Abstract
Diabetes mellitus (DM) has been emerging as one of the most serious health problems worldwide. Ocular complications of DM are currently one of the major causes of blindness in developed countries, among which diabetic retinopathy is relatively well studied and understood. However, although ocular surface complications of DM are common, diabetic complications of anterior segment of the eye, such as, cornea, conjunctiva, and lacrimal glands, are often overlooked. DM is associated with progressive damage to corneal nerves and epithelial cells, which increases the risk of anterior segment disorders including dry eye disease, corneal erosion, persistent epithelial defects, and even sight-threatening corneal ulcer. In this review, the authors will discuss the association of DM with disorders of anterior segment of the eye. Studies indicating the value of corneal nerve assessment as a sensitive, noninvasive, and repeatable biomarker for diabetic neuropathy will also be introduced. In addition, treatment modalities of anterior segment disorders associated with DM is discussed. The studies introduced in this review suggest that early and periodic screening of the anterior segment of the eye, as well as the retina, is important for the optimal treatment of DM.
Introduction
Diabetes mellitus (DM), defined as “a chronic disease that occurs when the pancreas does not produce enough insulin, or when the body cannot effectively use the insulin it produces”,Citation1 is a major global public health problem.Citation2 It is one of the most prevalent systemic diseases in the world with increasing prevalence.Citation3 DM was reported to affect 366 million people worldwide in 2011 and estimated to affect >555 and 640 million people by 2030 and 2040, respectively.Citation3
DM has also been increasingly prevalent in Korea, with an age-standardized prevalence among adults aged ≥30 years showing 8.6% in 2001, 9.6% in 2007, and 11.1% in 2013, according to the Korean National Health and Nutrition Examination Survey (KNHANES) data.Citation4 Data from the National Health Insurance Service also showed a rising trend in the prevalence of type 2 DM and impaired fasting glucose from 5.6% and 21.5% in 2006, to 8.0% and 25% in 2013, respectively.Citation4 As the prevalence of DM increases with age, the KNHANES data demonstrated a high prevalence of DM in age groups of ≥70 years old and 60–69 years of 27.6% and 25.2%, respectively, while the prevalence in age groups of 30–39 years and 40–49 years were only 2.5% and 7.3%, respectively.Citation4
DM leads to complications such as neuropathy, retinopathy, nephropathy, and cardiovascular disorders, in which hyperglycemia plays a major role.Citation3 Ophthalmologic complications have emerged as the leading cause of blindness in developed countries, of which retinopathy is the major manifestation that has been relatively well understood by health care providers.Citation3,Citation5
On the contrary, anterior segment complications associated with DM, including the cornea, conjunctiva, and lacrimal glands, are not well recognized, although up to two-thirds of patients are reported to experience diabetic keratopathy during the course of DM.Citation5,Citation6 Patients with DM demonstrate progressive decrease in corneal nerve density and reduction in corneal sensitivity,Citation7,Citation8 which subsequently result in the impairment of corneal epithelial wound healing process and increased susceptibility to persistent epithelial defects and corneal infections.Citation9–Citation11 These complications can potentially lead to blindness, which underscores the importance of understanding the impact of DM on anterior segment diorders.Citation12
In this review, we aimed to provide an overview of the association between DM and anterior segment diseases and discuss the underlying pathophysiologic mechanisms and treatment methods for anterior segment disorders associated with DM, as summarized in .
Diabetic corneal neuropathy
Diabetic peripheral neuropathy is the most common neuropathic presentation in DM.Citation13 Approximately half of the patients were reported to have diabetic peripheral neuropathy after a 25-year follow-up of DM.Citation14
Pathogenesis
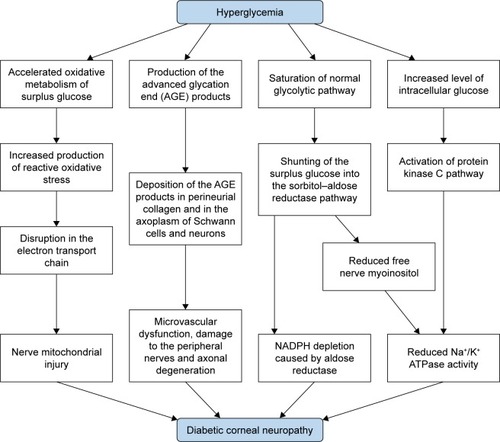
Chronic hyperglycemia is the core causative mechanism underlying the pathogenesis of diabetic neuropathy as well as other systemic complications.Citation15 It induces pathological pathways, such as generation of reactive oxidative stress (ROS), advanced glycation end (AGE) products, sorbitol– aldose reductase pathway, and protein kinase C activation.Citation16,Citation17
First, chronic hyperglycemia leads to excessive influx of glucose into the mitochondria, which promotes the production of ROS due to accelerated oxidative metabolism of glucose.Citation17 These ROS induce disruption in the mitochondrial electron transport chain, which results in mitochondrial injury.Citation17 Nerve fibers are more prone to mitochondrial damage due to their greater mitochondrial volume that subsequently leads to demyelination and conduction dysfunction.Citation16 Mitochondrial injury is also associated with decreased neurotrophic factors including the nerve growth factor (NGF).Citation18 An experimental study demonstrated that a marker of oxidative stress, 8-hydroxydeoxyguanosine, was increased in the diabetic rat cornea, suggesting the possible role of oxidative stress in the apoptosis of corneal cells in DM.Citation19
Second, AGE products generated by glycation may also play an important role in the pathogenesis of peripheral neuropathy.Citation20,Citation21 Glycation is the nonenzymatic bonding of sugar molecules including glucose or fructose to a protein or lipoprotein, which leads to the formation of AGE products with altered structure and function.Citation17 DM induces glycation of myelin proteins and deposition of AGE products in perineurial collagen and in the axoplasm of Schwann cells and neurons, which leads to microvascular dysfunction, damage to the peripheral nerves, and axonal degeneration.Citation21,Citation22 Moreover, the accumulation of AGE in the corneal epithelium promotes proapoptotic and antiproliferative local cell signaling pathways.Citation19
Third, elevated intracellular glucose level caused by chronic hyperglycemia leads to decreased Na+/K+ ATPase activity on cell membrane, which reduces nerve conduction velocity and inhibits nerve regeneration.Citation23,Citation24 Saturation of normal glycolytic pathway caused by hyperglycemia in nerve cells results in shunting of the surplus glucose into the sorbitol–aldose reductase pathway, in which the glucose is converted to fructose and sorbitol by the enzymes sorbitol dehydrogenase and aldose reductase.Citation25 In the shunted pathway, aldose reductase causes depletions of NADPH.Citation24 Moreover, accumulation of fructose and sorbitol results in decreased free nerve myoinositol, which causes reduced membrane Na+/K+ ATPase activity.Citation24,Citation26 Increased level of intracellular glucose is also associated with activation of protein kinase C pathway, which can inhibit the activity of membrane Na+/K+ ATPase ().Citation23,Citation27
An experimental study using a diabetic mouse model revealed a high concentration of antigen-presenting cells including Langerhans cells and dendritic cells in the cornea and aggregation of the cells around corneal nerve fibers.Citation28 The number of dendritic cells had a negative correlation with corneal nerve fiber density, suggesting the possible role of inflammation in the development of corneal neuropathy in DM.Citation28
Changes in corneal nerve parameters, such as a reduction in corneal subbasal nerve fiber density, length, and branch density, have been reported in both type 1 and type 2 DM,Citation29,Citation30 which show correlation with diabetic peripheral and autonomic neuropathy.Citation31–Citation34 Edwards et alCitation32 showed that patients with diabetic peripheral neuropathy had significantly decreased corneal subbasal nerve fiber length and branch density compared with individuals without DM and those with DM but without diabetic peripheral neuropathy. Pritchard et alCitation34 demonstrated that reduction in corneal nerve fiber length measured with corneal confocal microscopy (CCM) can predict the development of diabetic peripheral neuropathy. Misra et alCitation31 revealed that corneal subbasal nerve density changes assessed using CCM precede other functional changes detected by clinical and electrophysiology tests of neuropathy and that corneal sensitivity has a negative correlation with autonomic nerve analysis, suggesting that CCM and corneal sensitivity testing can be surrogate markers for the assessment of diabetic peripheral and autonomic neuropathy.Citation31 Tavakoli et alCitation35 demonstrated that parameters assessed using CCM had a significant correlation with the composite autonomic symptom scale and showed a high sensitivity and specificity for the diagnosis of diabetic autonomic neuropathy, indicating that CCM can be a rapid, noninvasive, and reliable diagnostic test for subclinical diabetic autonomic neuropathy.Citation35 Maddaloni et alCitation36 also suggested that CCM could be a noninvasive tool for the evaluation of cardiac autonomic neuropathy in patients with type 1 DM. A recent study on patients with type 1 DM revealed that among seven measures of diabetic peripheral neuropathy, including CCM, peroneal nerve conduction velocity, cold, warm and vibration perception, monofilament testing, and neuropathy disability score, corneal subbasal nerve morphology evaluated using CCM demonstrated the earliest and most consistent changes in neuropathy status during a 4-year follow-up period.Citation37 A meta-analysis reported in 2016 concluded that corneal nerve fiber density, length, and branch density assessed using CCM were significantly reduced in people with diabetic peripheral neuropathy, and CCM can be useful for early detection of nerve damage in diabetic peripheral neuropathy.Citation38
Changes in corneal cells and nerve fibers were also shown to predict the development of diabetic retinopathy.Citation39–Citation41 Alterations of the corneal subbasal nerve plexus have shown to progress in parallel with diabetic retinopathy and peripheral neuropathy.Citation39 In patients with type 1 DM, CCM demonstrated corneal cellular changes including decreased epithelial and endothelial cell densities and higher keratocyte cell density, as well as small nerve fiber changes, such as lower corneal nerve fiber density and length, lower nerve branch density, and greater nerve fiber width in patients without retinopathy, which worsens with the progression of diabetic retinopathy.Citation40 Bitirgen et alCitation41 revealed that changes in corneal nerve parameters including reduction of nerve fiber density, length, and branch density were observed in patients without diabetic retinopathy and aggravated with the progression of retinopathy.
Corneal nerve changes as a window to diabetic neuropathy
Although diabetic peripheral neuropathy involves both large and small nerve fibers, small nerve fibers can be more sensitive indicators of peripheral neuropathy as they are affected earlier.Citation42 Vibration perception using biothesiometry has been the gold standard for the diagnosis of diabetic peripheral neuropathy.Citation43 However, the method has its limitation based on the fact that only large nerve fiber functions are measured rather than small nerve fibers.Citation3 Visualization of small nerve fiber damage is possible using skin punch biopsy.Citation44 However, biopsy is invasive and nonrepeatable and is also associated with an increased risk of wound complications in patients with DM.Citation3 By contrast, CCM can allow noninvasive, direct visualization of subtle corneal nerve changes in vivo.Citation30 It can also detect diabetic nerve fiber damage earlier than vibration perception or corneal sensation testing.Citation30 Changes in corneal nerve fibers may be detected earlier compared with diabetic peripheral neuropathy in other parts of the body, ie, lower limb, due to high density of small nerve fibers in the cornea.Citation30
Alterations in the corneal subbasal nerve plexus assessed using CCM have close correlation with changes in the peripheral nerves.Citation11 These findings indicate the potential of CCM as a sensitive, noninvasive, and reliable surrogate marker for the evaluation of diabetic peripheral neuropathy,Citation11 which can enable early detection of diabetic peripheral neuropathy and prevention of serious neuropathic complications including diabetic foot ().Citation2 As retinal examination can be a window to systemic vascular changes in DM, visualization of corneal nerves using CCM can provide a window to systemic peripheral and autonomic nerve changes associated with DM.
Table 1 Studies suggesting corneal nerve assessment can be a reliable biomarker for peripheral and autonomic nerve damage in diabetes mellitus (DM)
Corneal nerve changes with diabetic control
Studies showed that diabetic neuropathy can be improved after interventions for diabetic control,Citation45 although it may not be completely reversed.Citation46 Smith et alCitation47 reported that significant improvements in intraepidermal nerve fiber density were detected using skin biopsy after 1 year of strict glycemic control and lifestyle modification. Improvement in risk factors for diabetic neuropathy, such as hyperglycemia, dyslipidemia, and hypertension, was associated with regeneration of corneal nerve fibers, which was confirmed using in vivo CCM.Citation45 In this study, improvement in HbA1c had significant correlation with an increase in corneal nerve fiber density.Citation45 In vivo CCM also demonstrated significant improvement in corneal nerve fiber length 1 year after simultaneous pancreas and kidney transplantation in patients with type 1 DM.Citation48 These results suggest that the evaluation of corneal nerve changes using CCM can be a viable option for monitoring the efficacy of therapy for diabetic control.
Ocular surface abnormalities in DM
DM can also cause alterations in the corneal epithelial basal cells and basement membrane, leading to corneal epitheliopathy and adhesion disorders.Citation49 In addition, loss of corneal nerves in DM leads to reduced neurotrophic support, resulting in accelerated loss and reduced proliferation of epithelial cells.Citation50,Citation51 DM also causes production of abnormal basal lamina and inadequate adhesion of epithelial cells to an abnormal basement membrane.Citation6,Citation10,Citation52
An experimental study demonstrated delayed corneal wound healing with decreased activation of endothelial growth factor receptor in a diabetic rat model.Citation53 Diminished expression of the tight junction proteins including β-catenin and zonula occludens-1 was also observed, indicating the disruption of cell to cell junctions in diabetic cornea.Citation53 Other animal studies showed detrimental effects of hyperglycemia on the corneal epithelium basement membrane complex and demonstrated findings suggesting compromise of corneal epithelial function, such as increase in corneal thickness, disruption of tight junctions, and loss of basal epithelial cells.Citation19,Citation54 The accumulation of AGE in the corneal epithelium basement membrane complex promotes proapoptotic and antiproliferative pathways, which results in corneal epithelial damage.Citation19 Di et alCitation55 demonstrated an excessive inflammatory response manifested by the accumulation of polymorphonuclear cells in mice diabetic corneas, resulting in increased levels of proinflammatory cytokines and delayed wound healing.
DM is consequently associated with damaged epithelial barrier function and impaired epithelial healing, which increases the risk of ocular surface diseases, such as dry eye disease (DED), superficial punctate keratitis, recurrent corneal erosion, persistent epithelial defects, and neurotrophic corneal ulcer.Citation6,Citation56,Citation57
Ocular surgery in DM
Corneal refractive surgery induces destruction and reconstruction of corneal basal nerves, and DM might conceivably impair the corneal epithelial wound healing process. Indeed, the Food and Drug Administration declared DM to be a relative contraindication to laser-assisted in situ keratomileusis (LASIK) in 2000.Citation58 Patients with DM had a substantially higher risk of corneal complications including erosions and persistent epithelial defects and worse visual outcome after LASIK compared with those without DM (47% vs 6.9%).Citation59
However, other studies revealed that LASIK did not increase the risk of corneal complications in individuals with well-controlled DM,Citation60 and there was no significant difference in anatomic and visual outcomes between the patients with DM and those without.Citation61 Although data regarding the safety and efficacy of LASIK in DM patients are limited, there are only few reports of significant corneal complications despite the large number of refractive surgery performed annually.Citation62 These findings indicate that LASIK can be performed effectively and safely at least in selected patients with well-controlled DM.Citation61,Citation62
Therefore, in 2005, the American Academy of Ophthalmology recommended that LASIK can be safely performed in a selected group of DM patients despite the risk of delayed corneal wound healing, although informed consent and close postoperative monitoring are mandatory.Citation62 The candidates eligible for LASIK should have stable and well-controlled fasting glucose with HbA1c <9 and no evidence of systemic DM complications including nephropathy or peripheral neuropathy.Citation62 Although those with mild DM retinopathy can be considered for LASIK on a case-by-case basis, individuals with significant DM retinopathy or a history of diabetic ocular complications should be excluded.Citation62
Because DM is associated with an increased risk of cataract even in the population younger than 65 years, increasing number of cataract surgery has been performed in patients with DM.Citation3 In addition to a higher risk of postoperative complications of the retina including cystoid macular edema and exacerbation of diabetic retinopathy, DM is also associated with an increased risk of corneal complications, such as persistent corneal edema, corneal abrasion, and delayed epithelial wound healing.Citation63 Cataract surgery can worsen the subbasal nerve damage in patients with reduced tear production or an impaired corneal epithelial integrity associated with DM.Citation64
DM is a significant risk factor for corneal complications following other procedures, such as trabeculectomy, vitrectomy, and laser photocoagulation.Citation65–Citation67 Dogru et alCitation65 demonstrated that patients with DM had increased corneal staining score, decreased corneal sensitivity, and decreased tear film breakup time after argon laser photocoagulation using coupling fluid and retinal laser lens compared with those without DM.Citation65 DM is also a risk factor for developing DED after intraocular surgery.Citation68
These findings indicate that special attention should be paid for corneal complications in patients with DM after any kind of ocular surgery, including even minor procedures.Citation69
DM and DED
Diabetic peripheral neuropathy can theoretically increase the risk of DED,Citation70 and half of patients with DM experience dry eye symptoms.Citation71 Misra et alCitation72 reported impairment of lipid layer quality and tear film stability in DM patients. Hyperglycemia can lead to microvascular damage to the lacrimal gland, and diabetic autonomic neuropathy is associated with impairment of lacrimal innervation, which both contribute to diminished tear production.Citation8 Diabetic peripheral neuropathy leads to decreased corneal nerve fiber density, which results in impaired corneal sensitivity and diminished reflex tearing.Citation71,Citation73 Corneal hypoesthesia can cause decreased mucin production by goblet cells, which leads to reduced tear film stability.Citation8 A higher tear osmolarity probably caused by reduced tear production or increased tear evaporation was also observed in patients with DM.Citation33
The severity of dry eye signs has close correlation with the degree of peripheral neuropathy and severity of diabetic retinopathy.Citation10,Citation71,Citation74 However, with prolonged disease duration, patients are often asymptomatic even in the presence of serious ocular surface damage due to reduced corneal sensitivity, which reflects the progression of diabetic peripheral neuropathy.Citation75
The presence of DED in DM patients further increases the risk of damage to the corneal epithelium.Citation51 Therefore, periodic screening of ocular surface damage and dry eye symptoms in addition to retinal examination would be necessary for patients with DM.Citation70,Citation75
Tear film abnormality in DM
Substance P plays a role in the maintenance of healthy ocular surface by providing neurotrophic support and promoting proliferation and migration of corneal epithelial cells.Citation76 Reduction in substance P level may result in impairment of corneal epithelial homeostasis, potentially leading to exacerbation of corneal complications associated with DM.Citation77 The concentration of substance P in the tear film is correlated with corneal nerve fiber density,Citation78 and corneal hypoesthesia is associated with diminished substance P level.Citation79 The tear substance P level was also shown to be related to the duration of DM and severity of diabetic retinopathy.Citation77
Insulin is found in the tear film, and insulin receptors are identified on the ocular surface.Citation80 Insulin is thought to provide neurotrophic support to the ocular surface and promote the metabolism and growth of the lacrimal glandCitation81: thus, decrease in insulin activity is postulated to be associated with damage to the corneal nerves in DM.Citation82 Upregulation of insulin-like growth factor binding protein 3 and activation of insulin-like growth factor receptor are observed in type 2 DM, which may cause corneal epithelial damage by the disruption of corneal epithelial cell–basement membrane complex.Citation83 Experimental studies showed that topical insulin can facilitate improvement of corneal epithelial integrity and sensitivity in animal models of diabetic keratopathy.Citation84–Citation86
DM and meibomian gland dysfunction
An experimental study revealed that insulin stimulated the proliferation of immortalized human meibomian gland epithelial cells in a dose-dependent manner, while excess glucose resulted in progressive cell loss, suggesting that insulin deficiency and hyperglycemia may be toxic for the meibomian gland epithelial cells and increase the risk of meibomian gland disorders.Citation81
Contact lens (CL) use in DM
CL wear is associated with theoretical risks as followsCitation10: 1) exacerbated corneal epithelial fragility increases the risk of corneal damage; 2) tear film instability could deteriorate DED; 3) impairment of corneal hydration control could lead to corneal edema; and 4) CL-induced endothelial polymegathism can cause corneal endothelial decompensation after intraocular surgery. These factors, together with reduced corneal sensitivity and the vulnerability to infection in DM, may increase the risk of corneal complications including infectious keratitis in the diabetic CL wearer.Citation10 Reports of ocular complications in diabetic CL wears do exist, mostly occurred in patients with advanced diabetic ocular complications or those using extended wear CL.Citation87
However, prospective studies suggest that CL wear might not be contraindicated in patients with DM.Citation88,Citation89 O’Donnell et alCitation89 demonstrated that there was no significant difference in conjunctival injection, corneal staining score, corneal thickness, or sensitivity after 1 year of CL wear between patients with DM and those without.Citation89 Another prospective study revealed that DM had no significant influence on corneal hydration and recovery, indicating that CL wear can be recommended in DM patients.Citation88 However, given the adverse impact of DM on the ocular surface, detailed advice to the patients and close monitoring for corneal complications are mandatory.Citation3
Based on the findings that tear glucose concentrations correlate with blood glucose levels,Citation3 tear glucose sensing devices for noninvasive monitoring of glycemic control using CL has been developed and may be widely used in the near future.
Treatment of anterior segment complications
As in other diabetic complications, strict glycemic control is essential for the treatment and prevention of anterior segment disorders associated with DM.Citation2 Topical artificial tears can be helpful for the maintenance of healthy ocular surface and clear visual axis. Topical anti-inflammatory medications, such as NSAIDs, steroids, and cyclosporine A, are also useful for alleviating ocular surface inflammation and encouraging re-epithelialization.Citation90 Bandage CL can be helpful for an irregular ocular surface, recurrent corneal erosion, and persistent epithelial defects.Citation2
Autologous serum is theoretically beneficial for facilitating corneal wound healing and nerve regeneration as it contains high amounts of growth factors.Citation2,Citation91 Topical autologous serum was shown to accelerate corneal epithelial healing after vitrectomy in DM patients.Citation92 Topical application of umbilical cord serum and platelet-derived plasma was also suggested to be effective in facilitating corneal nerve regeneration and epithelial healing.Citation91
Substance P can attenuate apoptosis of corneal epithelial cells induced by hyperglycemia and accelerate the epithelial healing process via the neurokinin-1 receptor signaling pathway.Citation37,Citation93 Insulin-like growth factor 1 (IGF-1) was also shown to promote regeneration of corneal nerves and ocular surface homeostasis.Citation94 Topical application of substance P and IGF-1 derivatives promote proliferation and migration of corneal epithelial cells in neurotrophic keratopathy including diabetic corneal neuropathy.Citation5,Citation76 A prospective clinical study demonstrated that topical administration of substance P and IGF-1 combination eye drops was effective in the prevention of postoperative superficial punctate keratopathy.Citation95
NGF can stimulate regeneration of damaged neurons and mucin production by goblet cells.Citation96 Topical NGF was shown to improve ocular surface integrity and corneal sensitivity in corneal neuropathy.Citation97 Park et alCitation98 reported that topical NGF can alleviate inflammation and hyperglycemia-induced apoptosis of epithelial cells in diabetic corneas. Kim et alCitation99 revealed that oral nicergoline promoted corneal wound healing in diabetic rat corneas, which was conceivably related to increased NGF in the cornea and lacrimal gland.Citation99
Aldose reductase inhibitor can theoretically reduce nerve damage and promote corneal epithelial regeneration by attenuating activation of the sorbitol–aldose reductase pathway.Citation3 Oral aldose reductase inhibitor was effective in improving corneal epithelial damage and corneal sensitivity after cataract surgery in DM patients.Citation69 Topical aldose reductase inhibitor has also shown to promote corneal epithelial wound healing in DM.Citation100
Naltrexone, an opioid antagonist, is useful for corneal wound healing by accelerating DNA synthesis.Citation101 DM is associated with the production of excessive opioid growth factors, which leads to inhibition of cell proliferation. Naltrexone is expected to promote wound healing in diabetic corneas as it blocks the opioid growth factor and its receptor pathway. Its topical application was shown to improve corneal regeneration and tear production in a type 1 diabetic rat model.Citation102 Topical naltrexone also promoted corneal epithelial repair in a type 2 diabetic mouse model.Citation103
Resolvin D, a docosahexaenoic acid-derived anti-inflammatory mediator, is also expected to be effective for the treatment of diabetic anterior segment disorders.Citation104 An in vitro study revealed that resolvin D attenuated the synthesis of inflammatory cytokines in the corneal epithelium.Citation105 An experimental study using a diabetic rat model also reported that oral administration of resolvin D alleviated corneal and peripheral nerve degeneration.Citation106
Antioxidants, such as carnosine and β-carotene, were also suggested to be beneficial for the prevention of DM-related corneal changes.Citation106,Citation107 Experimental treatment modalities including gene therapy, molecular, and stem cell therapies have been developed.Citation12,Citation55 Di et alCitation55 recently showed that bone marrow-derived mesenchymal stem cells could attenuate excessive inflammatory responses, activate corneal progenitor cells, and enhance would healing of diabetic cornea. However, these experimental methods still have to overcome myriad of barriers to be translated into clinical practice.Citation2 The effects of medications for anterior segment complications associated with DM are summarized in .
Table 2 Effects of the medications for anterior segment disorders associated with diabetes mellitus
Conclusion
DM has an adverse impact on ocular surface integrity, corneal sensitivity, corneal epithelial regeneration, and tear production.Citation3 Diabetic keratopathy is common, and its severity can vary from mild DED to sight-threatening corneal ulcer. The severity of diabetic corneal neuropathy correlates with diabetic peripheral and autonomic neuropathy of other organs. Subtle diabetic neuropathy in the cornea often precedes reti-nopathy and neuropathy of other parts of the body. Therefore, examination of the corneal nerve plexus using CCM can be a viable biomarker for the assessment of diabetic neuropathies. Although several medications were introduced to be effective for the treatment and prevention of anterior segment disorders associated with DM, further researches are still necessary for the development of better treatment methods.
The studies introduced in this review highlight the need for periodic screening of anterior segment diseases as well as retinal examination for patients with DM. Guidelines for screening ocular surface pathologies should also be established. An enhanced understanding in both patients and medical practitioners of the impact of DM on the anterior segment of the eye would be important for the optimal management of DM.Citation2
Acknowledgments
This study was supported by 2018 Research Grant from Kangwon National University (No.520180051).
Disclosure
The authors report no conflicts of interest in this work.
References
- AlbertiKGZimmetPZDefinitionZPZDefinition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultationDiabet Med19981575395539686693
- ShihKCLamKSTongLA systematic review on the impact of diabetes mellitus on the ocular surfaceNutr Diabetes201773e25128319106
- MarkoulliMFlanaganJTummanapalliSSWuJWillcoxMThe impact of diabetes on corneal nerve morphology and ocular surface integrityOcul Surf2018161455729113918
- NohJThe diabetes epidemic in KoreaEndocrinol Metab (Seoul)201631334935327586447
- AbdelkaderHPatelDVMcGheeCNJAlanyRGNew therapeutic approaches in the treatment of diabetic keratopathy: a reviewClin Exp Ophthalmol201139325927020973888
- Vieira-PotterVJKaramichosDLeeDJOcular complications of diabetes and therapeutic approachesBioMed Res Int201620163114
- PritchardNEdwardsKRussellAWCorneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetesDiabetes Care2015384671525573881
- DogruMKatakamiCInoueMTear function and ocular surface changes in noninsulin-dependent diabetes mellitusOphthalmology2001108358659211237914
- YoonKCImSKSeoMSChanges of tear film and ocular surface in diabetes mellitusKorean J Ophthalmol200418216817415635831
- O’DonnellCEfronNDiabetes and contact lens wearClin Exp Optom201295332833722537249
- PritchardNEdwardsKShahidiAMCorneal markers of diabetic neuropathyOcul Surf201191172821338566
- BikbovaGOshitariTBabaTDiabetic corneal neuropathy: clinical perspectivesClin Ophthalmol20181298198729872257
- SmithHSArgoffCEPharmacological treatment of diabetic neuropathic painDrugs201171555758921443281
- PirartJDiabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (3rd and last part)Diabete Metab197734245256 French598565
- NowotnyKJungTHöhnAWeberDGruneTAdvanced glycation end products and oxidative stress in type 2 diabetes mellitusBiomolecules20155119422225786107
- YagihashiSMizukamiHSugimotoKMechanism of diabetic neuropathy: where are we now and where to go?J Diabetes Investig2011211832
- BabizhayevMAStrokovIANosikovVVThe role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patientsCell Biochem Biophys20157131425144325427889
- TomlinsonDRFernyhoughPDiemelLTRole of neurotrophins in diabetic neuropathy and treatment with nerve growth factorsDiabetes199746Suppl 2S43S499285498
- KimJKimCSSohnEJeongIHKimHKimJSInvolvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathyGraefes Arch Clin Exp Ophthalmol2011249452953621104267
- AubertCEMichelPLGilleryPAssociation of peripheral neuropathy with circulating advanced glycation end products, soluble receptor for advanced glycation end products and other risk factors in patients with type 2 diabetesDiabetes Metab Res Rev201430867968524449227
- RyleCDonaghyMNon-enzymatic glycation of peripheral nerve proteins in human diabeticsJ Neurol Sci1995129162687751847
- SugimotoKYasujimaMYagihashiSRole of advanced glycation end products in diabetic neuropathyCurr Pharm Des2008141095396118473845
- ObrosovaIGDiabetes and the peripheral nerveBiochim Biophys Acta2009179293194019061951
- GreeneDASimaAAStevensMJFeldmanELLattimerSAComplications: neuropathy, pathogenetic considerationsDiabetes Care19921512190219251464245
- StavniichukRShevalyeHHirookaHNadlerJLObrosovaIGInterplay of sorbitol pathway of glucose metabolism, 12/15-lipoxygenase, and mitogen-activated protein kinases in the pathogenesis of diabetic peripheral neuropathyBiochem Pharmacol201283793294022285226
- ChungSSMHoECLamKSChungSKContribution of polyol pathway to diabetes-induced oxidative stressJ Am Soc Nephrol2003148 Suppl 3233S236S
- GeraldesPKingGLActivation of protein kinase C isoforms and its impact on diabetic complicationsCirc Res201010681319133120431074
- LeppinKBehrendtAKReichardMDiabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the corneaInvest Ophthalmol Vis Sci20145563603361524781935
- ZieglerDPapanasNZhivovAEarly detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetesDiabetes20146372454246324574045
- MessmerEMSchmid-TannwaldCZappDKampikAIn vivo confocal microscopy of corneal small fiber damage in diabetes mellitusGraefes Arch Clin Exp Ophthalmol201024891307131220490534
- MisraSLCraigJPPatelDVIn vivo confocal microscopy of corneal nerves: an ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitusInvest Ophthalmol Vis Sci20155695060506526241393
- EdwardsKPritchardNVagenasDRussellAMalikRAEfronNUtility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark studyClin Exp Optom201295334835422540156
- DemillDLHussainMPop-BusuiRShteinRMOcular surface disease in patients with diabetic peripheral neuropathyBr J Ophthalmol2016100792492826500330
- PritchardNEdwardsKDehghaniCLongitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristicsDiabetes Res Clin Pract2014104224825624629408
- TavakoliMBegumPMcLaughlinJMalikRACorneal confocal microscopy for the diagnosis of diabetic autonomic neuropathyMuscle Nerve201552336337025556884
- MaddaloniESabatinoFDel ToroRIn vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetesDiabet Med201532226226625251450
- EdwardsKPritchardNDehghaniCCorneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetesDiabetes20173113251327
- JiangMSYuanYGuZXZhuangSLCorneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysisBr J Ophthalmol2016100191425677672
- NitodaEKallinikosPPallikarisACorrelation of diabetic retinopathy and corneal neuropathy using confocal microscopyCurr Eye Res2012371089890622632054
- SzalaiEDeákEMódisLEarly corneal cellular and nerve fiber pathology in young patients with type 1 diabetes mellitus identified using corneal confocal microscopyInvest Ophthalmol Vis Sci201657385385826943147
- BitirgenGOzkagniciAMalikRAKerimogluHCorneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitusDiabet Med201431443143824117485
- MalikRAVevesATesfayeSSmall fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathyDiabetes Metab Res Rev201127767868421695760
- AlbersJWBrownMBSimaAAGreeneDANerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Tolrestat study group for the early diabetes intervention trialNeurology199646185918559426
- QuattriniCTavakoliMJeziorskaMSurrogate markers of small fiber damage in human diabetic neuropathyDiabetes20075682148215417513704
- TavakoliMKallinikosPIqbalACorneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathyDiabet Med201128101261126721699561
- YorekMSObrosovAShevalyeHEffect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J miceJ Peripher Nerv Syst2015201243125858759
- SmithAGRussellJFeldmanELLifestyle intervention for pre-diabetic neuropathyDiabetes Care20062961294129916732011
- TavakoliMMitu-PretorianMPetropoulosINCorneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantationDiabetes201362125426023002037
- HollandEJMannisMJLeeWBOcular Surface Disease: Cornea, Conjunctiva and Tear FilmLondonElsevier/Saunders2013
- LeongYYTongLBarrier function in the ocular surface: from conventional paradigms to new opportunitiesOcul Surf201513210310925881994
- CaiDZhuMPetrollWMKoppakaVRobertsonDMThe impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epitheliumAm J Pathol2014184102662267025102563
- BeuermanRWSchimmelpfennigBSensory denervation of the rabbit cornea affects epithelial propertiesExp Neurol19806911962017389846
- YinJHuangJChenCGaoNWangFYuFSCorneal complications in streptozocin-induced type I diabetic ratsInvest Ophthalmol Vis Sci20115296589659621715347
- KimECKimDJLeeSSKimMSUltrastructural changes of cornea after ethanol ingestion in Otsuka Long-Evans Tokushima fatty (OLETF) and Long-Evans Tokushima Otsuka (LETO) ratsGraefes Arch Clin Exp Ophthalmol2010248101457146620582705
- DiGDuXQiXMesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6–dependent stem cell activation and macrophage switchInvest Ophthalmol Vis Sci201758104344435428810264
- GöbbelsMSpitznasMOldendoerpJImpairment of corneal epithelial barrier function in diabeticsGraefes Arch Clin Exp Ophthalmol198922721421442721983
- GekkaMMiyataKNagaiYCorneal epithelial barrier function in diabetic patientsCornea2004231353714701955
- US Food and Drug Administration[homepage on the Internet] When is LASIK not for me? Available from: https://www.fda.gov/medi-caldevices/productsandmedicalprocedures/surgeryandlifesupport/lasik/ucm061366.htmAccessed April 20, 2018
- FraunfelderFWRichLFLaser-assisted in situ keratomileusis complications in diabetes mellitusCornea200221324624811917170
- HalkiadakisIBelfairNGimbelHVLaser in situ keratomileusis in patients with diabetesJ Cataract Refract Surg200531101895189816338557
- Cobo-SorianoRBeltránJBavieraJLASIK outcomes in patients with underlying systemic contraindicationsOphthalmology200611371118.e11118.e816647130
- MoshirfarMLASIK in patients with diabetes mellitus Available from: http://eyewiki.aao.org/LASIK_in_Patients_With_Diabetes_MellitusAccessed April 20, 2018
- PershingSMorrisonDEHernandez-BoussardTCataract surgery complications and revisit rates among three statesAm J Ophthalmol201617113013827615607
- GoebbelsMTear secretion and tear film function in insulin dependent diabeticsBr J Ophthalmol2000841192110611093
- DogruMKaderliBGeliskenOOcular surface changes with applanation contact lens and coupling fluid use after argon laser photocoagulation in noninsulin-dependent diabetes mellitusAm J Ophthalmol2004138338138815364219
- OnoTYukiKOzekiNShibaDTsubotaKOcular surface complications after trabeculectomy: incidence, risk factors, time course and prognosisOphthalmologica20132302939923774034
- FribergTROhjiMSchererJJTanoYFrequency of epithelial debridement during diabetic vitrectomyAm J Ophthalmol2003135455355412654382
- JiangDXiaoXFuTMashaghiALiuQHongJTransient tear film dysfunction after cataract surgery in diabetic patientsPLoS One2016111e014675226771186
- FujishimaHTsubotaKImprovement of corneal fluorescein staining in post cataract surgery of diabetic patients by an oral aldose reductase inhibitor, ONO-2235Br J Ophthalmol200286886086312140204
- AchtsidisVEleftheriadouIKozanidouEDry eye syndrome in subjects with diabetes and association with neuropathyDiabetes Care20143710e210e21125249675
- LvHLiAZhangXMeta-analysis and review on the changes of tear function and corneal sensitivity in diabetic patientsActa Ophthalmol2014922e96e10423782539
- MisraSLPatelDVMcGheeCNJPeripheral neuropathy and tear film dysfunction in type 1 diabetes mellitusJ Diabetes Res2014201484865925177708
- DehghaniCPritchardNEdwardsKNatural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathyInvest Ophthalmol Vis Sci201455127982799025406279
- Creuzot-GarcherCLafontainePOGualinoOD’AthisPPetitJMBronAStudy of ocular surface involvement in diabetic patientsJ Fr Ophtalmol2005286583588 French16141920
- DemillDLHussainMPop-BusuiRShteinRMOcular surface disease in patients with diabetic peripheral neuropathyBr J Ophthalmol2016100792492826500330
- NakamuraMOfujiKChikamaTNishidaTCombined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivoCurr Eye Res19971632752789088746
- YamadaMOgataMKawaiMMashimaYNishidaTSubstance P in human tearsCornea2003227 SupplS48S5414703707
- MarkoulliMYouJKimJCorneal nerve morphology and tear film substance P in diabetesOptom Vis Sci201794772673128650386
- YamadaMOgataMKawaiMMashimaYDecreased substance P concentrations in tears from patients with corneal hypesthesiaAm J Ophthalmol2000129567167210844065
- RochaEMCunhaDACarneiroEMBoscheroACSaadMJVellosoLAIdentification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surfaceInvest Ophthalmol Vis Sci200243496396711923235
- DingJLiuYSullivanDAEffects of insulin and high glucose on human meibomian gland epithelial cellsInvest Ophthalmol Vis Sci201556137814782026658502
- ChenDKFrizziKEGuernseyLSLadtKMizisinAPCalcuttNARepeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulinJ Peripher Nerv Syst201318430631524147903
- WuYCBucknerBRZhuMCavanaghHDRobertsonDMElevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epitheliumOcul Surf201210210010722482470
- ZagonISKlocekMSSassaniJWMcLaughlinPJUse of topical insulin to normalize corneal epithelial healing in diabetes mellitusArch Ophthalmol200712581082108817698755
- ZagonISSassaniJWMcLaughlinPJInsulin treatment ameliorates impaired corneal reepithelialization in diabetic ratsDiabetes20065541141114716567540
- KlocekMSSassaniJWMcLaughlinPJZagonISNaltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type I diabetic ratsExp Eye Res200989568669219576213
- SpoorTCHartelWCWynnPSpoorDKComplications of continuous-wear soft contact lenses in a nonreferral populationArch Ophthalmol19841029131213136591900
- O’DonnellCEfronNCorneal hydration control in contact lens wearers with diabetes mellitusOptom Vis Sci2006831222616432469
- O’DonnellCEfronNBoultonAJA prospective study of contact lens wear in diabetes mellitusOphthalmic Physiol Opt200121212713811261347
- HanSBYangHKHyonJYWeeWRAssociation of dry eye disease with psychiatric or neurological disorders in elderly patientsClin Interv Aging20171278579228553087
- GoyalSHamrahPUnderstanding neuropathic corneal pain – gaps and current therapeutic approachesSemin Ophthalmol2016311–2597026959131
- SchulzeSDSekundoWKrollPAutologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomyAm J Ophthalmol2006142220721116876497
- ThaiLCTomlinsonARidderWHContact lens drying and visual performance: the vision cycle with contact lensesOptom Vis Sci200279638138812086305
- WangCPengYPanSLiLEffect of insulin-like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusisNeurosci Lett201455816917424211688
- ChikamotoNChikamaTYamadaNNishidaTIshimitsuTKamiyaAEfficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patientsJpn J Ophthalmol200953546446919847599
- PriyadarsiniSRowseyTGMaJXKaramichosDUnravelling the stromal-nerve interactions in the human diabetic corneaExp Eye Res2017164223028827027
- LambiaseARamaPBoniniSCaprioglioGAloeLTopical treatment with nerve growth factor for corneal neurotrophic ulcersN Engl J Med199833817117411809554857
- ParkJHKangS-SKimJYTchahHNerve growth factor attenuates apoptosis and inflammation in the diabetic corneaInvest Ophthalmol Vis Sci201657156767677527978558
- KimSYChoiJSJooCKEffects of nicergoline on corneal epithelial wound healing in rat eyesInvest Ophthalmol Vis Sci200950262162518836171
- NakaharaMMiyataKOtaniSA randomised, placebo controlled clinical trial of the aldose reductase inhibitor CT-112 as management of corneal epithelial disorders in diabetic patientsBr J Ophthalmol200589326626815722300
- ZagonISSassaniJWMcLaughlinPJReepithelialization of the human cornea is regulated by endogenous opioidsInvest Ophthalmol Vis Sci2000411738110634604
- ZagonISKlocekMSSassaniJWMcLaughlinPJDry eye reversal and corneal sensation restoration with topical naltrexone in diabetes mellitusArch Ophthalmol2009127111468147319901212
- ZagonISSassaniJWImmonenJAMcLaughlinPJOcular surface abnormalities related to type 2 diabetes are reversed by the opioid antagonist naltrexoneClin Exp Ophthalmol201442215916823777539
- HampelUKrügerMKunnenCGarreisFWillcoxMPaulsenFIn vitro effects of docosahexaenoic and eicosapentaenoic acid on human meibomian gland epithelial cellsExp Eye Res201514013914826335632
- ErdinestNOvadiaHKormasRSolomonAAnti-inflammatory effects of resolvin-D1 on human corneal epithelial cells: in vitro studyJ Inflamm20141116
- ShevalyeHYorekMSCoppeyLJEffect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetesJ Neurophysiol2015114119920825925322
- Abdul-HamidMMoustafaNAmelioration of alloxan-induced diabetic keratopathy by beta-caroteneExp Toxicol Pathol2014661495924129090