among the races in South Africa (P=0.74).
Table 2 The classification of PIM received by the older persons in Nigeria and South Africa
presents the triggers found in the records of older persons evaluated in Nigeria and South Africa. The IHI tool provided triggers for suspected ADRs in Nigeria (67/268, 25.0%) and (86/339, 25.4%) South Africa. The level of correlations of IHI trigger with ADRs among hospitalized older persons in Nigeria was P=0.045 and in South Africans was P=0.72.
Table 3 The triggers for identifying suspected ADRs
presents the organ systems affected by the ADRs and the offending medications among the older persons investigated in Nigeria and South Africa. The hospitalized older persons in Nigeria (37/268, 13.8%) experienced 43 cases of ADRs, while the South Africans had 31 (31/339, 9.1%) within the study period (95% CI 0.38–1.04, P=0.07), with no significant racial difference (P=0.91). Of the total observed cases, only 9.3% (4/43) in Nigeria and 6.5% (2/31) in South Africa were formally documented as ADRs. Among the Nigerians, constipation (8/43, 18.6%) secondary to chlorpromazine, amitriptyline, tramadol, and diclofenac was observed, and in South Africa confusion (6/31, 19.4) was secondary to opioid and benzodiazepines administration.
Table 4 The ADRs and the offending medications among older persons
PIMs were responsible for only 34.9% (15/43) and 32.3% (10/31) ADR cases in Nigeria and South Africa, respectively (Table S2). The Naranjo algorithm measurement of the ADRs showed that 90.7% (39/43) Nigerian and 83.9% (26/31) South African cases were of probable ADR categories, while the remaining ADRs in Nigeria (4/43, 9.3%) and South Africa (5/31, 16.1%) were possible ADRs.
Associations between independent variables and ADRs
presents the associations between independent variables and ADRs. There was a significant difference in mean ages of older persons investigated in Nigeria with ADRs vs No ADRs being 67.49±7.81 years vs 71.02±8.20 years, respectively (95% CI 0.69–6.37, P=0.02). The length of hospital stays was 2.22±1.58 days vs 1.49±0.99 days, respectively (95% CI −1.11 to 0.35, P<0.001). The univariate analysis showed the presence of musculoskeletal disorders, exposure to psychoanaleptic medications, and duration of stay ≥12 days as being associated with ADRs in Nigeria.
Table 5 The univariate and multivariate analysis of independent variables and ADRs in Nigeria and South Africa
The mean ages of the South African with ADRs vs No ADRs were not significantly different (69.65±7.17 years vs 69.46±7.70 years, respectively, 95% CI −3.0 to 2.65, P=0.9). The length of hospital stays for those who experienced ADRs vs No ADRs in South Africa was 1.26±0.45 days vs 1.35±0.64 days, respectively (95% CI −0.14 to 0.32, P=0.45). There were no significant correlations between races and age (P=0.15), comorbidity index (P=0.77), PIM exposure (P=0.34), ADRs (P=0.07), and duration of hospital stay (P=0.51) in South Africa.
Discussion
Main findings
This study compared ADRs and PIMs among hospitalized older persons in Nigeria and South Africa and evaluated possible associations between PIMs and ADRs. However, no significant difference in PIMs and ADRs was found among hospitalized older persons evaluated in Nigeria and South Africa. Although the ADRs were not significantly associated with the 2015 AGS-Beers PIMs in both countries, psycho-analeptic medications were identified as being associated with ADRs among the Nigerians in the univariate analysis. The diseases of the musculoskeletal system were associated factors for ADRs among hospitalized older persons in Nigeria, but not in South Africa.
Comparison with previous studies
This study found no significant differences in the ADRs between hospitalized older persons in Nigeria and South Africa, despite the difference in the mean number of days spent in the hospital between the cohorts. However, other studies have associated length of hospital stay with ADRs.Citation1,Citation10 The prevalence of ADRs in the Nigerians (13.8%) and South Africans (9.1%) cohorts was lower than previously reported among hospitalized older Italians (25.0%) and Brazilians (21.1%).Citation10,Citation29 The result of this study compares with the global prevalence of ADRs (11.5%) reported among hospitalized older persons.Citation30 However, variations in the definition of older persons and methods of assessing ADRs can contribute to the different prevalence reported elsewhere. These variations are well-documented in the literature.Citation27,Citation30,Citation31
This study, found no significant difference in PIMs between the Nigerian and South African cohorts, or any significant association between the 2015 AGS-Beers PIMs and ADRs. The finding agrees with similar studies that applied the 2012 AGS-Beers Criteria among older Japanese, Italians, and Brazilians, Citation8–Citation10,Citation32 and are consistent with a recent study that applied the 2015 AGS-Beers Criteria among Indian older persons.Citation15 However, the present finding contradicts reports of studies that found an association between the 2012 AGS-Beers Criteria and ADRs.Citation7,Citation33,Citation34
While the 2015 AGS-Beers PIMs were generally not associated with ADRs among the hospitalized older persons at both study sites, a univariate analysis identified psycho-analeptic medications as associated factors for ADRs in Nigeria, similar to finding among French older persons.Citation34 Psychoanaleptics (N06CA01) including antidepressants and psycholeptic medications contribute to increased incidence of falls and reduction in the quality of life among older persons.Citation35 Physicians should, therefore, be vigilant when prescribing this class of medications to older persons.
Gastrointestinal bleeding was observed among the study population on NSAIDs in both study sites, and this can be potentially dangerous among older persons. The 2015 AGS-Beers Criteria recommend that long-term use of NSAIDs without gastroprotective agents be avoided in this cohort.Citation3 A case of acute kidney injury (AKI) was observed in Nigeria (due to NSAIDs) and South Africa (due to herbal medicine use). AKI is a serious ADR in this age group and efforts should be geared toward preventing their occurrence.Citation36 The cases in this study underscore the importance of taking a medication history before prescribing. Prescribers therefore, need to be cautious when prescribing NSAIDs for older persons, and improve on the practice of medication history assessment and documentation, especially in developing countries where herbal medicines are a part of the health care culture.
The diseases of the musculoskeletal system were consistently associated with ADRs among Nigerians, but not South Africans. This is possibly the first study to associate musculoskeletal disorders with ADRs among hospitalized older persons. Previous studies identified neoplasm, diseases of the urinary system, and circulatory system disorders as risk factors for ADRs among hospitalized older persons.Citation10,Citation30,Citation37 Although the diseases identified by other studies are also presented in this study, they were not associated with ADRs. Age ≥80 years and comorbidity index ≥4.0 were not associated with ADR in this study in contrast with previous reports.Citation27,Citation28
Clinical implications
ADRs constitute a growing burden to older persons and health care system worldwide and is associated with poor clinical outcomes and increased health care costs.Citation10,Citation14 Interventions to reduce ADRs among older persons are therefore of utmost clinical priority. The lack of association between the 2015 AGS-Beers Criteria PIMs and ADRs in this study indicates that avoiding the Criteria PIMs may not be sufficient to reduce ADRs among older persons. Physicians should, therefore, consider other interventions in addition to the AGS-Beers PIMs when prescribing to older persons. The present study suggests that with proper monitoring and good clinical practice, the length of hospital stay may not be a significant determinant of ADRs among older persons. The finding also highlights the need for clinicians to be vigilant when prescribing medications to older persons with musculoskeletal diseases.
Strengths and limitations of the study
This study’s strengths were it applied both objective criteria and triggers tool to identify ADRs, which will provide more reliability and detection. It provided information about other predictors for ADRs among older persons apart from PIMs. It was a comparative study in the two countries with the largest population of older persons in Africa. The findings of the study may, therefore, be relevant in other African and developing countries.
Its limitations were while a few medications that could prolong QT interval were prescribed in both health care settings, radio imaging data which could have provided information about ADR occurrence due to these medications were not evaluated. The study was retrospective and could therefore have been affected by shortcomings in data collection and recording in the medical charts. Furthermore, not all the participants were managed by geriatricians. The study involved only hospitalized older persons in teaching hospitals, with the South African study population not being stratified. Despite the study strengths, these factors may limit the generalizations of the study findings to other settings.
Conclusion
The prevalence of PIMs and ADRs in the Nigerian and South African cohorts was similar. While the 2015 AGS-Beers PIMs were not significantly associated with ADRs in both countries, the presence of musculoskeletal system disorders were predictors for ADRs in Nigeria. A multidimensional approach should be adopted to prevent/reduce ADRs among older persons. Prospective and multicentered studies may be needed to validate the finding of this study.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Table S1 PIM utilization among participants in Nigeria and South Africa
Table S2 Potential inappropriate medicines that resulted in ADRs among study participants
References
- DaviesEAO’MahonyMSAdverse drug reactions in special populations – the elderlyBr J Clin Pharmacol201580479680725619317
- World Health OrganizationSafety of medicines: a guide to detecting and reporting adverse drug reactions2002 Available from: http://apps.who.int/medicinedocs/en/d/Jh2992e/Accessed October 1, 2018
- American Geriatrics Society 2015 Beers Criteria Update Expert PanelAmerican Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adultsJ Am Geriatr Soc201563112227224626446832
- GallagherPRyanCByrneSKennedyJO’MahonyDStopp O’MahonyDSTOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validationInt J Clin Pharmacol Ther2008462728318218287
- Renom-GuiterasAMeyerGThürmannPAThe EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countriesEur J Clin Pharmacol201571786187525967540
- JanoEAparasuRRHealthcare outcomes associated with beers’ criteria: a systematic reviewAnn Pharmacother200741343844817311835
- ReichORosemannTRapoldRBlozikESennOPotentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalizationPLoS One201498e10542525136981
- GalliTBReisWCAndrzejevskiVMPotentially inappropriate prescribing and the risk of adverse drug reactions in critically ill older adultsPharm Pract2016144818
- IshiiSKojimaKEzawaKThe association of change in medication regimen and use of inappropriate medication based on beers criteria with adverse outcomes in Japanese long-term care facilitiesGeriatr Gerontol Int201717459159727228966
- de FigueiredoTPde Souza GroiaRCBarrosoSCCdo NascimentoMMGReisAMMFactors associated with adverse drug reactions in older inpatients in teaching hospitalInt J Clin Pharm201739467968528466398
- BrownJDHutchisonLCLiCPainterJTMartinBCPredictive validity of the Beers and Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP) Criteria to detect adverse drug events, hospitalizations, and emergency department visits in the United StatesJ Am Geriatr Soc2016641223026782849
- CorsonelloAPrannoLGarastoSFabiettiPBustacchiniSLattanzioFPotentially inappropriate medication in elderly hospitalized patientsDrugs Aging200926Suppl 1313920136167
- HamiltonHGallagherPRyanCByrneSO’MahonyDPotentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patientsArch Intern Med2011171111013101921670370
- LevyHBMarcusELChristenCBeyond the beers criteria: a comparative overview of explicit criteriaAnn Pharmacother201044121968197521081709
- RawatRSEvaluation of potentially inappropriate medication use and risk of adverse drug reactions in hospitalized older adults: an observational study in a tertiary care hospitalInt J Pharm Res20181127985
- YusupovEChenDKrishnamachariBMedication use and falls: applying Beers criteria to medication review in Parkinson’s diseaseSAGE Open Med2017542050312117743677
- Parameswaran NairNChalmersLPetersonGMBereznickiBJCastelinoRLBereznickiLRHospitalization in older patients due to adverse drug reactions – the need for a prediction toolClin Interv Aging20161149750527194906
- BelhekarMNTaurSRMunshiRPA study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactionsIndian J Pharmacol201446111712024550597
- MurayamaHSakumaMTakahashiYMorimotoTImproving the assessment of adverse drug reactions using the Naranjo algorithm in daily practice: the Japan Adverse Drug Events StudyPharmacol Res Perspect201861e00373
- NaranjoCABustoUSellersEMA method for estimating the probability of adverse drug reactionsClin Pharmacol Ther19813022392457249508
- GriffinFAResarRKInstitute for Healthcare ImprovementIHI Global Trigger Tool for Measuring Adverse EventsSecond edCambridge, Massachusetts: IHI Innovation Series white paper2009 Available from https://oig.hhs.gov/…/IHI%20Guidance%20Document%20-%20Hospital%20Trigger&percntAccessed August 13, 2018
- SakaSAOosthuizenFNlootoMInterventions towards reducing adverse drug reactions among geriatric population in Africa: a scoping review of the literature from 1990–2016PULA: Botswana Journal of African Studies2017311180194
- United Nations DoEaSAPopulation Division World Population Ageing 2017-Highlights (ST/ESA/SERA/397)2017 Available from: http://www.un.org/en/development/desa/population/.../ageing/WPA2017_Highlights.pdfAccessed August 13, 2018
- do NascimentoMMMambriniJVLima-CostaMFFirmoJOPeixotoSWde Loyola FilhoAIPotentially inappropriate medications: predictor for mortality in a cohort of community-dwelling older adultsEur J Clin Pharmacol201773561562128108781
- CharanJBiswasTHow to calculate sample size for different study designs in medical research?Indian J Psychol Med201335212112624049221
- CharlsonMSzatrowskiTPPetersonJGoldJValidation of a combined comorbidity indexJ Clin Epidemiol19944711124512517722560
- Obreli-NetoPRNobiliAde Oliveira BaldoniAAdverse drug reactions caused by drug-drug interactions in elderly outpatients: a prospective cohort studyEur J Clin Pharmacol201268121667167622644345
- TangiisuranBScuttGStevensonJDevelopment and validation of a risk model for predicting adverse drug reactions in older people during hospital stay: Brighton Adverse Drug Reactions Risk (BADRI) modelPLoS One2014910e11125425356898
- ConfortiACostantiniDZanettiFMorettiUGrezzanaMLeoneRAdverse drug reactions in older patients: an Italian observational prospective hospital studyDrug Healthc Patient Saf201247522888275
- AlhawassiTMKrassIBajorekBVPontLGA systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care settingClin Interv Aging201492079208625489239
- HarugeriAParthasarathiGRameshMGuidoSBasavanagowdappaHFrequency and nature of adverse drug reactions in elderly inpatients of two Indian medical college hospitalsJ Postgrad Med201157318919521941055
- TosatoMLandiFMartoneAMPotentially inappropriate drug use among hospitalised older adults: results from the CRIME studyAge Ageing201443676777324637848
- PriceSDHolmanCDSanfilippoFMEmeryJDD’ArcyJAssociation between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patientsAnn Pharmacother201448161624396090
- MontastrucFDuguetCRousseauVBagheriHMontastrucJLPotentially inappropriate medications and adverse drug reactions in the elderly: a study in a PharmacoVigilance databaseEur J Clin Pharmacol20147091123112724925091
- de JongMRVan der ElstMHartholtKADrug-related falls in older patients: implicated drugs, consequences, and possible prevention strategiesTher Adv Drug Saf20134414715425114778
- RosnerMHAcute kidney injury in the elderlyClin Geriatr Med201329356557823849008
- SikdarKCDowdenJAlaghehbandanRMacdonaldDPeterPGadagVAdverse drug reactions in elderly hospitalized patients: a 12-year population-based retrospective cohort studyAnn Pharmacother2012467–896097122739715
