Abstract
Purpose
Producing tongue pressure (TP) by pushing the tongue against the palate consists of lifting the tongue muscles and elevating the floor of the mouth via suprahyoid muscle contraction. Though studies have shown that tongue-pressure resistance training (TPRT) increases tongue function, none have focused on suprahyoid muscle function enhancements. Our study aimed to verify whether TPRT improves both tongue function and hyoid movement during swallowing.
Materials and methods
Eighteen patients (mean age: 76.8±6.2 years) with presbyphagia presenting with symptoms such as coughing and choking were enrolled. All patients performed daily living activities independently. None of the participants had diseases causing dysphagia or previous oral or pharyngeal surgery. Participants were instructed to push their tongues against the palate as hard as possible with their mouths closed for 10 seconds, and then resting for 10 seconds. A set consisted of five consecutive exercise and resting periods; two sets per day were performed for a month. TP and the oral diadochokinetic rate (ODKR), measured by repetitions of the syllables /ta/ and /ka/, assessed tongue function. The extent of anterior and superior hyoid movement and parameters related to swallowing, including the penetration aspiration scale (PAS) and the normalized residue ratio scale (NRRS) in the valleculae (NRRSv) and piriform sinus (NRRSp), were evaluated based on videofluoroscopic data.
Results
The anterior (P=0.031) and superior hyoid movement (P=0.012), TP (P=0.002), ODKR/ta/ (P=0.034), ODKR/ka/ (P=0.009), and the width of the upper esophageal sphincter (P=0.001) were larger at follow-up than at baseline. NRRSp (P=0.022), PAS (P=0.016), and pharyngeal transit times (P=0.004) were smaller at follow-up than at baseline.
Conclusion
TPRT improved tongue strength, dexterity, both anterior and superior hyoid elevation, and swallowing functions. Therefore, TPRT could improve tongue function and suprahyoid muscle function simultaneously and contribute to prevention of sarcopenic dysphagia.
Introduction
Presbyphagia refers to the characteristic changes in the swallowing mechanism of healthy older adults that result from the normal aging process.Citation1 These changes have an impact on each stage of deglutition. Recently, dysphagia due to sarcopenia, known as sarcopenic dysphagia, was reported as a new concept.Citation2 It is well known that sarcopenia affects not only the general skeletal muscles, but also the swallowing muscles.Citation2–Citation5 For instance, decreased volume of the suprahyoid muscle is correlated with aspiration in healthy elderly adults.Citation4 Suprahyoid muscle plays an important role for opening upper esophageal sphincter (UES)Citation6,Citation7 and helps with swallowing. Decreasing suprahyoid strength affect UES opening can cause insufficient UES opening resulting in aspiration.Citation8 Furthermore, the tongue is affected by sarcopenia and is reportedly associated with dysphagia in healthy elderly people as well.Citation5 The tongue plays a significant role in not only transporting a bolus from the oral cavity into the pharynx, but also in pushing the bolus into the esophagus against the pharyngeal wall.Citation9 Therefore, a decline of tongue strength can cause oropharyngeal dysphagia.
To prevent sarcopenia, resistance training has been already reported as effective,Citation10,Citation11 and a resistance training regimen should be clearly designed to target vulnerable muscles for maximum effectiveness. Regarding preventing sarcopenia that could affect the tongue muscle, exercising the tongue by pushing it against the palate, thus producing tongue pressure (TP), increases the tongue strength and volume.Citation12 TP is generated by lifting the tongue against the palate and elevating the mouth floor. Specifically, immediately before producing TP, hyoid elevation caused by contraction of the suprahyoid muscle, resulting in mouth floor elevation, was observed.Citation13 Briefly, the action of producing TP consists of lifting the tongue via extrinsic and intrinsic tongue muscles and elevating the floor of the mouth by suprahyoid muscle contraction. Given these facts, the action of pushing the tongue against the palate to produce pressure has the potential to simultaneously enhance both tongue and suprahyoid muscle function. However, though there are many studies indicating that tongue-pressure resistance training (TPRT) increases tongue function,Citation12,Citation14 none have shown that the exercises enhance suprahyoid muscle function. Hence, the aim of our study was to verify whether TPRT improves both tongue function and hyoid movement during swallowing.
Materials and methods
Trial design
This before–after study was carried out in accordance with the Declaration of Helsinki of 1975, as revised in 2013, and was approved by the ethics committee of the School of Tokyo Medical Dental University (D2018-007). Written informed consent was obtained from all participants.
Participants
Eighteen independent, elderly people were assessed for recruitment in the Kobayashi Dental Clinic and Tokyo Medical and Dental University. Eligibility criteria included age >65 years, no history of illnesses causing dysphagia, such as cerebral vascular disease and neuromuscular disease, the ability to eat regular food, and complaints of swallowing problems such as coughing and choking during eating (presbyphagia). In addition, an overall stable condition and no previous experience with swallowing muscle exercises were the other inclusion criteria. Exclusion criteria were an inability to comprehend our instructions due to poor cognitive function, severe dysphagia, and the presence of malignant tumors. In addition, individuals who had previously had surgery on the oral cavity or pharynx were excluded.
Intervention
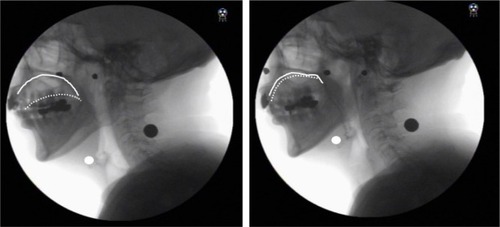
Participants were informed of the TPRT methods by a dentist. To perform TPRT, participants began by pushing their entire tongue against their palate as hard as possible for 10 seconds with their mouth closed (), and then rested for 10 seconds. They repeated the exercise and the resting period five times as a set and performed two sets a day for a month. To confirm each participant’s feasibility for performing TPRT, the examiner observed and checked hyoid elevation resulting from suprahyoid muscle contraction via videofluoroscopy during TPRT. Videofluoroscopy confirmed that all participants could perform TPRT. The subjects recorded their training history and submitted it at the end of the month-long training program, and a researcher collected the data. The achievement rate (exercise achievement days/30 days × 100%) was calculated as an index of the feasibility of TPRT.
Figure 1 Tongue-pressure resistance training.
Outcomes
Outcome variables were assessed at baseline and at a 4-week follow-up session. The primary outcomes included assessments of hyoid movement during swallowing and the position of the hyoid at rest. The secondary outcomes included tongue function and swallowing function.
Measurements
Videofluoroscopic measurement
Participants sat in a chair in a relaxed upright position during the assessment and were instructed to swallow 10 mL of thin barium (40% W/V). Then, the examiner instructed the participant to hold a bolus in his or her mouth with a neutral neck position before swallowing. Lateral videofluoroscopic images were taken at a speed of 30 frames/s using the digital X-ray television system Flexavision (Shimadzu Medical Systems Corp, Osaka, Japan) and video was recorded with a digital recorder (DV-AC82; Sharp Corporation, Osaka, Japan). Afterward, the video was converted to an MP4 format and loaded on to a personal computer. A coordinate system was established with the y-axis forming a straight line connecting the lower edges of the second and fourth cervical vertebrae and the x-axis forming a straight line drawn perpendicular from the lower end of the fourth cervical vertebra. The hyoid was defined as the anterior-upper edge of the hyoid bone (). To evaluate displacement of the hyoid during swallowing, two frames (the maximum anterior-superior position of the hyoid during swallowing and the resting position of the hyoid) were selected.
Figure 2 Lateral radiogram demonstrating the x- and y-axes.
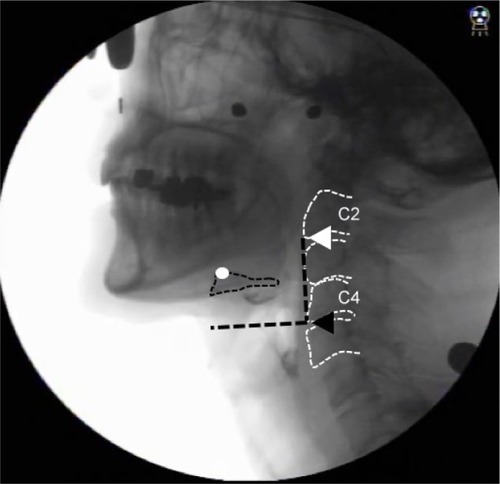
Based on a previous study,Citation15 the resting position of the hyoid was defined as the lowest position of the hyoid bone post-swallow, with concurrent epiglottic return and pharyngeal relaxation.
Hyoid anterior movement was defined as the distance between the resting position of the hyoid on the x-axis and the maximum anterior-superior position on the x-axis. Hyoid superior movement was defined as the distance between the resting position of the hyoid on the y-axis and its maximum anterior-superior position on the y-axis.
The UES opening was measured when the esophagus was maximally opened during the barium swallow by identifying the minimum anterior-posterior distance in the segment between cervical vertebrae C3 and C6. Pharynx transit time (PTT) was measured from the arrival of the bolus head at the ramus of the mandible to the time when the bolus tail passed through the UES.
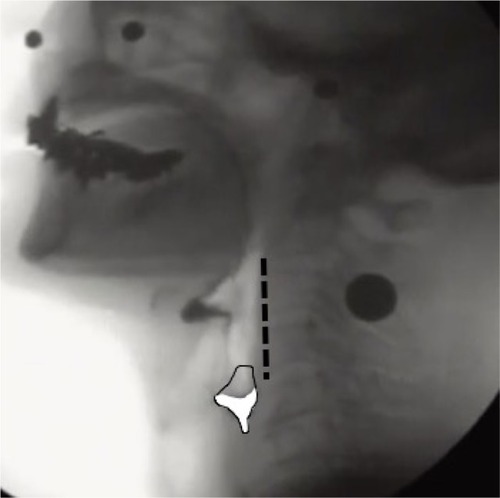
Following a previous studyCitation16 demonstrating an approach to avoid the effects of gender differences and height on the study results, measurements including hyoid movement and the width of UES opening were normalized by dividing them by the length of the straight line connecting the lower ends of the second and fourth cervical vertebrae on the y-axis (length C2 to C4); these measurements were obtained as a ratio (). Furthermore, we evaluated the presence of penetration and aspiration using the penetration aspiration scale (PAS).Citation17 For measuring the amount of pharyngeal residue, a continuous pixel-based scale of residue measurement, the normalized residue ratio scale (NRRS)Citation15,Citation18 was used. The complete procedure has been described in detail in earlier studies.Citation8,Citation15,Citation18 Briefly, NRRS was calculated separately for post-residue in the valleculae (NRRSv) and the piriform sinus (NRRSp) to determine the amount of residue (residue area), size of the sinus (spatial housing area), and the length from C2 to C4 as an internal anatomical reference using the software ImageJ (version 1.37; National Institutes of Health, Rockville, MD, USA). The frame of the resting position of the hyoid (as mentioned earlier) was used for analyzing the NRRS, based on a previous study.Citation18 NRRS was calculated using the formula: NRRS = (residue area/housing area) × (residue area/[the length from C2 to C4]Citation2×10) ().
Figure 3 Example of pixel-based measurement of pharyngeal residue in the piriform sinus.
To assess the reliability of videofluoroscopic measurements, we calculated the interclass correlation coefficients (ICCs). The intra- and interrater reliability ICCs of hyoid anterior movement were 0.93 and 0.99, respectively; of hyoid superior movement were 0.97 and 0.94, respectively; of the UES width were 0.97 and 0.95, respectively; of PTT were 0.96 and 0.99, respectively; of NRRSv were 0.847 and 0.866, respectively; and of NRRSp were 0.899 and 0.879, respectively.
In addition, the researcher who analyzed the videofluo-roscopic measurement data was blinded to whether it was baseline or follow-up data.
Assessment of tongue function
In this study, tongue function was divided into two categories: strength and dexterity. Tongue strength was defined as the maximum TP measured using a JMS tongue pressure manometer (JMS Co. Ltd, Tokyo, Japan). The balloon on the device was positioned on the dorsal side of the tongue with the lips closed. The participants were instructed to lift their tongue and compress the balloon toward the palate as hard as possible. The measurement was performed three times for each participant and the average was recorded. Tongue dexterity was defined as oral diadochokinesis;Citation19,Citation20 participants were given instructions to repeat the syllables /ta/ and /ka/ as quickly as possible for 5 seconds each. Pronouncing the syllable /ta/ is used as an index of dexterity of the anterior part of tongue, while pronouncing the syllable /ka/ is used as an index of dexterity of the base of tongue. The total number of appropriately pronounced syllables was automatically counted by a device. The repetition speeds were calculated as repetitions per second (times/s) and were recorded as the oral diadochokinetic rates ODKR/ta/ and ODKR/ka/.
Sample size calculation
We calculated our sample size by using G*power software (G*Power 3.1 software; Kiel University, Kiel, Germany). To determine an appropriate effect size (ES), we referred to a previous studyCitation10 about the effect of TPRT on TP (ES, d=1.0, large ES) because TPRT should affect suprahyoid muscles similar to tongue muscles. We determined that our sample size should include at least eleven individuals with a power of 0.8, an ES of 0.8, and an alpha of 0.05.
Statistical analyses
To verify the normality of the data, the Shapiro–Wilk test was utilized. Afterward, a paired t-test for parametric data and the Wilcoxon signed-rank test were used to compare the measurements for nonparametric data between baseline and follow-up. A P-value <0.05 was considered statistically significant. The ES was calculated and defined as large for r>0.5, medium for 0.3< r<0.5, small for 0.1< r<0.3, and no effect for r<0.1. Furthermore, correlations among PAS, NRRSv, and NRRSp and the other variables including hyoid movement and tongue function were assessed using bivariate correlation analysis with Pearson’s correlation analysis for parametric data and Spearman’s rank test for nonparametric data. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
The mean participant age was 76.8±6.2 years (men: eleven, women: seven). Some participants took medicine for ailments such as hypertension and diabetes. However, none of the participants took medicines that affected swallowing function. The median achievement rate for TPRT was 100% (IQR, 100%–100%). Improvement in symptoms was seen in 15 of 18 (72.2 %) participants. shows that the anterior and superior hyoid movements during swallowing were significantly larger at follow-up than at baseline (anterior hyoid movement: P=0.031, r=0.51, large ES; superior hyoid movement: P=0.012, r=0.65, large ES). Furthermore, TP, ODKR/ta/, and ODKR/ka/ were significantly larger at follow-up than at baseline (TP: P=0.002, r=0.65, large ES; ODKR/ta/: P=0.034, r=0.49, large ES; ODKR/ka/: P=0.009, r=0.61, large ES). Regarding changes in the swallowing function, UES opening widths were larger at follow-up than at baseline (P=0.001, r=0.82, large ES), while PTT was shorter at follow-up than at baseline (P=0.004, r=0.68, large ES) (). The PAS scores at follow-up were smaller than that at baseline (P=0.016, r=0.57, large ES). NRRSp at follow-up was smaller than that at baseline (P=0.022, r=0.54, large ES), but there was no significant difference in the NRRSv between follow-up and baseline (P=0.09, r=0.40, medium ES) (). shows that the PAS scores at baseline significantly correlated with TP (r=0.610) and UES opening width (r=0.675). In addition, NRRSv at baseline was significantly correlated with ODK/ta/ (r=−0.516) and ODK/ ka/ (r=−0.499). There was a significant correlation between NRRSv and ODKR/ka/ (r=−0.530) at follow-up. NRRSp at follow-up was significantly correlated with UES opening width (r=−0.497).
Table 3 Correlations between PAS scores and NRRS and the other variables
Table 2 Pre- and post-exercise swallowing function comparison
Table 1 Pre- and post-exercise comparison of hyoid bone kinematics
Discussion
The effectiveness of TPRT in assessing tongue and suprahyoid muscle function
Our study revealed that TPRT increased TP, which is consistent with previous studies.Citation12,Citation14 In addition, our study showed that TPRT increased ODKR/ta/ and ODKR/ka/, which we used as an index of tongue dexterity. Our study is the first to demonstrate that tongue resistance training improves tongue dexterity. ODKR is widely used for evaluating oral motor skillsCitation19 less affected by lingual factors.Citation20 Speech motor control disruptions are generally reported in older adults who perform movements more slowly.Citation21 Therefore, the ODKR decline associated with aging affects tongue muscles and motor neurons that control tongue muscles. The time course for gaining strength with resistance training accounts for initial neural adaptations, such as an increased motor unit discharge rate and a progressive enhancement in motor unit synchro-nization, prior to the muscle hypertrophy.Citation22 Therefore, it is considered that TPRT enhances motor neurons that control the tongue, resulting in an increased ODKR.
TP is generated by lifting the tongue itself and elevating the mouth floor, which occurs due to hyoid elevation caused by the contraction of the suprahyoid muscle.Citation13 Importantly, a surface electromyogram study showed that TP is correlated with the activity of the suprahyoid muscle.Citation23 Therefore, it is reasonable that TPRT could enhance suprahyoid muscle function, resulting in improved superior and anterior hyoid elevation during swallowing.
TPRT and swallowing function
Superior hyolaryngeal excursions during swallowing are thought to contribute to airway protection and prevent aspiration; anterior hyolaryngeal excursions are thought to be related to UES opening.Citation6,Citation7 Therefore, our finding that the width of the UES was improved at follow-up compared to baseline measurements is reasonable. Furthermore, PAS scores at follow-up significantly decreased compared with that at baseline (P=0.016, r=0.57, large ES). The PAS scores at baseline were moderately correlated with superior hyoid movement despite not being significantly different (r=−0.411). It is likely that increased superior hyoid movement after the TPRT program might have contributed to improvement in the PAS scores. In addition, there was a significant correlation between the TP at baseline and the PAS scores (r=−0.475). As the tongue plays a significant role in pushing the bolus into the esophagus against the pharyngeal wall,Citation9 decreased TP causes oropharyngeal dysphagia. However, contrary to our expectations, there was a strong positive correlation between the width of the UES and the PAS scores at baseline. The reason for this might have been the presence of a confounding variable or compensatory mechanism of increase in the width of the UES for preventing aspiration and penetration. Interestingly, at follow-up, the correlation coefficient value between the width of the UES and the PAS scores was acceptable (r=−0.216). Given these results, the occurrence of aspiration or penetration in elderly with presbyphagia might be correlated with both decreased tongue muscle strength and decreased superior hyoid elevation during swallowing.
Regarding pharyngeal residue, we measured the amount of pharyngeal residue using a pixel-based method for quantitative analysis. Pharyngeal residue is influenced by different parameters, including movement at the base of the tongue,Citation24 pharyngeal constriction,Citation25 hyoid laryngeal elevation,Citation16,Citation26 narrower UES,Citation27 and pharyngeal shortening.Citation28 Regarding pharyngeal residue in the valleculae, NRRSv at follow-up was smaller than that at baseline (P=0.09, r=0.40, medium ES). Tongue driving forces reportedly play a major role in avoiding residue in the vallecula because the tongue base motions during swallowing diminish the amount of residue.Citation24 In our study, the ODKR/ta/ and ODKR/ka/ at baseline were significantly correlated with NRRSv (r=−0.516 and r=−0.449, respectively) (). In addition, ODKR/ta/ and ODKR/ ka/ at follow-up were correlated with NRRSv (r=−0.448 and r=−0.530, respectively). We believe that ODKR/ta/ and /ka/ might reflect decline of tongue movement. Nevertheless, the reason why a significant correlation between TP and NRRSv was not observed in this study remained unclear. One reason could be that the presence of confounding variables was not adjusted in this analysis due to the small sample size. Another possible explanation could be that there were not enough participants with remarkably low TPs. However, we presume that improved tongue function, including TP and ODKR/ta/ and /ka/, contributed to the decreased NRRSv rather than improved hyoid movement during swallowing, after the TPRT program.
Regarding pharyngeal residue in the piriform sinus, NRRSp at follow-up was significantly smaller than that at baseline (P=0.022, r=0.54, large ES). In addition, UES opening width at follow-up was significantly larger than that at baseline (P=0.001, r=0.82, large ES). Decreased anterior hyolaryngeal displacement contributes to reduced opening of the UES, resulting in the piriform sinus residue.Citation27 Therefore, it is reasonable that there was significant correlation between the UES opening width and NRRSp (r=−0.497). However, there was unexpected correlation at baseline between NRRSp and anterior hyoid movement (r=0.449). UES were opening archived by hyolaryngeal elevation during swallowing along with cricopharyngeal muscle relaxation.Citation6,Citation7 It is possible that some participants with insufficient cricopharyngeal muscle relaxation were included in the present study. However, it is notable that the median (IQR) of NRRSp at follow-up was 0 (0–0), which indicates that the pharyngeal residue in the piriform sinus in many of the participants had almost disappeared at follow-up. Therefore, the unreasonable correlation between NRRSp and anterior hyoid movement at follow-up was probably caused by sample bias. However, we presume that improved UES opening width resulting from improved suprahyoid muscle function contributed to the decreased pharyngeal residue in the piriform sinus.
On the other hand, as the participants in the present study did not have severe dysphagia, both NRRSv and NRRSp values were smaller compared with those reported in participants with dysphagia,Citation8,Citation28 even though the values of NRRSv and NRRSp at baseline in the present study were above the reported normal values of NRRS (NRRSv: mean 0.01, 95% CI 0.00–0.02; NRRSp: mean 0.00, 95% CI 0.00–0.00).Citation28 Therefore, further studies are needed in participants with dysphagia for verifying the effect of TPRT on the swallowing function. However, a previous study that demonstrated that TPRT using balloon tubes improved the swallowing function in stroke dysphagia patients supports the results of our study.Citation14
Clinical implications
Some researchersCitation29,Citation30 suggest that older adults are more vulnerable in terms of transitioning from the healthy swallowing, seen normally in older individuals, to experiencing dysphagia, due to the decreased muscle reserve with aging; this is especially seen with additional stressors such as acute illnesses or certain medications. Regarding the suprahyoid muscle, the amount of hyoid elevation during swallowing is affected by aging, especially in older men,Citation29 which is thought to be caused by the loss of muscle reserve leading to decline of suprahyoid muscle strength.Citation3 In addition, another study showed that for safe swallowing, older women may be working harder by increased hyoid elevation during swallowing, compared with young womenCitation31 to compensate for the decreased strength in swallowing-related muscles.Citation3 Therefore, it is essential to perform effective and efficient resistance training for the swallowing muscles in elderly with presbyphagia.
For elderly individuals with age-related decline of both tongue and suprahyoid muscle strength, TPRT should be performed to improve the strength of both the tongue and the suprahyoid muscles. Regarding the use of TPRT for improving the strength of the suprahyoid muscle and the hyoid elevation during swallowing, some regimens target the suprahyoid muscle, such as the Shaker exerciseCitation32 and the jaw-opening exercise.Citation33 However, the Shaker exercise has some limitations including a low achievement feasibility and a strenuous protocol. In a study of 26 elderly adults (aged 66–93 years) without swallowing problems, only 50% of participants completed the prescribed isometric goals and only 70% completed the prescribed isokinetic goals in an exercise regimen that spanned 6 weeks.Citation34 In addition, some researchers reported that the strenuous protocol decreased compliance due to sternocleidomastoid muscle discomfort, especially in elderly, frail patients.Citation34,Citation35 Furthermore, patients who cannot lift their heads and flex their necks, such as those with cervical spondylosis, cannot perform this exercise, nor can individuals with a temporomandibular joint disorder. However, TPRT can be performed by individuals with temporomandibular joint disorders and can be easily carried out without using a tool. Further, TPRT enhances tongue and suprahyoid function simultaneously.
Limitations
Though our study suggests new findings, there are some limitations. First, there were no participants with severe dysphagia. Second, the sampling data of hyoid bone movement during swallowing were inadequate. As the participants did not have severe dysphagia, we avoided giving the participants large volumes of barium to drink due to ethical considerations in view of the side effects of ingesting barium such as vomiting, discomfort, and intestinal obstruction. In addition, the reason for avoiding performing multiple swallowing movements during the videofluoroscopic study was to avoid radiation exposure in the participants. In future studies, we would ensure obtaining appropriate sampling data of hyoid movement during swallowing in participants with severe dysphagia. In addition, we need to assess the effects of TPRT for individuals with severe dysphagia. Third, this study was a before–after study; thus, information bias could not be adjusted, even though videofluoroscopy measurements were blinded with regard to whether the data were before or after. Further studies are needed to verify the effectiveness of TPRT in randomized, controlled studies.
Conclusion
TPRT improved tongue strength, tongue dexterity, both anterior and superior hyoid elevation, and swallowing function. Therefore, our results suggest that TPRT can improve tongue and suprahyoid muscle function simultaneously, and is important for preventing sarcopenic dysphagia.
Ethics approval and informed consent
This study was approved by the ethics committee of the School of Tokyo Medical Dental University (D2018-007). Informed consent was obtained from all participants.
Disclosure
The authors report no conflicts of interest in this work.
References
- WakabayashiHPresbyphagia and sarcopenic dysphagia: association between aging, sarcopenia, and deglutition disordersJ Frailty Aging2014329710327049901
- OgawaNMoriTFujishimaIUltrasonography to measure swallowing muscle mass and quality in older patients with sarcopenic dysphagiaJ Am Med Dir Assoc201819651652229287693
- HaraKToharaHKobayashiKAge-related declines in the swallowing muscle strength of men and women aged 20–89 years: a cross-sectional study on tongue pressure and jaw-opening force in 980 subjectsArch Gerontol Geriatr201878647029902686
- FengXToddTLintzenichCRAging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adultsJ Gerontol A Biol Sci Med Sci201368785386023112114
- ButlerSGStuartALengXThe relationship of aspiration status with tongue and handgrip strength in healthy older adultsJ Gerontol A Biol Sci Med Sci201166445245821300744
- JacobPKahrilasPJLogemannJAShahVHaTUpper esophageal sphincter opening and modulation during swallowingGastroenterology1989976146914782583413
- CookIJDoddsWJDantasROOpening mechanisms of the human upper esophageal sphincterAm J Physiol19892575 Pt 1G748G7592596608
- MolfenterSMSteeleCMThe relationship between residue and aspiration on the subsequent swallow: an application of the normalized residue ratio scaleDysphagia201328449450023460344
- FeltonSMGaigeTAReeseTGWedeenVJGilbertRJMechanical basis for lingual deformation during the propulsive phase of swallowing as determined by phase-contrast magnetic resonance imagingJ Appl Physiol (1985)2007103125526517395759
- LiuCJLathamNKProgressive resistance strength training for improving physical function in older adultsCochrane Database Syst Rev20093CD002759
- PetersonMDSenAGordonPMInfluence of resistance exercise on lean body mass in aging adults: a meta-analysisMed Sci Sports Exerc201143224925820543750
- RobbinsJGangnonRETheisSMKaysSAHewittALHindJAThe effects of lingual exercise on swallowing in older adultsJ Am Geriatr Soc20055391483148916137276
- HoriKTaniguchiHHayashiHRole of tongue pressure production in oropharyngeal swallow biomechanicsPhysiol Rep201316e0016724400166
- KimHDChoiJBYooSJChangMYLeeSWParkJSTongue-to-palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagiaJ Oral Rehabil2017441596427883209
- MolfenterSMSteeleCMVariation in temporal measures of swallowing: sex and volume effectsDysphagia201328222623323271165
- SteeleCMBaileyGLChauTThe relationship between hyoid and laryngeal displacement and swallowing impairmentClin Otolaryngol2011361303621414151
- RosenbekJCRobbinsJARoeckerEBCoyleJLWoodJLA penetration-aspiration scaleDysphagia199611293988721066
- PearsonWGMolfenterSMSmithZMSteeleCMImage-based measurement of post-swallow residue: the normalized residue ratio scaleDysphagia201328216717723089830
- FletcherSGTime-by-count measurement of diadochokinetic syllable rateJ Speech Hear Res19721547637704652397
- KentRDKentJFRosenbekJCMaximum performance tests of speech productionJ Speech Hear Disord19875243673873312817
- WohlertABReflex responses of lip muscles in young and older womenJ Speech Hear Res19963935785898783136
- MoritaniTDevriesHANeural factors versus hypertrophy in the time course of muscle strength gainAm J Phys Med1979583115130453338
- PalmerPMJaffeDMMccullochTMFinneganEMvan DaeleDJLuscheiESQuantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generationJ Speech Lang Hear Res200851482883518658054
- DejaegerEPelemansWPonetteEJoostenEMechanisms involved in postdeglutition retention in the elderlyDysphagia199712263679071804
- EkbergONylanderGPharyngeal constrictor paresis in patients with dysphagia: a cineradiographic studyClin Radiol19823332532587075127
- MurrayJManual of Dysphagia Assessment in AdultsSan Diego, CASingular Publication Group Inc19999495
- OlssonRCastellJJohnstonBEkbergOCastellDOCombined videomanometric identification of abnormalities related to pharyngeal retentionAcad Radiol1997453493549156231
- PalmerJBTanakaEEnsrudEMotions of the posterior pharyngeal wall in human swallowing: a quantitative videofluorographic studyArch Phys Med Rehabil200081111520152611083359
- LogemannJAPauloskiBRRademakerAWColangeloLAKahrilasPJSmithCHTemporal and biomechanical characteristics of oropharyngeal swallow in younger and older menJ Speech Lang Hear Res20004351264127411063246
- RobbinsJLevineRWoodJRoeckerEBLuscheiEAge effects on lingual pressure generation as a risk factor for dysphagiaJ Gerontol A Biol Sci Med Sci1995505M257M2627671027
- LogemannJAPauloskiBRRademakerAWKahrilasPJOropharyngeal swallow in younger and older women: videofluoroscopic analysisJ Speech Lang Hear Res200245343444512068997
- ShakerRKernMBardanEAugmentation of deglutitive upper esophageal sphincter opening in the elderly by exerciseAm J Physiol19972726 Pt 1G1518G15229227489
- WadaSToharaHIidaTInoueMSatoMUedaKJaw-opening exercise for insufficient opening of upper esophageal sphincterArch Phys Med Rehabil201293111995199922579648
- EasterlingCGrandeBKernMSearsKShakerRAttaining and maintaining isometric and isokinetic goals of the Shaker exerciseDysphagia200520213313816172822
- YoonWLKhooJKPRickard LiowSJChin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exerciseDysphagia201429224324824337867
