Abstract
Background
We aimed to evaluate the abilities of a 21-item frailty index based on laboratory blood and urine tests (FI-Lab21) assessed at different points in time, ie, at admission to hospital (FI-Lab21admission) and before discharge from hospital (FI-Lab21discharge), and the change of the FI-Lab21 during the hospital stay to predict 6-month and 1-year mortality in hospitalized geriatric patients.
Methods
Five hundred hospitalized geriatric patients aged ≥65 years were included in this analysis. Follow-up data were acquired after a period of 6 months and 1 year.
Results
The FI-Lab21admission and FI-Lab21discharge scores were 0.33±0.15 and 0.31±0.14, respectively (P<0.001). The FI-Lab21admission and FI-Lab21discharge both predicted 6-month and 1-year mortality (areas under the receiver operating characteristic curves: 0.72, 0.72, 0.77, and 0.75, respectively, all P<0.001). The predictive abilities for 6-month and 1-year mortality of the FI-Lab21admission were inferior compared with those of the FI-Lab21discharge (all P<0.05). Patients with a reduction in or stable FI-Lab21 score during the hospital stay revealed lower 6-month and 1-year mortality rates compared with the persons whose FI-Lab21 score increased during the hospital stay (all P<0.05). After adjustment for age, sex, and FI-Lab21admission, each 1% decrease in the FI-Lab21 during the hospital stay was associated with a decrease in 6-month and 1-year mortality of 5.9% and 5.3% (both P<0.001), respectively.
Conclusion
The FI-Lab21 assessed at admission or discharge and the changes of the FI-Lab21 during the hospital stay emerged as interesting and feasible approaches to stratify mortality risk in hospitalized geriatric patients.
Plain language summary
We evaluated the prognostic significance of a 21-item frailty index based on laboratory blood and urine tests (FI-Lab21) assessed at different points in time, ie, at admission to hospital (FI-Lab21admission) and before discharge from the hospital (FI-Lab21discharge), and the changes of the FI-Lab during the hospital stay of 513 geriatric inpatients on 6-month and 1-year mortality. The FI-Lab21admission and FI-Lab21discharge predicted 6-month and 1-year mortality. The FI-Lab21discharge was more powerful in predicting 6-month and 1-year mortality compared with the FI-Lab21admission. The mean FI-Lab21 score could be reduced during the hospital stay. Patients with a reduction or stable FI-Lab21 score during the hospital stay demonstrated lower mortality rates after 6 months and 1 year of follow-up in comparison with the individuals whose FI-Lab21 score increased during the hospital stay. Moreover, taking potential confounders into account, an association between the quantity of the change of the FI-Lab during the hospital stay and 6-month and 1-year mortality was found. Thus, the FI-Lab21 evaluated at admission and/or assessed before discharge and the change of the FI-Lab during the hospital stay emerged as interesting and feasible instruments to evaluate the mortality risk of older individuals hospitalized on geriatric wards.
Introduction
Frailty describes a syndrome or clinical state accompanied by loss of physiological reserves, causing an increased vulnerability of an individual.Citation1 Thereby frail individuals respond sensitively to external or internal stressor events with an even sudden deterioration of their health status.Citation2 Due to this fluid condition, frail older people are at higher risk for adverse health outcomes such as falls, hospitalization, institutionalization, and mortality among others than robust persons.Citation1,Citation3–Citation8 Adverse medical conditions going beyond physiological aging processes such as sarcopenia,Citation9,Citation10 malnutrition,Citation11,Citation12 multimorbidity,Citation13 cognitive degradation,Citation14 mental illness,Citation15 mobility impairment,Citation16 and chronic diseasesCitation17 were found to conduce to frailty. Of clinical interest, frailty was proposed to be sensitive to adequate interventions and at least in part reversible in some individuals.Citation18 There is general agreement among the importance of screening and/or assessing older people in relation to frailty and the imperative for developing feasible measure methods in older people.Citation19
Several approaches to screen and/or assess individuals in relation to frailty have been elaborated, discussed, and validated.Citation20,Citation21 The most accepted, applied, and widely studied are probably the frailty phenotypeCitation22 and the frailty index (FI) approaches.Citation23 A limitation of the frailty phenotype is that it is inappropriate to investigate fine grades of frailty.Citation24 This feasibility is a strength of an FI, enhancing precision and reliability for predicting adverse outcomes.Citation25,Citation26 FI is calculated by counting the number of present deficits (eg, clinical signs, symptoms, diseases, measures of function among others) of an individual divided by the number of all evaluated potential deficits.Citation27 A limitation of FI established in clinical practice is that evaluation of a long checklist of clinical parameters and diseases is time-consuming.Citation28 To overcome this aforementioned limitation of FI, recently FIs operationalized on the basis of standard laboratory tests (eg, blood tests, urine tests, blood pressure, and/or heart rate) (FI-Lab) have been introduced and validated.Citation28–Citation32
Several studies in different clinical settings found that FI-Lab predicts mortality in older people.Citation28,Citation33 We had previously found that FI-Lab operationalized from common blood and urine tests based on data that were obtained before discharge of the patients from hospital predicted 6-month and 1-year mortality in hospitalized patients at geriatric wards.Citation28 Recent studies now indicate that FI operationalized from the data obtained at admission to hospital is able to predict mortality.Citation3,Citation33,Citation34 From a clinician’s perspective, FI-Lab obtained from data at admission to hospital might be preferable to FI-Lab before discharge, thereby allowing the information incorporated in the FI-Lab to be used for risk stratification and care planning already at the beginning of the hospital stay. Moreover, it might be of interest whether changes in FI-Lab during the hospital stay of the hospitalized geriatric patients reveal prognostic significance for mortality. Of note, no study has so far evaluated whether changes in FI-Lab during a hospital stay reveal prognostic power for mortality.
Several other authors have so far evaluated the power of an FI-Lab on mortality in different settings.Citation29,Citation30,Citation32,Citation42 Howlett et alCitation42 assayed a strong relationship with mortality operationalizing an FI based on 21 routine blood tests combined with systolic and diastolic blood measurement in 1,013 community-dwelling and long-term institutionalized people aged 65 years or older during a 6-year follow-up evaluation. Rockwood et alCitation32 detected a predictive value of a 23-item FI-Lab in 595 long-term care residents aged 82.7±8 years in Canada during a 6-year follow-up analysis. A previous study by Blodgett et alCitation29 in 8,888 participants composing a nationally representative sample (mean age of 49.4±19 years) demonstrated higher mortality rates in individuals for higher FI-Lab categories during a period of 10-year follow-up. Another study by Blodgett et alCitation30 in a sample of 3,369 community-dwelling men aged 60±11 years accomplished in eight European countries found that FI-Lab predicted mortality, institutionalization, frequency of doctor visits, high number of medications, falls, and poor self-reported health status during a mean follow-up period of 4.4 years. In contrast to aforementioned studies, we operationalized FI-Lab21 twice, first at admission and second before discharge.
In the study presented here, we aimed to evaluate whether 1) an FI-Lab operationalized from data obtained at admission to hospital predicts 6-month and 1-year mortality; 2) an FI-Lab operationalized from data obtained at admission to hospital differs from an FI-Lab operationalized from data obtained before discharge from hospital in the ability to predict 6-month and 1-year mortality; and 3) changes in the FI-Lab score during the hospital stay reveal predictive information for 6-month and 1-year mortality in patients hospitalized on geriatric wards.
Methods
Study design and study population
This investigation was a secondary analysis concluding two recent prospective, longitudinal studies and summarized 513 patients, hospitalized on the geriatric wards of the Malteser Hospital Waldkrankenhaus St Marien in Erlangen, Germany. Criterion for inclusion was a minimum age of 65 years or older. Exclusion criteria were the missing of a written informed consent by the participant or by legal guardian on behalf of the participant. The patients were examined and evaluated for a variety of clinical characteristics and tests (eg, age, sex, weight, height, body mass index, functional impairment, diseases, Mini-Mental Status Examination, Barthel Index, Geriatric Depression Scale, Timed “Up and Go” Test). Furthermore, venous blood sampling and the analysis of spot urine took place at admission, before discharge, and, if necessary, at various points in time in-between during the hospital stay of the patients. Follow-up data were collected 6 months and 1 year after the baseline examination. These data included information with respect to death due to any cause among other clinical endpoints. The follow-up investigation was acquired by telephone interviews with participants, legal guardians, relatives, and/or general practitioners. The study protocol followed the Declaration of Helsinki and Good Clinical Practice and was approved by the Ethics Committee of the University of Erlangen-Nuremberg. Written informed consent was obtained from the study participants or their legal guardian prior to inclusion into the study.
FI based on 20 laboratory blood tests and one urinary test (FI-Lab21)
The FI-Lab21 was composited out of 20 routine blood parameters and one parameter of a urine sample. In order to take care of a well-balanced combination, focus was on respecting various organ, health, and functional systems. Therefore, the FI-Lab21 as operationalized in this study presented here contained thyroid, liver, renal, hematological, inflammatory, electrolyte, and coagulation parameters. To prevent any imbalance, the number of parameters related to the red blood cell system was reduced compared with the 23-item FI-Lab in our previous study (ie, by removing the parameters hematocrit and hemoglobin).Citation28 Much importance was attached to select parameters, which are available in any routine laboratory analysis. As also done in our preceding study,Citation28 every laboratory parameter was coded binary either 0 or 1; 0 denoted values within normal range, 1 demonstrated pathological values (ie, (sub)clinical deficits). For calculating FI-Lab21, a ratio between summed number of laboratory parameters that were out of the normal range and total number of examined parameters was built for each participant. To allege an example, an individual with pathological laboratory values for potassium, C-reactive protein, and total protein in spot urine, with normal values for the 18 further examined laboratory parameters, would have a summed number of three deficits, which yielded divided by 21 (total number of potential deficits) an FI-Lab21 score of 0.143. As explained in this example comprehensibly, the FI-Lab21 score ranked in magnitude from 0 to 1 for each participant. If one or more parameter was not surveyed, the total number by which divided FI-Lab21 was given, decreased by the count of unavailable parameters. To set an example for this particular case, a patient with a deficit in five variables, two missing values and 14 values within normal range would have an FI-Lab21 of 0.263 (5/(21–2)). FI-Lab21 was calculated only up to a minimum number of 17 parameters, which guarantees >80% of given variables for each case.
The FI-Lab21 was assessed twice during the hospital stay. One time with blood and urine sample obtained at admission (FI-Lab21admission) and second time with samples gained before discharge (FI-Lab21discharge). Moreover, the change in the FI-Lab21 during the hospital stay (∆FI-Lab21) of the study participants was calculated as difference between both aforementioned FI-Lab21s (FI-Lab21admission − FI-Lab21discharge). If ∆FI-Lab21 assumed positive values, FI-Lab21 decreased during hospital stay (ie, FI-Lab21 at admission > FI-Lab21 before discharge), a negative ∆FI-Lab21 signals an increment of FI-Lab21discharge compared with FI-Lab21admission.
Statistical analyses
Statistical analyses were accomplished by using IBM SPSS Statistics software (IBM Corp. Released 2017, IBM SPSS Statistics for Windows, version 25.0, IBM Corp., Armonk, NY, USA). Kolmogorov–Smirnov test was applied for testing normal distribution of values. Unless otherwise stated, results are presented as percentages, mean ± SD, or median (interquartile range). Comparing clinical characteristics was performed using chi-squared test and Mann–Whit-ney U-test where appropriate. Comparison of the change of the FI-Lab21 during the hospital stay, ie, FI-Lab21admission −FI-Lab21discharge, was performed using Wilcoxon signed-rank test. Furthermore, receiver operating characteristic curves were used to estimate area under the receiver operating characteristic curve (AUC) for FI-Lab21admission, FI-Lab21discharge, and various laboratory parameters considered solely in relation to 6-month and 1-year mortality. An AUC value of 0.5 indicates a model of random classification. AUC values >0.9 denotes “very good,” >0.8 “good,” >0.7 “useful” predictive ability.Citation35 Comparisons among AUCs were performed using the method of Hanley and McNeil.Citation36 Kaplan–Meier survival analyses were applied to estimate the increased odds of mortality for each category increase in FI-Lab21 score (0, 0.001–0.1, 0.101–0.2, 0.201–0.3, 0.301–0.4, 0.401–0.5, and >0.5) and ∆FI-Lab21 score (≥0 and <0). For this model, P-value was observed by Breslow test. To determine predictive accuracy of FI-Lab21admission and FI-Lab21discharge for survival, Cox proportional hazard models were applied. For this aforementioned analysis, FI-Lab21 scores were multiplied by 100 and rounded to integers, converting FI-Lab21 scores to percentage values for modeling. HRs for the FI-Lab21 scores were both analyzed separately and adjusted for age and sex to take possible confounders into consideration. The level of statistical significance was set a priori at P<0.050.
Results
Clinical characteristics of the study cohort
A flowchart describing the process of inclusion and follow-up of the study participants is given in . Five hundred thirteen patients were included in the study (see ). The clinical characteristics of these 513 individuals are given in . For 13 of these 513 participants, <80% of the laboratory parameters that construct the FI-Lab21 were available (see ). These 13 patients were excluded from further analyses. The clinical characteristics of these 13 patients are given in . From all, the remaining 500 participants with availability of 80% or more of the laboratory parameters that construct the FI-Lab21 follow-up data could be obtained after 6 months of follow-up (see ). For six individuals, follow-up data could not be collected after 1-year of follow-up (see ). The clinical characteristics of the 500 patients with 80% or more FI-Lab21 parameters and the six individuals that were lost to follow-up after 1 year are shown in . Among aforementioned 500 individuals, the male participants were taller, revealed a higher body weight, and more often suffered from cancer, diabetes, and coronary infarction compared with the female individuals (see ). After 6 months of follow-up, 86 of the 500 participants (17.2%) had died due to any cause. After 1 year of follow-up, 114 of the 494 remaining study participants (23.1%) had died.
Table 1 Clinical characteristics at baseline examination for all study participants (n=513) and participants with <80% available FI-Lab21 parameters (n=13)
Table 2 Clinical characteristics at baseline examination for all study participants with >80% available FI-Lab21 parameters (n=500), divided by sex (n=165 and n=335, respectively) and participants lost to follow-up at 1-year follow-up after baseline examination (n=6)
Distribution of the FI-Lab21 at admission to hospital and before discharge from hospital
The FI-Lab21 did not show a normal distribution irrespective of whether it was assessed at admission to hospital or before discharge from hospital (both P<0.001). With the FI-Lab21admission, mean score was 0.33±0.15, the median 0.33, minimum 0, maximum 0.76, the first, fifth, 95th, and 99th percentiles observed were 0.05, 0.09, 0.57, and 0.71, respectively. With the FI-Lab21discharge, the mean score was 0.31±0.14, median 0.32, minimum measured 0, maximum 0.71, the first, fifth, 95th, and 99th percentiles ascertained were 0.00, 0.10, 0.53, and 0.67, respectively.
Ability of the FI-Lab21 at admission to hospital and before discharge from hospital to predict 6-month and 1-year mortality
The FI-Lab21admission and FI-Lab21discharge were both able to discriminate between individuals who had died and those who still were alive after 6 months and 1 year of follow-up (all P<0.05) (see ). The ability of the FI-Lab21 to predict 6-month as well as 1-year mortality was greater at the point in time before discharge from hospital compared with the point in time at admission to hospital (P=0.046 and P=0.040, respectively). Among the individual laboratory parameters that construct the FI-Lab21, white blood cells, red blood cells, platelets, quick value, partial thromboplastin time, calcium, urea, total protein in serum, creatinine, aspartate aminotransferase, gamma-glutamyl transferase, lactate dehydrogenase, albumin, C-reactive protein, and total protein in spot urine were able to predict 6-month and/or 1-year mortality when assessed at admission to hospital or before discharge from hospital (all P<0.05) (see ). With increasing scores of the FI-Lab21, the mortality rates of the study participants for 6 months and 1 year increased when the FI-Lab21 was applied at admission to hospital or before discharge from hospital (all P<0.001) (see and and ).
Table 3 FI-Lab21 at admission to hospital (FI-Lab21admission), FI-Lab21 before discharge to hospital (FI-Lab21discharge), and standard laboratory parameters compiling the FI-Lab21
Table 4 FI-Lab21 at admission to hospital (FI-Lab21admission), FI-Lab21 before discharge from hospital (FI-Lab21discharge), and the change of the FI-Lab21 during the hospital stay (∆FI-Lab21) stratified according to score with 6-month and 1-year mortality
Figure 2 Kaplan–Meier survival function: FI-Lab21 at admission to hospital stratified into seven groups with increasing scores.
Abbreviation: FI-Lab21, 21-item frailty index based on laboratory blood and urine tests.
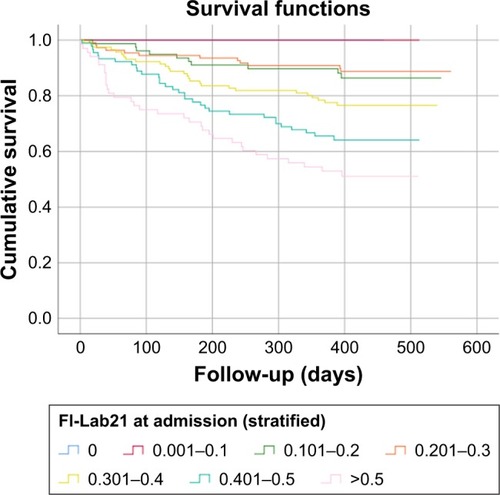
Figure 3 Kaplan–Meier survival function: FI-Lab21 before discharge from hospital stratified into seven groups with increasing scores.
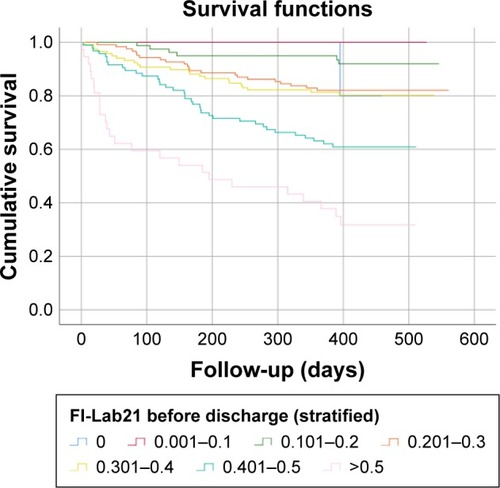
Each 1% increase in score of the FI-Lab21admission and FI-Lab21discharge was associated with an increase in mortality after 6 months and 1 year of follow-up ranging between 4.6% and 6.9%, respectively (all P<0.001). After adjustment for the analyses of age and sex, each 1% increase in score of the FI-Lab21admission and FI-Lab21discharge was associated with an increase in mortality after 6 months and 1 year of follow-up of 4.9% and 7.1%, respectively (all P<0.001) (see ).
Table 5 HR and age- and sex-adjusted HR for each 0.01 increment in score of FI-Lab21admission and FI-Lab21discharge in relation to 6-month and 1-year mortality
Ability of the change of the FI-Lab21 between the point in time at admission to hospital and before discharge from hospital to predict 6-month and 1-year mortality
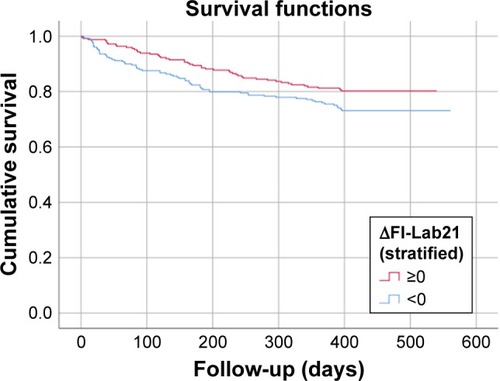
Among the individuals, the score of the FI-Lab21 decreased during the hospital stay with a mean reduction of the FI-Lab21 of 0.02±0.11 (P<0.001), with a minimum of −0.48 and a maximum of 0.43. The first, fifth, 95th, and 99th percentiles of the change in the FI-Lab21 during the hospital stay were −0.25, −0.14, 0.19, and 0.29, respectively (). Individuals whose FI-Lab21 score decreased or did not change during the hospital stay had a lower risk to die during the 6-month and 1-year follow-up period compared with those individuals whose FI-Lab21 score increased during the hospital stay (P=0.004 and P=0.046, respectively) (see ). Each 1% decrease of FI-Lab21 during the hospital stay was associated with a decrease in 6-month mortality of 2.0% (P=0.040) and, with reference to statistical nonsignificant terms, a reduction in 1-year mortality of 1.3% (P=0.126) (see ). After adjustment of the analysis for age, sex, and FI-Lab21admission, each 1% decrease in the FI-Lab21 during the hospital stay was associated with a decrease in 6-month and 1-year mortality of 5.9% and 5.3% (both P<0.001), respectively (see ).
Table 6 HR and HR adjusted for age, sex, and FI-Lab21admission for each 0.01 increment in score of ∆FI-Lab21 in relation to 6-month and 1-year mortality
Figure 4 Mean change of the FI-Lab21 during the hospital stay (n=500; mean ± standard error of the mean).
Abbreviation: FI-Lab21, 21-item frailty index based on laboratory blood and urine tests.
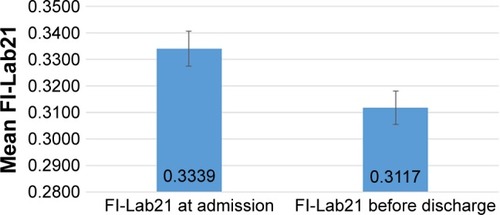
Figure 5 Kaplan–Meier survival function: change of the FI-Lab during the hospital stay (∆FI-Lab21) stratified into patients who suffered from an increase of their FI-Lab (∆FI-Lab21 ≥0) and those patients whose FI-Lab did not change or decreased (∆FI-Lab21 <0) during the hospital stay.
Discussion
In this study, we show that FI-Lab21 operationalized from common blood and urine tests obtained at admission to hospital of hospitalized geriatric patients reveals predictive ability for 6-month and 1-year mortality. We thereby expand the findings of our previous study showing a predictive ability of FI-Lab based on blood and urine parameters obtained before discharge from hospital of hospitalized geriatric patients for 6-month and 1-year mortality.Citation28 Our findings are in line with several other studies that demonstrated a predictive value of frailty instruments incorporating data that were obtained from patients at admission to the hospital for mortality or other adverse clinical outcomes.Citation3,Citation33,Citation34 Klausen et alCitation33 found a strong relation between FI-Lab based on 17 routine laboratory tests and 3-year mortality for 4,005 acutely admitted older patients aged 65 years or older at the Emergency Department of Copenhagen University Hospital, Denmark. Evans et alCitation3 showed that FI based on a comprehensive geriatric assessment (CGA) that was administered at admission to general medical wards in a cohort of 752 hospitalized participants aged 75 years or older predicted institutionalization, length of hospital stay, and 30-day mortality. In a study by Hubbard et al in 1,418 Australian hospitalized patients aged 70 years or older, FI obtained within the first days of hospitalization on general medical units was predictive for length of hospital stay, discharge to residential aged care, falls, pressure ulcer incidence, and in-hospital mortality.Citation34 This FI was operationalized based on a CGA including physical, mental, and cognitive functions, medications, medical diagnoses, and discharge destination.Citation34 In contrast to aforementioned studies,Citation3,Citation33,Citation34 we obtained data for the FI-Lab21 not only at admission but also before discharge.
A further interesting finding of our study is that the FI-Lab21 operationalized from blood and urine tests before discharge from hospital was more powerful compared with the FI-Lab21 obtained at admission to hospital. These findings indicate that the clinical state as evaluated with the FI-Lab21 before discharge from hospital after initial treatment of the acute disease or exacerbation of the chronic disease that had initially resulted in hospital admission stronger predicts 6-month and 1-year mortality than the more acute clinical state at hospital admission. The clinical state before discharge from hospital might probably better reflect to overall health status of a geriatric person than the clinical state driven by acute disease or exacerbation of the chronic disease at admission to the hospital geriatric ward of an older individual.
Of note, in our study during the hospital stay of the patients, the FI-Lab21 score decreased, ie, the FI-Lab21 operationalized from the data before discharge to hospital was lower compared with the FI-Lab21 based on data at admission to hospital. This indicates that the frailty severity of the patients, as assessed by the FI-Lab21, has decreased during their hospital stay. Possibly, the change in the FI-Lab21 between admission and discharge visualized performance and achievement of clinical interventions and therapies. Clearly, during their stay at the hospital geriatric wards, the patients received medical treatment and therapies by the physicians and other members of the multidisciplinary geriatric team. Our findings are thus in line with the concept that frailty reveals a potentially reversible state at least in some patients and is sensitive to adequate treatment and therapy.Citation37–Citation41 In line with the findings of the study presented here, Basile et alCitation37 revealed that a 46-item FI based on laboratory and clinical data tended to decrease during the hospital stay in 156 hospitalized patients on geriatric wards being followed up to 12 months. Fairhall et alCitation38 observed an increase in walking speed, more extended mobility, and a reduction of mobility-related disability in a randomized, controlled trail comprising 241 frail community-dwelling elderly in Sidney during a 12-month interventions program. Another randomized, controlled trial that was performed in 1994 by Fiatarone et al,Citation39 showed an improvement of muscle size and strength as a result of a 10-week high-intensity resistance training of older frail people compared with nonexercising persons (mean age of the 100 nursing home residents was 87.1±0.6 years). KimCitation40 investigated the effects of a 3-month exercise program and nutritional supplementation on improving frailty according to the frailty phenotype in 131 community-dwelling older people, finding an increase of muscle strength and bone mineral content. A recent random-ized, controlled study performed in Singapore in 246 participants with mean age of 70±4.7 years detected that nutritional, physical, and cognitive interventions were effective in reducing frailty (measured based on frailty phenotype criteria) during a follow-up period of 12 months.Citation41 In contrast to our study, none of these authors operationalized an FI based solely on laboratory parameters. In line with the findings of this study all aforementioned studies showed changeability and improvement of frailty by purposive interventions.Citation37–Citation41
A further major finding of our study presented here is that the individuals who showed an increase of the FI-Lab21 during the hospital stay had a higher 6-month and 1-year mortality rate compared with those persons whose FI-Lab21 score did not change or decreased (ie, improved) during the hospital stay at the hospital geriatric wards. This indicates that not only the FI-Lab21 irrespective of whether assessed at admission to hospital or before discharge from hospital but also the change of the FI-Lab21 reveals predictive power for 6-month and 1-year mortality in hospitalized geriatric patients. Our data thus show that patients who did not suffer from a deterioration of their frailty severity, as assessed by the FI-Lab21, during their hospital stay, revealed a better survival compared with those patients whose frailty status, as assessed by the FI-Lab21, worsened during the hospital stay at the geriatric wards. Moreover, after adjustment of the analysis for age, sex, and FI-Lab21admission, we found an association of the quantity of improvement of the FI-Lab21 during the hospital stay and the reduction in 6-month and 1-year mortality risk of the hospitalized geriatric patients. This indicates that also the changes of the FI-Lab21 during the hospital stay might be an interesting and additional tool to stratify mortality risk in hospitalized geriatric patients.
This study has some major strengths. To the best of our knowledge, this is the first study evaluating an FI based solely on laboratory parameters at different points in time for patients during hospital stay. The FI-Lab21 used in the present study was based solely on few routine laboratory parameters enabling easy implementation of this FI-Lab into clinical practice. Of note, the FI-Lab21 as evaluated in this study shows key characteristics of FI such as an upper limit of FI of ~0.7.Citation43,Citation44
This study has limitations. The study was a single-center analysis. Single-center studies might not be generalizable to a broad population. Of note, in some cases, single-center studies have been contradicted in subsequently larger multicenter studies.Citation45 In addition, the predominant part of our cohort was Caucasian. Further investigation is needed to extrapolate our findings to other ethnic groups. Apparently, according to the findings of Chen et al, comorbidity burden, mobility-related disability, and the impact of acute and chronic diseases might have driven the FI-Lab21admission, the FI-Lab21discharge, and the change of the FI-Lab21 during the hospital stay, intensifying their predictive power on individuals risk at death.Citation46 Furthermore, a total number of 21 health deficits as operationalized in our study might underestimate adverse outcomes. More robust estimates are measured with FIs consisting of 50 or more deficits.Citation47 Thus, our data have to be considered with caution.
In conclusion, the FI-Lab21 irrespective of whether assessed at admission to hospital or before discharge from hospital and the changes of the FI-Lab21 during the hospital stay at the geriatric wards emerged as interesting and feasible approaches to stratify the risk for 6-month and 1-year mortality in hospitalized individuals on geriatric wards. Thereby, the FI-Lab21 evaluated at the point in time before discharge from hospital might be more powerful than the FI-Lab21 operationalized from the data obtained at admission to hospital in predicting 6-month and 1-year mortality in aforementioned group of persons. Furthermore, also the quantity of change of the FI-Lab21 during the hospital stay reveals predictive power for mortality in hospitalized geriatric patients.
Acknowledgments
We acknowledge the support by Deutsche Forschungsge-meinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing. The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med.” by the Friedrich-Alexander-Universität Erlangen-Nürnberg (JJ).
Disclosure
The authors report no conflicts of interest in this work.
References
- RockwoodKA global clinical measure of fitness and frailty in elderly peopleCan Med Assoc J2005173548949516129869
- CleggAYoungJIliffeSRikkertMORockwoodKFrailty in elderly peopleLancet2013381986875276223395245
- EvansSJSayersMMitnitskiARockwoodKThe risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessmentAge Ageing201443112713224171946
- MousaASavvaGMMitnitskiAIs frailty a stable predictor of mortality across time? Evidence from the cognitive function and ageing studiesAge Ageing201847572172729905755
- PilottoARengoFMarchionniNComparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patientsPLoS One201271e2909022247767
- RittMBollheimerLCSieberCCGaßmannKGPrediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patientsArch Gerontol Geriatr201666667227259029
- TheouOBrothersTDMitnitskiARockwoodKOperationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortalityJ Am Geriatr Soc20136191537155124028357
- HoogendijkEOTheouORockwoodKOnwuteaka-PhilipsenBDDeegDJHHuismanMDevelopment and validation of a frailty index in the longitudinal aging study AmsterdamAging Clin Exp Res201729592793327896796
- CesariMLandiFVellasBBernabeiRMarzettiESarcopenia and physical frailty: two sides of the same coinFront Aging Neurosci2014619225120482
- KemmlerWTeschlerMGoisserSPrevalence of sarcopenia in Germany and the corresponding effect of osteoarthritis in females 70 years and older living in the community: results of the FORMoSA studyClin Interv Aging2015101565157326491272
- DornerTELugerETschinderleJAssociation between nutritional status (MNA®-SF) and frailty (SHARE-FI) in acute hospitalised elderly patientsJ Nutr Health Aging201418326426924626753
- ChristnerSRittMVolkertDWirthRSieberCCGaßmannKGEvaluation of the nutritional status of older hospitalised geriatric patients: a comparative analysis of a Mini Nutritional Assessment (MNA) version and the Nutritional Risk Screening (NRS 2002)J Hum Nutr Diet201629670471327298113
- HanlonPNichollBIJaniBDLeeDMcqueenieRMairFSFrailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participantsLancet Public Health201837e323e33229908859
- HalilMCemal KizilarslanogluMEmin KuyumcuMYesilYCruz JentoftAJCognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairmentJ Nutr Health Aging201519327628325732212
- Ní MhaoláinAMFanCWRomero-OrtunoRFrailty, depression, and anxiety in later lifeInt Psychogeriatr20122481265127422333477
- RittMSchüleinSLubrichHBollheimerLCSieberCCGaßmannKGHigh-technology based gait assessment in frail people: associations between spatiotemporal and three-dimensional gait characteristics with frailty status across four different frailty measuresJ Nutr Health Aging201721334635328244577
- WeissCOFrailty and chronic diseases in older adultsClin Geriatr Med2011271395221093721
- MorleyJEVellasBvan KanGAFrailty consensus: a call to actionJ Am Med Dir Assoc201314639239723764209
- MuscedereJAndrewMKBagshawSMScreening for frailty in Canada’s health care system: a time for actionCan J Aging2016350328129727211065
- RittMRádiKHSchwarzCBollheimerLCSieberCCGaßmannKGA comparison of frailty indexes based on a comprehensive geriatric assessment for the prediction of adverse outcomesJ Nutr Health Aging201620776076727499310
- RittMSchwarzCKronawitterVAnalysis of Rockwood et al’s clinical frailty scale and Fried et al’s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric wardJ Nutr Health Aging201519101043104826624218
- FriedLPTangenCMWalstonJFrailty in older adults: evidence for a phenotypeJ Gerontol A Biol Sci Med Sci2001563M146M15711253156
- MitnitskiABMogilnerAJRockwoodKAccumulation of deficits as a proxy measure of agingScientificWorldJournal2001132333612806071
- RockwoodKTheouOMitnitskiAWhat are frailty instruments for?Age Ageing201544454554725824236
- RittMRittJISieberCCGaßmannKGComparing the predictive accuracy of frailty, comorbidity, and disability for mortality: a 1-year follow-up in patients hospitalized in geriatric wardsClin Interv Aging20171229330428223787
- RockwoodKAndrewMMitnitskiAA comparison of two approaches to measuring frailty in elderly peopleJ Gerontol A Biol Sci Med Sci200762773874317634321
- RockwoodKRockwoodMRMitnitskiAPhysiological redundancy in older adults in relation to the change with age in the slope of a frailty indexJ Am Geriatr Soc201058231832320370858
- RittMJägerJRittJISieberCCGaßmannKGOperationalizing a frailty index using routine blood and urine testsClin Interv Aging2017121029104028721031
- BlodgettJMTheouOHowlettSERockwoodKA frailty index from common clinical and laboratory tests predicts increased risk of death across the life courseGeroScience201739444745528866737
- BlodgettJMTheouOHowlettSEWuFCRockwoodKA frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomesAge Ageing201645446346827076524
- HowlettSEStanleyJWongHMitnitskiARockwoodKA procedure to create a frailty index based on routinely-collected laboratory and clinical safety data in the setting of an Alzheimer’s disease clinical trialAlzheimers Dement2017137P1519
- RockwoodKMcMillanMMitnitskiAHowlettSEA frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilitiesJ Am Med Dir Assoc2015161084284725952475
- KlausenHHPetersenJBandholmTAssociation between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort studyBMC Geriatr20171716228249621
- HubbardREPeelNMSamantaMGrayLCMitnitskiARockwoodKFrailty status at admission to hospital predicts multiple adverse outcomesAge Ageing201746580180628531254
- SwetsJAMeasuring the accuracy of diagnostic systemsScience19882404857128512933287615
- HanleyJAMcNeilBJA method of comparing the areas under receiver operating characteristic curves derived from the same casesRadiology198314838398436878708
- BasileGCatalanoAMandraffinoGFrailty modifications and prognostic impact in older patients admitted in acute careAging Clin Exp Res Epub2018626
- FairhallNSherringtonCKurrleSELordSRLockwoodKCameronIDEffect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trialBMC Med201210112023067364
- FiataroneMAO’NeillEFRyanNDExercise training and nutritional supplementation for physical frailty in very elderly peopleN Engl J Med199433025176917758190152
- KimHInterventions for frailty and sarcopenia in community-dwelling elderly womenNihon Ronen Igakkai Zasshi201249672673023883636
- NgTPFengLNyuntMSNutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trialAm J Med2015128111225123626159634
- HowlettSERockwoodMRMitnitskiARockwoodKStandard laboratory tests to identify older adults at increased risk of deathBMC Med201412117125288274
- ArmstrongJJMitnitskiALaunerLJWhiteLRRockwoodKFrailty in the Honolulu-Asia aging study: deficit accumulation in a male cohort followed to 90% mortalityJ Gerontol A Biol Sci Med Sci201570112513124973228
- SearleSDMitnitskiAGahbauerEAGillTMRockwoodKA standard procedure for creating a frailty indexBMC Geriatr2008812418826625
- BellomoRWarrillowSJReadeMCWhy we should be wary of single-center trialsCrit Care Med200937123114311919789447
- ChenCSiaIMaHMThe synergistic effect of functional status and comorbidity burden on mortality: a 16-year survival analysisPLoS One201498e10624825170612
- RockwoodKMitnitskiAHow might deficit accumulation give rise to frailty?J Frailty Aging20121181227092931