Abstract
Objective
To bridge the efficacy and compare the safety of the 24-week teriparatide treatment in a Chinese osteoporosis study (NCT0414973) to a large international trial (FPT, NCT0670501) to determine whether long-term results from the international study were applicable to Chinese patients.
Methods
In this post-hoc analysis, a propensity score matching method was used to select patients with similar baseline characteristics. Patients were female with osteoporosis at high risk of fracture, aged ≥55 years, and had no history of rheumatoid arthritis or corticosteroid use. Outcomes included percentage changes in lumbar-spine bone mineral density (LS-BMD) from baseline to 24 weeks, safety in matched-pair patients, and long-term percentage changes in LS-BMD and fragility fracture incidence in the matched fracture prevention trial (FPT) population. The determination of the acceptability of bridging results was based on the International Conference on Harmonization E5 guidelines.
Results
A total number of 228 patients from each study were matched and paired. Patients were similar at baseline (P-values >0.33) except for ethnicity (98% Caucasian for FPT). For changes in LS-BMD from baseline to week 24, treatment with teriparatide showed significantly greater increases (P-values <0.001; least-squares mean difference: 5.0% in the Chinese study and 5.4% in FPT) than comparator (calcitonin/placebo). The safety profiles over 24 weeks were similar between two studies. For matched-pair FPT patients, long-term changes in LS-BMD were significantly greater (least-squares mean difference: 11.5%, P<0.001) and the fragility fracture rate was marginally lower in the teriparatide group compared with the placebo group (13.1% vs 22.3%, P=0.070).
Conclusion
Assuming similar pharmacokinetic profiles for teriparatide between populations, comparable increases in LS-BMD and consistent safety profiles within 24 weeks of the treatment suggest long-term LS-BMD results from the FPT may be applicable to Chinese population.
Introduction
Osteoporosis is a systemic skeletal disease that leads to an increased risk of fracture. The osteoporosis prevalence for women in nine industrialized countries ranged from 9% to 38%Citation1 and it is reported the prevalence of osteoporosis in China increased over the past decades and reached 28% during the period spanning 2012–2015.Citation2 Teriparatide, the 1–34 N-terminal fragment of parathyroid hormone, is an anabolic medication that increases bone mineral density (BMD) and improves bone quality by stimulating both bone formation and bone resorption.Citation3 Compared to placebo, teriparatide treatment reduces risk of vertebralCitation4–Citation6 and nonvertebralCitation7 fractures in postmenopausal women with osteoporosis.
One-year fracture risk can be equally well predicted in Caucasian, Blacks, Hispanics, Native Americans, and Asians using BMD.Citation8 Although the efficacy and safety of teriparatide have been assessed in randomized, controlled trials of men and women with osteoporosis for up to 21 months, study populations were largely Caucasian.Citation4–Citation6 There is no data showing the comparison in the response of teriparatide between Chinese and Caucasian. Therefore, it is important to know whether the efficacy and safety of teriparatide treatment in Chinese and Caucasian patients with osteoporosis are comparable. The International Conference of Harmonization (ICH) E5 guidelines on ethnic factors in the acceptability of foreign clinical dataCitation9 allow investigators to use bridging studies to extrapolate data from large foreign studies to smaller domestic trials. This strategy has been used to evaluate osteoporosis therapies, including raloxifene, alendronate, and risendronate.Citation10
The goal of our post-hoc analysis was to compare efficacy and safety of 24 weeks of teriparatide treatment in paired Chinese and Caucasian patients with similar baseline characteristics from two studies. If the efficacy of teriparatide was shown to be similar in the matched pairs at 24 weeks, this analysis would support the bridging of data between the Chinese osteoporosis studyCitation11 and the international fracture prevention trial (FPT),Citation12 thus allowing for a reasonable estimate of long-term lumbar spine-bone mineral density (LS-BMD) change and risk reduction of new fractures in Chinese women with osteoporosis. We hypothesize that efficacy and safety between matched Chinese and Caucasian patients will show similar efficacy and safety at 24-weeks.
Materials and methods
Study designs
Response to teriparatide in Chinese and Caucasians was evaluated by selecting patients from two randomized trials. The Chinese osteoporosis study (ClinicalTrials.gov identifier NCT0414973) was an open-labeled, multicenter, active comparator, randomized phase III study. It was carried out in 10 study centers and included 362 postmenopausal Chinese women and men with established osteoporosis.Citation11 Patients were randomly assigned to receive teriparatide 20 µg once daily or calcitonin 200 IU once daily. Efficacy was assessed by absolute and percent changes in LS-BMD and hip BMD, and changes in biochemical bone markers (osteocalcin; OC) after 24 weeks of treatment.Citation11
The study for comparison, the FPT (ClinicalTrials.gov identifier NCT0670501), took place at 99 centers in 17 counties that included 1,637 postmenopausal women with prior vertebral fracture, of whom 98.5% were Caucasian.Citation12 Patients were randomly assigned to teriparatide treatment (20 or 40 µg once daily) or placebo for a median of 19 months. Efficacy was assessed by radiographic and clinical vertebral fracture, nonvertebral fracture, changes in BMD in spine and hip and changes in the bone marker including procollagen 1 carboxyterminal propeptide (P1CP).
Overview of both study designs are shown in .
Table 1 Overview of study designs
Outcomes
The short-term efficacy of teriparatide was evaluated in matched Chinese and Caucasian patients based on absolute and percent changes in mean LS-BMD from baseline to week 24. LS-BMD for both studies was measured by dual-energy X-ray absorptiometry with the use of Hologic (Hologic; Marlborough, MA, USA), Lunar (GE Healthcare; E Healthcare, Little Chalfont, UK), or Norland (Swissray; Piscataway, NJ, USA) scanners. Manufacturer-specific BMD data, not scanner-generated standardized data, were collected. Standardized BMD (sBMD) data were the basis for any statistical analysis.Citation13 The following standardization methodology was used:
For Hologic instruments: sBMD (mg/cm2) =1,000 [1.091× BMDHologic −0.016]
For Lunar instruments: sBMD (mg/cm2) =1,000 [BMDLunar −0.0552]
For Norland instruments: sBMD (mg/cm2) =1,000 [1.005× BMDNorland +0.070].
Efficacy measurements also included bone turnover markers (OC in the Chinese study, P1CP in the FPT). Safety was assessed by the frequency and proportion of patients in each study who experienced a treatment-emergent adverse event (TEAE) and serious adverse event (SAE). Laboratory tests included serum, calcium, phosphorus, hepatic enzyme, renal function, and uric acid analyses. Changes in mean LS-BMD and the rate of fracture after 24 months treatment for matched patients from the FPT were evaluated. In addition, the entire FPT and Chinese study populations were pooled for an analysis that estimated the relative risk reduction for fracture of teriparatide compared to comparator.
Statistical analysis
To make the patients comparable, only those who met the selection criteria for both the FPT and the Chinese study were considered. Therefore, patients who were female with osteoporosis at high risk of fracture, aged ≥55 years, and had no history of rheumatoid arthritis or use of corticosteroids were included for matching.
Following the initial trimming, a propensity score (PS) matching method was used to generate matched-pair patients (one patient from each study) that balanced baseline characteristics between ethnic groups. All key unbalanced characteristics (including baseline age, weight, height, body mass index, smoking status, previous osteoporosis medication usage and baseline LS-BMD) were included in the PS model. A 1:1 (Chinese:Caucasian) greedy matching algorithm without replacement and with a specified caliper distance of 0.15 was used to identify matched-pair patients.
The balance of the matched pairs from the two studies was determined by hypothesis testing (two-sample t-test for continuous variable and chi-squared test for categorical variables) or by assessing the standardized difference, which compares the difference in means in units of the pooled SD. A standardized difference of 0.1 was taken to indicate a negligible difference in the mean or prevalence of a covariate between two studies.Citation14
Change in mean LS-BMD from baseline to post-baseline visit within each treatment arm was tested by paired t-test. The difference between teriparatide and comparator groups in LS-BMD was tested by analysis of covariance adjusted for investigator and baseline LS-BMD. Least-squares mean and 95% CI were provided.
The frequency and proportion of patients with TEAEs/SAEs through week 24 were summarized using preferred terms of the Medical Dictionary for Regulatory Activities (MedDRA; version 12.0). The relative risk (95% CI) was provided. Changes from baseline to week 24 in standard laboratory measures and in bone biomarker values was tested by Wilcoxon signed rank test. The difference between teriparatide and comparator groups was tested by Wilcoxon rank sum test.
The difference between teriparatide and comparator groups in the rate of fracture from baseline to month 19 was analyzed by chi-squared test. The relative risk (95% CI) was provided. Further, we combined all study data from the two studies to determine the difference in fracture at endpoint for teriparatide versus comparator groups. The OR, 95% CI, and P-values were provided by simple logistic regression.
The two-sided significance level of 0.05 was used. Due to the exploratory nature of this analysis, no adjustments for multiplicity were made. Statistical comparisons were accomplished using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
A total of 228 patients each from the Chinese study and the FPT were matched based on baseline characteristics (). Matched patients from the Chinese study included 148 randomized to teriparatide and 80 to calcitonin, while those from the FPT included 107 randomized to teriparatide and 121 to placebo. In the Chinese study, paired patients were Chinese and had a median study duration of 24.3 weeks (5.7 months). In the FPT, 97.8% of paired patients were Caucasian (1.32% East Asian and 0.88% Hispanic) and had a median study duration of 81.6 weeks (19.0 months). Except for ethnicity, baseline characteristics of Chinese and Caucasian patients were similar in patients selected using the PS method (). All P-values were >0.33 and all standard differences were <10%.
Figure 1 Patient flow resulting from predetermined criteria to select patients eligible for matching.
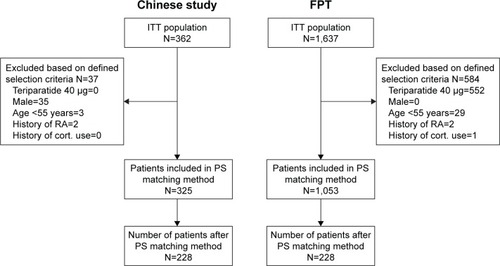
Table 2 Baseline characteristics of matched-pair patients from the Chinese study and from the FPT
Short-term efficacy (24 weeks following randomization)
In both the Chinese and Caucasian patients, LS-BMD in the teriparatide groups increased significantly compared with baseline (6.4% for the Chinese study and 8.6% for the FPT, P<0.001 in both groups) (). For the primary efficacy endpoint, the 24-week teriparatide treatment showed significantly greater increases in LS-BMD (P<0.001) for both absolute and percent change from baseline compared to either comparator (calcitonin or placebo) in both Chinese and Caucasian women with osteoporosis at high risk of fracture. The least-squares mean (95% CI) of absolute change difference between the teriparatide group and the calcitonin/placebo group were 33.9 mg/cm2 (95% CI: 22.4–45.4) in the Chinese study and 39.9 mg/cm2 (95% CI: 20.7–59.0) in the FPT (P<0.001 in both studies). The least-squares mean on percent change difference in LS-BMD between the teriparatide group and the calcitonin/placebo group were 5.0% (95% CI: 3.2–6.7) in the Chinese study and 5.4% (95% CI: 2.5–8.2) in the FPT (P<0.001 in both studies).
Table 3 Change in lumbar spine-bone mineral density from baseline to week 24 for matched-pair patients by treatment and by study
Biomarkers: OC and P1CP
In the Chinese study, the bone formation biomarker of interest was OC. In 200 out of 228 (88%) patients, the OC data had been collected from baseline to week 24. The median of the percent change from baseline was 139% in the teriparatide group and −6% in the calcitonin group, for a significantly different magnitude of change (P<0.001). In the FPT, the biomarker of interest was P1CP. In 62 out of 228 (27%) patients, the P1CP data changed from baseline to week 24. The median of the percent change from baseline was 10.1% in the teriparatide group and −3.5% in the placebo group. This difference was not statistically significant. The median values of both biomarkers increased numerically in the teriparatide treatment groups compare with comparators in both studies (Table not shown).
Short-term safety (24 weeks following randomization)
During the 24-week treatment period, one death was reported in all matched-pair patients from placebo group in FPT (). SAEs were reported in 4.7% of the patients randomized to teriparatide and 1.3% of the patients assigned to calcitonin in the Chinese study. For FPT, 7.5% of teriparatide-treated patients and 9.9% of placebo-treated patients in matched patients reported SAEs. The percentage of patients with ≥1 TEAE was 32.4% and 27.5% in the teriparatide and calcitonin groups, respectively, in the Chinese study (RR: 1.18; 95% CI: 0.77–1.80). In FPT, patients with ≥1 TEAE were reported between 66.4% and 65.3% in the teriparatide and placebo groups, respectively (RR: 1.02; 95% CI: 0.84–1.23). The adverse event of “blood uric acid increased” was reported in eight patients (5.4%) randomized to the teriparatide group but none in calcitonin group in the Chinese study. The adverse event of “urinary tract infection” was reported in seven patients from teriparatide (6.5%) and placebo (5.8%) groups, respectively, in the FPT. The trend of common TEAEs in the Chinese study was similar to that in the FPT in the 6-month follow-up period after randomization. In teriparatide-treated Chinese and Caucasian patients, similar increases in alkaline phosphatase, uric acid, and calcium were seen. In Chinese and Caucasian patients there were mild changes in phosphorus, ALT, AST, urea nitrogen, and creatinine that were not clinically significant.
Table 4 Overview of safety, laboratory values, and bone markers in matched-pair patients over 24 weeks after randomization
Long-term efficacy (19 months following randomization)
Long-term data from matched-pair patients in FPT (), exhibited significantly increased mean LS-BMD from teriparatide treatment compared with placebo (P<0.001). The least-squares mean (95% CI) on absolute difference of LS-BMD between teriparatide and placebo groups from the baseline visit to last visit was 75.3 mg/cm2 (95% CI: 61.4–89.2). The least-squares mean (95% CI) on percent difference was 11.5% (95% CI: 9.2–13.8). The difference in the number of matched FPT patients who experienced a fragility fracture in the long-term analysis () was numerically less compared to placebo, but the difference was not statistically significant: teriparatide 14/107, (13.1%); placebo 27/121, (22.3%); RR: 0.59; 95% CI: 0.32–1.06; P=0.070.
Table 5 Analysis of lumbar spine-bone mineral density and fragility fracture prevalence at endpoint (median 19.0 months) for patients from the FPT selected for matching
In an analysis of fracture incidence during the 19-month study duration in the entire FPT population (N=1,053), the OR (95% CI) for fracture between the teriparatide and placebo groups was (OR: 0.43; 95% CI: 0.30–0.61; P<0.001). Over 24 weeks, there were very few fractures in the entire population of the Chinese study (N=325), which is inconclusive given the short duration of the study. However, after a pooled analysis of the 6-month Chinese study and the 19-month FPT, the OR (95% CI) for fracture between teriparatide and comparator groups in the full analysis set from both studies (N=1,378) was (OR: 0.36; 95% CI 0.25–0.51; P<0.001).
Discussion and conclusion
Teriparatide is a recombinant form of the parathyroid hormone that has been shown to decrease the risk of vertebral and nonvertebral fractures, increase bone mineral density, and have only minor side effects over a median treatment period of 19 months in a large international study of postmenopausal women with osteoporosis.Citation15 Whether the short-and long-term results from this study can be extrapolated to a Chinese population was not known; in particular, the long-term effects of teriparatide on bone mass and fracture risk were not studied. In the present analysis, we compared results from a 24-week osteoporosis study in China to the FPT and found that data from matched-pair patients with osteoporosis from the two studies showed similar increases in least-squares mean of LS-BMD following 24 weeks of teriparatide treatment, and have a similar rate of TEAEs, SAEs, and change from baseline in specific laboratory values. The FPT showed a median treatment duration of 19 months, which is three times larger than that of the Chinese study and provided important information on further changes in LS-BMD and the rate of fracture in the long-term. These results suggested that long-term results of the FPT could reasonably be extrapolated to a Chinese population.
There are known ethnic and racial differences in the incidence of osteoporosis and osteoporotic fractures throughout the world.Citation16,Citation17 In addition, genetic variability related to ethnicity is one factor that accounts for the observed differences in both pharmacokinetics and pharmacodynamics of drugs, resulting in variability in response to drug therapy.Citation18–Citation20 Bridging studies exist to allow safe approval of drugs in ethnically different nations without duplicating research efforts. This study utilized bridging methodology developed by the ICH, a body that brings together regulatory authorities of Europe, Japan, and the United States, and experts from the pharmaceutical industry in the three regions. The ICH E5 guidelines were introduced in 1998 and, except for the development of Bayesian statistical methods to assess the degree of similarity, have remained unchanged since that time.
On comparing increase in LS-BMD after 24 weeks of teriparatide 20 µg in Chinese and Caucasian patients, we found that both had significant (P<0.001) increases compared to their comparator (calcitonin/placebo). Teriparatide is known to improve LS-BMD over timeCitation21 and the increases were of a similar magnitude (5.0% in Chinese vs 5.4% in Caucasian) and were consistent with results from the entire population of each study (5.0% at 24 weeks in the Chinese study and 9.7% at 19 months for the FPT). In a Japanese teriparatide bridging study, which compared 203 patients from a phase III randomized clinical trial to patients in the FPT, the difference between the teriparatide 20 µg group and the placebo group in the percent change in LS-BMD from baseline to 12 months was 10.20% in the Japanese study and 7.41% in the FPT, thereby confirming superiority of teriparatide 20 µg to placebo in both studies.Citation22 Likewise, increase in LS-BMD was shown in a systematic review of ten randomized clinical trials of teriparatide 20 µg for bone-related conditions from China, Hong Kong, Japan, Republic of Korea, Philippines, Singapore, and Taiwan.Citation23 Efficacy data was mainly taken from randomized clinical trials for treatment of postmenopausal osteoporosis and percent change in LS-BMD increased with increasing duration of treatment, from 4.3% at 24 weeks of treatment to greater than 10% at 18–24 months of treatment. This review provided an appraisal of the few long-term studies of teriparatide available in Asian patients.
Safety over 24 weeks was similar in the Chinese study and the FPT. Teriparatide was well tolerated and the only death was reported from the placebo group.Citation11 SAEs occurred infrequently and individual SAEs tended to occur singularly. TEAEs were twice as likely to occur in FPT patients as in patients from the Chinese study. However, the similarity between relative risks for teriparatide versus comparator in both the Chinese trial and the FPT suggests that the differences in TEAE rates between studies may reflect differences in reporting systems, rather than differences in the action of the drug between ethnic groups. The changes in laboratory values from baseline following teriparatide treatment that were seen in both studies have been well documented in prior teriparatide trials.Citation24 Teriparatide has been shown to induce modest but transient increases in serum calcium, which is consistent with the known effects of endogenous parathyroid hormone (PTH), and no unexpected safety findings were observed in patients who experienced hypercalcemia during treatment with teriparatide. The increase of alkaline phosphatase, calcium, and uric acid demonstrate pharmacologic response of teriparatide (similar to PTH) in both Chinese and Caucasian patients. Phosphorus had a clinically insignificant increase at week 24 in the Chinese study but not in the FPT. Also, the observed increases in both calcium and uric acid have been documented in the product insert and no nephrolithiasis was reported in either study. Both studies showed an increase in bone formation biomarkers reflecting bone formation over 12 weeks.
For the long-term analysis of paired patients in the FPT, the P-value for fracture reduction compared with placebo was 0.070. The result was not significant, primarily because after matching, the sample size was markedly reduced. In this study, the proportion of matched patients from the FPT with fracture was 13.1% for teriparatide and 22.3% for placebo. Assuming there were roughly 120 patients in each study arm, the power to detect the statistically significant difference between two arms was 47%.
Patients utilized in this comparison were chosen through a PS matching method. At baseline, patients selected by matching from the Chinese teriparatide study and the global FPT looked very similar, with no significant differences for standard baseline characteristics thought to be risk factors of osteoporosis that may influence the disease outcome (duration of osteoporosis, body mass index, etc). This met our expectations and suggested that we had created a model for PS matching that had successfully selected two groups of patients who were balanced in most of the risk factors except for ethnicity. However, the PS method only controls observed variables. Any hidden bias due to latent variables may remain.
These studies had two different comparators: placebo and calcitonin. Calcitonin is a weak antiresorptive agent; for example, at the end of 1 year, calcitonin 200 IU/day increased LS-BMD by <1.5%,Citation25 which is below the sensitivity of the test (estimated at 3%). In our 24-week study, LS-BMD increased 1.4% after 6 months of calcitonin 200 IU (compared with 6.5% in the teriparatide group). These differences seen with calcitonin treatment were minimal and would only marginally affect the results of this comparison.
A limitation of the current study was that the pharmacokinetics of teriparatide in these matched-pair patients were not compared because such parameters were not evaluated in the Chinese study as would be optimal per ICH E5 guidelines. A literature search revealed that a pharmacokinetic analysis of teriparatide had not been performed in Chinese versus Caucasian population. However, the pharmacokinetics of the 20 µg dose was evaluated in 9 healthy Chinese volunteersCitation26 and similar findings were obtained in a subgroup of 360 global patients from the FPT in whom pharmacokinetic parameters were measured.Citation27 According to the package insert, teriparatide 20 µg, once daily has high bioavailability (~95%), rapid onset (~30 minutes), and short exposure (t1/2 of 1 hour)Citation24 and these pharmacokinetic properties would not be expected to reduce drug availability in different ethnic groups. Tsujimoto et al analyzed data from healthy Japanese and Caucasian women with postmenopausal osteoporosis.Citation22 This study compared results from the FPT to results from three Japanese studies, one of which included pharmacokinetic data. Findings from this bridging study concluded that there were no major differences in bodyweight-adjusted pharmacokinetics of teriparatide in Japanese patients and patients in the FPT. Chinese and Japanese populations feature similar regional geography and similar genes encoding drug-metabolizing enzymes and transporters.Citation28 It may be reasonable to assume that they would have similar pharmacokinetic characteristics for teriparatide and, by extension, that pharmacokinetic parameters in Chinese patients would be similar to those of patients in the FPT.
Results of this analysis help fill the gap created by the lack of data from long-term head-to-head studies of teriparatide versus comparator in Chinese postmenopausal women. Weaknesses of this study include use of different comparators in the Chinese study (calcitonin) and the FPT (placebo), though at 24 weeks, calcitonin has been shown to have a placebo-like effect on LS-BMD in this population.Citation29 In addition, the dose–response data were limited, and assumptions were made based on existing data in similar populations. We estimated the relative risk of teriparatide compared with comparator; however, we could not estimate the absolute risk, as there are different fracture rates in Chinese and Caucasian patients. Moreover, long-term Chinese fracture and safety data is desired to validate the findings from the bridging study.
In conclusion, this bridging study of patients with osteoporosis from two separate trials is the first one to compare Chinese and Caucasian populations. It showed similar increases in LS-BMD in Chinese and Caucasian patients following 24 weeks of therapy with teriparatide 20 µg daily. This analysis, which conforms to ICH E5 guidelines, suggests that it might be reasonable to extrapolate the 19-month fracture results of the FPT to Chinese patients. Thus, long-term treatment with teriparatide would be expected to help reduce the risk of new fractures in Chinese women at a rate similar to that seen in other ethnic groups.
Compliance with ethical standards
Per the original publications for the Chinese study and the FPT, both had been approved by the appropriate institutional and/or national research ethics committee and had been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The patient consent forms were collected in both trials. For this post-hoc analysis by using existing data from the two original trials for research purpose, new formal consent is not required.
Acknowledgments
This study was funded by Eli Lilly and Company. The authors wish to thank Tao Wu of Eli Lilly for editorial assistance.
Disclosure
Zhongjian Xie has no conflicts of interest to disclose in this work. Yun Chen, Sirel Gurbuz, Bin Zhang, Yujie Li, Fan Bai, and Yu Chen are employees of and minor stock holders in Eli Lilly and Company.
References
- WadeSWStraderCFitzpatrickLAAnthonyMSO’MalleyCDEstimating prevalence of osteoporosis: examples from industrialized countriesArch Osteoporos2014918224847682
- ChenPLiZHuYPrevalence of osteoporosis in China: a meta-analysis and systematic reviewBMC Public Health2016161103927716144
- CompstonJESkeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structureBone20074061447145217045858
- GallagherJCGenantHKCransGGVargasSJKregeJHTeriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fracturesJ Clin Endocrinol Metab20059031583158715613428
- OrwollESScheeleWHPaulSThe effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosisJ Bone Miner Res200318191712510800
- GreenspanSLBoneHGEttingerMPEffect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trialAnn Intern Med2007146532633917339618
- VestergaardPJorgensenNRMosekildeLSchwarzPEffects of parathyroid hormone alone or in combination with antiresorptive therapy on bone mineral density and fracture risk – a meta-analysisOsteoporos Int2007181455716951908
- Barrett-ConnorESirisESWehrenLEOsteoporosis and fracture risk in women of different ethnic groupsJ Bone Miner Res200520218519415647811
- NaitoCEthnic factors in the acceptability of foreign clinical dataTher Innov Regul Sci19983211283S1292S
- UyamaYShibataTNagaiNHanaokaHToyoshimaSMoriKSuccessful bridging strategy based on ICH E5 guideline for drugs approved in JapanClin Pharmacol Ther200578210211316084844
- DaiKChenDZhangZComparison of the effects of subcutaneous teriparatide and intranasal calcitonin in treating established osteoporosis in postmenopausal Chinese womenChin J Osteoporos Bone Miner Res201142331
- NeerRMArnaudCDZanchettaJREffect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Engl J Med2001344191434144111346808
- LiuYYeKMathurAKHuiSFuerstTPGenantHKComparative calibration without a gold standardStat Med19971616188919059280040
- AustinPCAn introduction to propensity score methods for reducing the effects of confounding in observational studiesMultivariate Behav Res201146339942421818162
- PrevrhalSKregeJHChenPGenantHBlackDMTeriparatide vertebral fracture risk reduction determined by quantitative and qualitative radiographic assessmentCurr Med Res Opin200925492192819250060
- KanisJAJohnellODe LaetCJonssonBOdenAOgelsbyAKInternational variations in hip fracture probabilities: implications for risk assessmentJ Bone Miner Res20021771237124412096837
- CauleyJADefining ethnic and racial differences in osteoporosis and fragility fracturesClin Orthop Relat Res201146971891189921431462
- YasudaSUZhangLHuangSMThe role of ethnicity in variability in response to drugs: focus on clinical pharmacology studiesClin Pharmacol Ther200884341742318615002
- International HapMapCThe international HapMap projectNature2003426696878979614685227
- WilsonJFWealeMESmithACPopulation genetic structure of variable drug responseNat Genet200129326526911685208
- Obermayer-PietschBMMarinFMcCloskeyEVEffects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatmentJ Bone Miner Res200823101591160018505369
- TsujimotoMUenakaKIwataAHigashiuchiYSowaHEffects of teriparatide in Japanese and non-Japanese populations: bridging findings on pharmacokinetics and efficacyJ Bone Miner Metab201230332633721938381
- ChenJFYangKHZhangZLA systematic review on the use of daily subcutaneous administration of teriparatide for treatment of patients with osteoporosis at high risk for fracture in AsiaOsteoporos Int2015261112825138261
- Forteo [package insert]Indianapolis INEli Lilly and Company2016
- ChesnutCH3rdSilvermanSAndrianoKA randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study GroupAm J Med2000109426727610996576
- ChuNNLiXNChenWLXuHRPharmacokinetics and safety of recombinant human parathyroid hormone (1–34) (teriparatide) after single ascending doses in Chinese healthy volunteersPharmazie2007621186987118065105
- SatterwhiteJHeathmanMMillerPDMarinFGlassEVDobnigHPharmacokinetics of teriparatide (rhPTH[1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosisCalcif Tissue Int201087648549220953593
- YiSAnHLeeHKorean, Japanese, and Chinese populations featured similar genes encoding drug-metabolizing enzymes and transporters: a DMET Plus microarray assessmentPharmacogenet Genom20142410477485
- HwangJSTuSTYangTSChenJFWangCJTsaiKSTeriparatide vs. calcitonin in the treatment of Asian postmenopausal women with established osteoporosisOsteoporos Int200617337337816421647
