Abstract
Objectives
To assess the effect of continuous positive airway pressure (CPAP) on nocturia in ischemic stroke patients with obstructive sleep apnea (OSA).
Methods
This was a prospective and non-randomized controlled study in which ischemic stroke patients with OSA being treated in a rehabilitation ward were enrolled. The participants who tolerated CPAP were classified as the CPAP group, while those who refused or could not tolerate CPAP were classified as the control group. The percentage change of nocturia before and after 2 weeks of CPAP therapy between the two groups were compared.
Results
A total of 44 participants were enrolled in and 35 participants (mean age= 59.8±11.7 years old; mean apnea hypopnea index=42.9±16.7/h) completed the study (control group: 14, CPAP group: 21). Overall, 69% of the participants had nocturnal polyuria and 69% of them had more than one nocturia episode per night. The baseline and initial nocturia characteristics did not differ significantly between the two groups. As compared to the control group, CPAP therapy significantly decreased the nocturnal polyuria index (mean percentage change: 9% vs −21% (P=0.005)) and nocturnal urine output (mean percentage change: 6% vs −26% (P=0.04)), but not the nocturia episodes or 24-hours total urine output.
Conclusion
Nocturia due to nocturnal polyuria is very common in post-stroke patients with OSA. Treating OSA by CPAP significantly reduces nocturnal polyuria, but not nocturia frequency, in ischemic stroke patients.
Introduction
Obstructive sleep apnea (OSA) is a common chronic disorder, with an estimated prevalence of ~4%–7% among the adult general populationCitation1 and an independent risk factor for ischemic stroke and cardiovascular death.Citation2 Stroke is the second leading cause of death worldwide, and a major cause of mental and physical impairment.Citation3 The prevalence of OSA iŝ60% in stroke patients,Citation4 which is substantially higher than the prevalence among the general population. Previously published data revealed that patients who survive a stroke and have OSA have an increased rate of mortality, especially mortality caused by cardiovascular events, compared to stroke survivors who do not have OSA.Citation2 The OSA patients also have worse neurological and functional statuses and longer periods of hospitalization than the patients without OSA.Citation5 Overall, OSA negatively affects short-term and long-term stroke outcomes, length of hospitalization, and recurrence risk.Citation6 These effects may occur via a number of pathogenic pathways influenced by intermittent hypoxemia, sympathetic stimulation, and hypertension that in turn influence cerebral vasoregulation and atrial fibrillation.Citation7
Nocturia is a common problem among the elderly. More than one nocturia episode per night is considered pathological, and nearly 60%–80% of elderly people have more than one episode per night.Citation8 Nocturia has been found to be an important risk factor for falls in ambulatory elders,Citation9 and elderly individuals who void three or more times per night have a greater mortality rate than those who void less often.Citation10 Global polyuria is defined as a 24-hour urinary output that exceeds 40 mL/kg body weight and results in increased 24-hour urinary frequency. Nocturnal polyuria is defined as >20% of daily urine output occurring at night in young patients and >33% occurring at night in elderly patients.Citation11 Fitzgerald’s studies showed that nocturia is a common symptom in OSA, with the prevalence of nocturia in OSA patients being positively correlated with OSA severity.Citation12 Another study also found that nocturia is an independent predictor for severe OSA after adjusting for age, gender, and neck circumference, and suggested that nocturia might be used to screen for OSA in patients with ischemic stroke.Citation13
Continuous positive airway pressure (CPAP) is the standard of therapy for OSA. CPAP therapy can reduce cardiovascular mortality, improve cognitive function, and improve quality-of-life.Citation14 Moreover, long-term CPAP treatment for OSA in ischemic stroke patients has been found to be associated with a reduction in the excess risk of mortality and improved functional neurologic outcomes.Citation15 Another study also found that CPAP significantly reduces the frequency of nocturia in non-stroke OSA patients;Citation16 however, the effects of CPAP on nocturia in ischemic stroke patients with OSA have yet to be well investigated. As such, the purpose of this study was to investigate the effects of CPAP on nocturia in ischemic stroke patients with OSA.
Materials and methods
Study subjects
This study was conducted in the rehabilitation wards of a teaching hospital from October 2013 to October 2016. All of the participants had been admitted to the wards for recent ischemic stroke (that is, a stroke that occurred more than 4 weeks before, but within the last 12 months) and were diagnosed with OSA. The diagnosis of ischemic stroke for each participant was made on the basis of a full clinical assessment with detailed neurological examinations and neuroimaging studies. OSA was defined by an apnea hypopnea index (AHI) of >15/hour with >50% of respiratory events being of the obstructive or mixed type. Participants with central sleep apnea (>50% of respiratory events being of the central type), sleep hypoventilation (arterial PCO2>55 mmHg for ≧10 minutes or increased arterial PCO2>10 mmHg in comparison to when awake with arterial PCO2>50 mmHg for ≧10 minutes), chronic obstructive airway disease, orofacial abnormality, urinary retention or incontinence, as well as patients who received other therapies like an oral appliance or soft palate surgery, were excluded. In addition, those patients who had an adjustment in their diuretic or medication for benign prostate hyperplasia (BPH) during the study period were also excluded, as were any potential participants who had a neurogenic bladder or active urinary tract infection during the study period. Meanwhile, those participants who refused CPAP therapy or could not tolerate it were classified as the control group. This study was approved by the institutional review board of Chang Gung Memorial Hospital (105–2690C), and all the subjects signed an informed consent form.
Risk factors survey and clinical presentation recording
All of the participants were screened for hypertension (a history of hypertension or systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg on the second day of admission) and diabetes mellitus (a history of diabetes mellitus or fasting blood glucose >126 mg/dL). The functional status (Barthel index),Citation17 polysomnography parameters, body weight, age, sex, and serum creatinine level of each participant was recorded. The presence of significant renal dysfunction (serum creatinine >1.4 mg/dL), congestive heart failure, poorly controlled diabetes mellitus, other genitourinary system diseases, or global polyuria (24-hour urine output of more than 40 mL/kg) was also recorded and reported because these factors might interfere with nocturia and make the association between nocturia and OSA more complicated. Medications for BPH, hypertension, and diabetes mellitus were considered confounding factors. Other medications, including hypnotics, anti-depressants, and lipid-lowering medications, may also influence nocturia. Thus, those medications were monitored during the urine frequency and volume recording period at both baseline and second-time measurements. If the medications were changed between the baseline and second-time measurements, then those changes were also recorded and reported.
Polysomnography
Standard overnight polysomnograms were performed using a computerized polysomnography system (N7000 Embla, Broomfield, CO). The parameters recorded were electroencephalograms, bilateral electrooculograms, submental and bilateral anterior tibialis electromyograms, electrocardiograms, nasal and oronasal airflow (nasal pressure and thermistor), arterial oxygen saturation by finger probe pulse oximetry, chest and abdominal movements (inductance plethysmography), body position, and sound intensity. Sleep stages were scored manually in 30-second epochs. Obstructive apnea was defined as the absence of airflow for at least 10 seconds in the presence of respiratory effort, while central apnea was defined as the absence of airflow without concurrent respiratory effort. Hypopnea was defined as any decrease in airflow >30% lasting >10 seconds and followed by either an oxygen desaturation of at least 3% or EEG arousal. The oxyhemoglobin desaturation index, defined as the number of desaturations per hour, was also calculated. All of the sleep stage and respiratory event scoring was completed according to the criteria of the America Academy of Sleep Medicine version 2012.Citation18
CPAP intervention protocol
The CPAP efficacy and compliance of the participants affected the study results. Therefore, a standard CPAP protocol was arranged to minimize the factors that influence CPAP compliance and to optimize the CPAP efficacy. Before receiving CPAP therapy, all the participants underwent education about the consequences of OSA and the benefits of CPAP treatment. Mask selection and mask wearing training were done before the initial night of CPAP therapy. Most of the subjects started with automated CPAP (AutoSet Spirit S8, ResMed, Sydney, Australia). The CPAP pressure generally started with a maximum pressure of 10–16 cm H2O and a minimum pressure of 4–6 cm H2O, after which the maximum and minimum pressures were adjusted according to the given patient’s residual AHI record and subjective complaints. Because the residual AHI and duration of CPAP usage are important, regular checks were conducted to ensure that the CPAP recording included the residual AHI, average hours of daily use, and leak value. CPAP tracking was performed on days 3, 7, 14, and 28 to ensure good mask fitting and adequate CPAP usage. Moreover, any CPAP-related side-effects were immediately addressed in order to optimize CPAP compliance.
Measurements of nocturia before and after CPAP therapy
The urinary frequency and volume of each subject were recorded at two time periods, with measurements over 3 consecutive days for each period. The baseline urine recording was conducted before the CPAP trial. The second urine recording for the CPAP group was only conducted for those subjects who could adequately use CPAP. Specifically, if a participant met the criteria of using CPAP longer than 4 hours every day and for >70% of the days in 1 week, then the urinary frequency and volume of that participant were measured. For the control group, if a participant did not take part in any CPAP trial, his or her urinary frequency and volume were measured for a second time beginning 2 weeks after the baseline measurement. The reason why the measurements were conducted 2 weeks apart was that it generally takes 1–2 weeks to evaluate CPAP intolerance or adequate usage. Meanwhile, if subjects in the control group had tried CPAP but found it intolerable, the second set of nocturia measurements was conducted 2 weeks after the patient stopped using CPAP, in order to minimize any effect from the CPAP. All of the participants were instructed to record the time and volume of each void, as well as their waking time and bedtime. Nocturia episodes are voids that occur between an individual’s bedtime and waking time. The first voiding of urine by a participant after waking in the morning without going back to sleep was regarded as nocturnal urine. The nocturnal polyuria index (NPI) was defined as the ratio of nocturnal urine production to 24-hours total urine production.Citation19 The frequency of nocturia, 24-hours total urine output, nocturnal urine output, and NPI were calculated by averaging the results of the 3 days of recordings. The percentage changes of nocturia episodes, 24-hours total urine output, nocturnal urine output, and NPI between the baseline and the second set of measurements were also calculated.
Statistics
The basic characteristics of the control and CPAP groups were compared with independent t-tests, and underlying diseases and medication usage were compared with the chi-squared test and Fisher’s exact probability test. The primary end-point consisted of comparing the improvement in nocturia (in terms of nocturia episodes, nocturnal urine output, 24-hours total urine output, and NPI) between those two groups. Those parameters were compared with independent t-tests. Statistical evaluations were performed using the SPSS 16.0 Software (SPSS Inc., Chicago, IL). Data were presented as mean±SD. P<0.05 was considered statistically significant.
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of Chang Gung Memorial Hospital (105-2690C) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
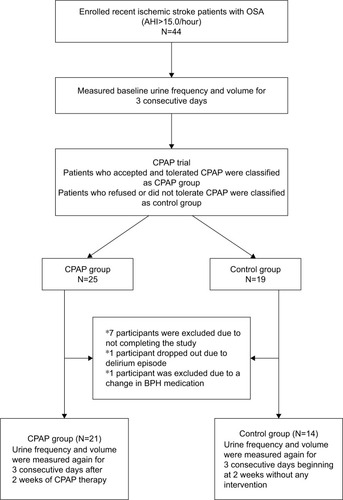
Forty-four participants were initially enrolled in our study, and the study process is shown in . There were seven participants who dropped out of the study because they were discharged from the hospital early and, thus, could not complete the study. One participant suffered from an episode of delirium during the study period and refused to finish the study. Another subject was excluded because the medication he was receiving for benign prostate hyperplasia was adjusted during the study period. As such, a total of 35 subjects (mean age=59.8±11.7 years old) completed the study. All of them were able to follow our study protocol. Most of the participants had moderate-to-severe OSA (mean AHI=42.9±16.7/hour). There were four participants who refused CPAP without taking part in any CPAP trial, and 10 participants who took part in a CPAP trial but could not tolerate CPAP. Those 10 participants tried CPAP only for a few days. These 14 participants were classified as the control group. The other 21 participants who could tolerate and accept CPAP were classified as the CPAP group. All of participants in the CPAP group used a nasal mask in the study period. The basic characteristics, underlying diseases, and medication adjustments of these two groups were not statistically different (). There were no participants who had a genitourinary system disease or global polyuria.
Table 1 Basic characteristics, underlying diseases, and medication adjustments
Figure 1 Flow diagram of the study.
The average AHI of the CPAP group was decreased from 43.0±17.3/hour to 6.5±4.9/hour after CPAP therapy. The average time of CPAP usage was 7.3±1.0 hours per night, and all of the participants used it for more than 70% of the days during the study period. Overall, 69% (control n=10, CPAP n=14) of the subjects had nocturnal polyuria according to the definition of a NPI >33%. Otherwise, there were 24 (69%; control n=9, CPAP n=15) patients who had more than one nocturia episode per night and 11 (31%; control n=4; CPAP n=7) patients with three or more nocturia episodes per night. There were no significant differences between the two groups in terms of baseline nocturia episodes, 24-hours total urine output, nocturnal urine output, and NPI (). As compared to the control group, CPAP therapy significantly decreased NPI (mean percentage change=9% vs −21% (P=0.005)) and nocturnal urine output (mean percentage change=6% vs −26% (P=0.04)), but not the nocturia episodes or 24-hours total urine output ().
Table 3 Percentage changes of nocturia between the control and CPAP groups after CPAP therapy
Table 2 Baseline nocturia of the control and CPAP groups
Discussion
Nocturia due to nocturnal polyuria is very common in post-stroke patients with OSA. To the best of our knowledge, this is the first study to report that CPAP therapy can significantly reduce nocturnal polyuria but not nocturia frequency in ischemic stroke patients with OSA.
Due to multiple comorbidities, CPAP therapy does not significantly improve nocturia frequency in ischemic stroke patients with OSA. Oztura et al’sCitation20 study revealed that the prevalence of nocturia is 52% in patients with primary snoring, 57.2% in patients with mild OSA, 64.3% in patients with moderate OSA, and 76.9% in patients with severe OSA. Their results show that the prevalence of nocturia increases with increasing OSA severity. In addition, several previous studies found that CPAP could effectively reduce the frequency of nocturia and night time urine volume, while also improving the quality-of-life in subjects without ischemic stroke.Citation16,Citation21,Citation22 The reasons why the nocturia frequency of the stroke patients in this study was not improved by CPAP use are not clearly understood, but we speculate that ischemic stroke patients are generally elderly individuals with multiple comorbidities, such as insomnia, BPH, bladder dysfunction, sleep disorder, and heart failure, who also usually use several kinds of medications. Those are all possible factors influencing nocturia and could result in large variations in nocturia frequency. A previous study also found that younger OSA patients experienced better nocturia improvements than elderly patients when receiving CPAP.Citation23 The results of this study support that finding and suggest that age-related urinary diseases and voiding dysfunction may mask the influence of CPAP on nocturia in ischemic stroke patients. Therefore, the effects of CPAP on nocturia in stroke patients are less obvious than in non-stroke and younger patients.
Hajduk et al’sCitation24 study reported that nocturnal polyuria was found in 47.8% of non-ischemic OSA patients, a rate which is considerably lower than the 69% rate found in our study. The older ages, ischemic strokes, and multiple comorbidities of the patients in the present study may all have played a role in that difference. Miyazato et al’sCitation22 study proved that CPAP could effectively reduce NPI and nocturnal urine output in non-stroke OSA patients, but no previous study had investigated the effects of CPAP on nocturia in ischemic stroke patients with OSA and multiple comorbidities. Our study is, thus, the first investigation of that type. OSA is caused by upper airway obstruction during sleep. The obstructed site is usually on the tongue or soft palate, and the obstruction leads to intermittent hypoxemia, increasing sympathetic activity, and altering hormone regulation.Citation25 In addition, airway obstruction following continuous breathing generates an abnormal airway pressure change. This results in the development of an extremely high negative airway pressure level caused by sucking through an obstructed airway. This in turn causes the heart to receive a false signal of volume overload. The hormonal response to this signal is increased atrial natriuretic peptide (ANP) secretion. The normal physiological response to atrial stretch is to excrete ANP, which is a natriuretic, diuretic, and vasorelaxant cardiac hormone.Citation26 ANP can also inhibit the secretion of arginine vasopressin (AVP), rennin-angiotensin, and aldosterone, while increasing glomerular filtration and further increasing nocturnal urine output.Citation27 AVP is the major hormone responsible for the regulation of urine formation. In healthy adults, the diurnal release of AVP into plasma has its peak during night time. The net effect of this AVP secretion is diminished urine production during the sleep interval.Citation28 Sakakibara et al’sCitation29 study revealed that post-stroke patients had nocturnal polyuria with abnormal circadian rhythm in terms of plasma AVP secretion, and Umlauf et al’sCitation26 study showed that plasma ANP levels were significantly higher among subjects with higher AHI levels. The possible mechanism of OSA in increasing nocturnal urine output could be that the interrupted sleep and increased ANP secretion cause diminished AVP levels. Meanwhile, CPAP could eliminate the respiratory events and reduce the airway pressure swings seen in OSA. Thus, the mechanism by which CPAP reduces nocturnal polyuria would be by abolishing the negative pressure swings and intermittent hypoxia of OSA by reducing ANP and normalizing AVP levels. As nocturnal urine output is less affected by insomnia, BPH, and other genitourinary system diseases, CPAP could thus significantly decrease nocturnal polyuria, but not the frequency of nocturia in stroke patients.
There are several limitations to this study. First, it recruited patients in a rehabilitation ward. Most of the patients were survivors of a recent ischemic stroke. However, some transient ischemic attack or minor stroke patients are not admitted for rehabilitation. Thus, such patients with minor strokes were not included in this study. In addition, patients who are comatose, too drowsy, and have an unstable clinical condition are not transferred to the rehabilitation ward. Therefore, the present study may have had a selection bias and may not represent the entire ischemic stroke population, and thus the results of the study may not be generalizable to minor or severe ischemic stroke patients. The second limitation was the lack of upper airway evaluation. Previous studies showed that the upper airway status, such as nasal obstruction and a floppy epiglottis, might influence OSA severity and CPAP compliance.Citation30 Because the upper airway was not routinely evaluated in our study, the impact of any upper airway abnormality on group allocation could not be assessed. Further studies are recommended to clarify this issue. The third limitation of this study was its relatively small sample size. This may have influenced the statistical power of the study, especially with respect to nocturia episodes. It is, thus, suggested that a large randomized controlled study be conducted to address this limitation.
Conclusion
In conclusion, CPAP treatment can significantly reduce nocturnal polyuria, but not nocturia frequency, in ischemic stroke patients with OSA. Treating comorbidities associated with nocturia while also providing CPAP is suggested as a way to substantially reduce nocturia in ischemic stroke patients with OSA.
Acknowledgments
The authors would also like to thank Chang Gung Memorial Hospital and Medical Research Council, Keelung, Taiwan, ROC, for assistance and financial support. This work was funded by the Chang Gung Medical Research Council of Keelung, Taiwan, ROC (Grant number CMRPG2C0263). The sponsor had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- PeppardPEYoungTBarnetJHPaltaMHagenEWHlaKMIncreased prevalence of sleep-disordered breathing in adultsAm J Epidemiol201317791006101423589584
- YaggiHKConcatoJKernanWNLichtmanJHBrassLMMohseninVObstructive sleep apnea as a risk factor for stroke and deathN Engl J Med2005353192034204116282178
- MurrayCJLopezADMortality by cause for eight regions of the world: global burden of Disease StudyLancet19973499061126912769142060
- HermannDMBassettiCLSleep-related breathing and sleep-wake disturbances in ischemic strokeNeurology200973161313132219841384
- AaronsonJAvan BennekomCAHofmanWFObstructive sleep apnea is related to impaired cognitive and functional status after strokeSleep20153891431143725669178
- MansukhaniMPBellolioMFKollaBPEnduriSSomersVKSteadLGWorse outcome after stroke in patients with obstructive sleep apnea: an observational cohort studyJ Stroke Cerebrovasc Dis201120540140520656506
- IferganeGOvanyanAToledanoRObstructive sleep apnea in acute stroke: a role for systemic inflammationStroke20164751207121227073238
- BoschJLWeissJPThe prevalence and causes of nocturiaJ Urol20131891 SupplS86S9223234639
- StewartRBMooreMTMayFEMarksRGHaleWENocturia: a risk factor for falls in the elderlyJ Am Geriatr Soc19924012121712201447437
- AsplundRMortality in the elderly in relation to nocturnal micturitionBJU Int199984329730110468725
- van KerrebroeckPAbramsPChaikinDThe standardisation of terminology in nocturia: report from the standardisation Sub-committee of the International continence SocietyNeurourol Urodyn200221217918311857672
- FitzgeraldMPMulliganMParthasarathySNocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatmentAm J Obstet Gynecol2006194513991403
- ChenCYHsuCCPeiYCYuCCChenYSChenCLNocturia is an independent predictor of severe obstructive sleep apnea in patients with ischemic strokeJ Neurol2011258218919420725736
- SteiropoulosPTsaraVNenaEEffect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndromeChest2007132384385117573492
- Martínez-GarcíaMASoler-CataluñaJJEjarque-MartínezLContinuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up studyAm J Respir Crit Care Med20091801364119406983
- MargelDShochatTGetzlerOLivnePMPillarGContinuous positive airway pressure reduces nocturia in patients with obstructive sleep apneaUrology200667597497716635510
- ShahSCooperBMaasFThe Barthel index and ADL evaluation in stroke rehabilitation in Australia, Japan, the UK and the USAAust Occup Ther J199239151321790640
- BerryRBBudhirajaRGottliebDJRules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions Task Force of the American Academy of sleep medicineJ Clin Sleep Med20128559761923066376
- van KerrebroeckPAnderssonKETerminology, epidemiology, etiology, and pathophysiology of nocturiaNeurourol Urodyn201433Suppl 1S2S5
- OzturaIKaynakDKaynakHCNocturia in sleep-disordered breathingSleep Med20067436236716564213
- MiyauchiYOkazoeHOkujyoMEffect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndromeUrology201585233333625623679
- MiyazatoMTohyamaKTouyamaMEffect of continuous positive airway pressure on nocturnal urine production in patients with obstructive sleep apnea syndromeNeurourol Urodyn201736237637926633747
- MaedaTFukunagaKNagataHObstructive sleep apnea syndrome should be considered as a cause of nocturia in younger patients without other voiding symptomsCan Urol Assoc J2016107–8E241E24528255415
- HajdukIAStrolloPJJasaniRRAtwoodCWHouckPRSandersMHPrevalence and predictors of nocturia in obstructive sleep apnea-hypopnea syndrome – a retrospective studySleep2003261616412627734
- MøllerDSLindPStrungeBPedersenEBAbnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apneaAm J Hypertens200316427428012670743
- UmlaufMGChasensERGreevyRAArnoldJBurgioKLPillionDJObstructive sleep apnea, nocturia and polyuria in older adultsSleep200427113914414998251
- SuttnerSWBoldtJNatriuretic peptide system: physiology and clinical utilityCurr Opin Crit Care200410533634115385748
- AbdelfatahDShakerHIsmailMEzzatMNocturnal polyuria and nocturnal arginine vasopressin (AVP): a key factor in the pathophysiol-ogy of monosymptomatic nocturnal enuresisNeurourol Urodyn200928650650919260089
- SakakibaraRUchiyamaTLiuZNocturnal polyuria with abnormal circadian rhythm of plasma arginine vasopressin in post-stroke patientsIntern Med200544428128415897635
- DedhiaRCRosenCASooseRJWhat is the role of the larynx in adult obstructive sleep apnea?Laryngoscope201412441029103424353028
