Abstract
Purpose
We measured dorsal and ventral thoracic 12 vertebral (T12V) body heights as a way to predict lumbar vertebral fracture (LVF) in postmenopausal women. MRI of dorsal and ventral T12V body heights has not yet been used to investigate their association with LVF. We hypothesized that the dorsal and ventral T12V body height are important morphologic parameters in the prediction of LVF.
Patients and methods
In total, 80 osteoporotic patients with LVF (LVF group) and 80 osteoporotic patients without LVF (control group) were examined by MRI at the lumbothoracic level. Sagittal T2-weighted MRI images in the T12 level were obtained from all subjects. We analyzed both the dorsal and ventral T12V body height. The difference in dorsal and ventral body heights of the control and LVF patients was calculated at the T12V level.
Results
The average dorsal T12V body height was 21.25±1.64 mm in the control group and 20.11±1.49 mm in the LVF group. The average ventral T12V body heights were 19.51±1.54 mm and 17.62±1.95 mm, respectively. The LVF group had significantly lower dorsal and ventral T12V body heights (both P<0.001). ROC curve analysis showed the best cut-off value for dorsal T12V body height value of 20.74 mm, with 62.5% sensitivity and 60.0% specificity. The best cut-off point of ventral T12V body height was 18.76 mm, with 68.8% sensitivity and 67.5% specificity.
Conclusion
This study confirmed the association between dorsal and ventral T12V body height and occurrence of LVF in postmenopausal women with osteoporosis. Dorsal and ventral T12V body height were both significantly associated with LVF, with ventral T12V body height being a more sensitive measurement parameter. Thus, to predict risk of LVF in patients, the treating physician should carefully inspect the ventral T12V body height.
Introduction
Numerous risk factors influence the occurrence of osteoporotic lumbar vertebral fracture (LVF). LVF is the most common osteoporotic fracture.Citation1–Citation5 The ability to predict risk factors for LVF is crucial because of the negative consequences of LVF that include chronic back pain, related functional disability, kyphosis, and height loss, all of which have major impacts on a patient’s quality-of-life; as well as the associated increases in morbidity and mortalitiy.Citation6–Citation20 Analysis of bone mineral density (BMD) using dual-energy X-ray absorptiometry (DEXA) is the most commonly used index of bone power, and a low BMD is the most important risk factor for LVF prediction.Citation21–Citation24 But, almost 50% of LVF occurs in patients with BMD above the World Health Organization (WHO) diagnosis threshold of osteoporosis (T-score≤2.5).Citation25 Along with vertebral bone strength, the contribution of microarchitecture to LVF has been investigated clinically and biomechanically.Citation26,Citation27 The incidence of spinal deformity and LVF increases with age. LVF results in decreased quality-of-life due to functional disability, pain that may last for months, and affects morbidity.Citation28 Various approaches have been used to find factors predictive of LVF. The strength of a vertebral body is determined by bone shape, size, bone mineral density, and indirectly by intrinsic properties of bone matrix, micro damage, and bone microarchitecture. Bone size has been demonstrated to be positively correlated with bone strength.Citation29 The macroarchitecture of bone has also been investigated semiquantitatively.Citation30 Vertebrae morphology is measured by depth, width, diameter, height, volume, and cross-sectional area, according to different imaging modalities (eg, DEXA, X-rays, and CT scan). Vertebral volume is considered to be an independent LVF risk factor.Citation31 However, no studies have evaluated the dorsal and ventral T12V body height as a morphological parameter for prediction of LVF. T12V is unique in that is represents a transition from thoracic to lumbar vertebrae. It is thoracic-like in that it contains superior articular facets and costal facets that allow for flexion and rotation. It is lumbar-like in that it has articular processes that do not allow for rotation, only flexion and extension. The T12V endures the most weight of all thoracic vertebrae, making it the strongest thoracic vertebra, but also the most susceptible to stress-related damage.Citation32 Thus, we hypothesized that the dorsal and ventral T12V body heights are important morphologic parameters in the prediction of LVF. Therefore, we used MRI to compare the dorsal and ventral T12V body heights between osteoporotic patients, with and without LVF. To our knowledge, dorsal and ventral T12V body heights have not yet been used to predict LVF.
Patients and methods
Protocol approval and patient consent
This research protocol was approved and reviewed by the Catholic Kwandong University, International St. Mary’s Hospital, Incheon, Institutional Review Board (IRB) (IRB protocol number: IS18RISI0077). Written informed consent according to the Declaration of Helsinki was obtained from each subject involved in this research.
Study population
We reviewed patients who underwent MRI between February 2018 and September 2018 and had been diagnosed with osteoporosis. We only included postmenopausal women over age 60 if they had clinical manifestations and radiological findings compatible with LVF (sudden manifestation of backache, limited spinal mobility, worsened pain on walking or standing, pain relief lying on back, height loss, disability, or deformity), and MRI performed within 1 month of the diagnosis that was available for review. We excluded vertebral abnormalities due to reasons other than osteoporosis, such as Scheuermann’s disease and osteoarthritis. And we also excluded patients with a history of previous neuropathic conditions, such as lumbar spinal cord injury, lumbar spine surgery, congenital lumbar spine defects, or space occupying lesions, such as tumors or cysts.
We enrolled a total of 80 patients after the osteoporotic LVF diagnosis was confirmed by two experienced neuroradiologists. The mean age of women in the LVF group was 69.26±9.29 years (range=60–80 years; ). To compare the dorsal and ventral T12V body heights between patients with and without LVF, we enrolled a group of control patients who had undergone MRI as part of a medical examination, but had no LVF-related diseases. The control group consisted of 80 participants with a mean age of 69.74±5.27 years (range=60–86 years; ). We examined the dorsal and ventral T12V body heights in the control group and the LVF group at the T12V level.
Table 1 Comparison of the characteristics of the control and lumbar vertebral fracture (LVF) groups
Imaging parameters
MRIs were performed with a 3T Avanto (Siemens Healthcare, Erlangen, Germany) with 1.5 T scanners (Achieva; Philips Healthcare, Best, the Netherlands). For MRI examinations, sagittal T2-weighted images were obtained with a slice thickness <4.0 mm, 0.9 mm intersection gap, 2,710-ms/95-ms repetition time/echo time, 300×300 field of view, and a 512×358 matrix. MRI imaging data were transferred from the MRI unit to an INFINITT system (INFINITT Healthcare Co., Seoul, Korea).
Image analysis
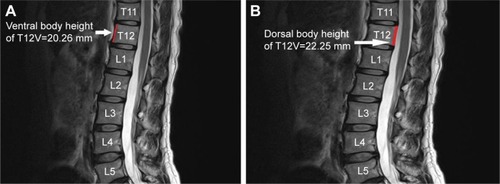
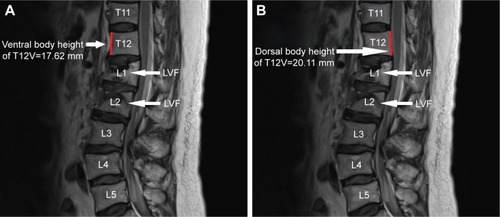
The dorsal and ventral heights (sagittal images) of the T12V body were recorded in millimeters ( and ). Measurement points were placed at the most inferior and superior points of the T12V body.
Statistical analyses
We expressed all data as means±SD, and we used student t-tests to compare the dorsal and ventral T12V body heights between the control and LVF groups, setting significance at P<0.05. The validity of the dorsal and ventral T12V body heights as predictors of LVF was estimated by the Receiver Operator Characteristics (ROC) curves, area under the curve (AUC), cut-off values, sensitivity, and specificity with 95% CIs. We performed all statistical analyses with SPSS for Windows version 22 (IBM SPSS, IBM Corp, NY, USA).
Results
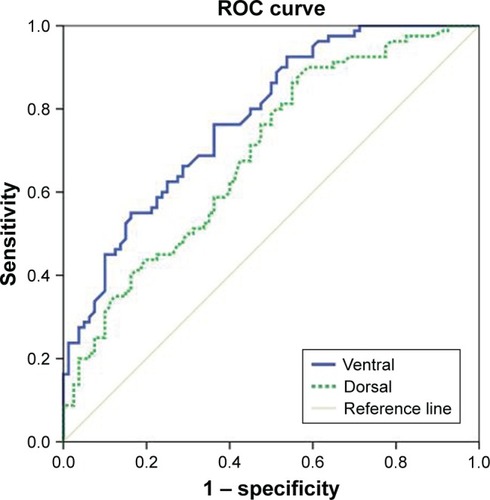
Demographic data were not significantly different between the two groups (). The average dorsal T12V body heights were 21.25±1.64 mm in the control group and 20.11±1.49 mm in the LVF group, and the average ventral T12V body heights were 19.51±1.54 mm in the control group and 17.62±1.95 mm in the LVF group. The LVF group had significantly lower dorsal T12V body heights (P<0.001) and ventral T12V body heights (P<0.001; see ) than the control group. Regarding the validity of both the dorsal and ventral T12V body height as predictors of LVF, the ROC curve analysis showed that the best cut-off point of the dorsal T12V body height was 20.74 mm, with 62.5% sensitivity and 60.0% specificity (), and AUC of 0.69 (95% CI=0.61–0.77) (). The optimal cut-off point of the ventral T12V body height was 18.76 mm, with 68.8% sensitivity and 67.5% specificity (), and AUC of 0.78 (95% CI=0.71–0.85) ().
Table 3 Sensitivity and specificity of each cut-off point of the ventral T12V body height
Table 2 Sensitivity and specificity of each cut-off point of the dorsal T12V body height
Figure 3 ROC curve of ventral body height and dorsal body height for prediction of lumbar vertebral fracture.
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
Osteoporosis is defined as a metabolic bone disorder, characterized by micro architectural deterioration and low bone mass, which leads to a higher risk of LVF and bone fragility.Citation33,Citation34 LVF account for 5% of total fractures and are most commonly found in the upper lumbar spine, where 75% of the vertebral fractures occur.Citation35 Conservative treatment, including analgesics, bed rest, physical therapy, and bracing, may fail to relieve pain and lead to prolonged bed rest, which is associated with increased healthcare costs and a negative effect on quality-of-life. LVF patients with spinal canal invasion and neurological deficits of soft-tissue or bone fragments may require surgical management.Citation35 BMD measured by DEXA is the current standard for predicting LVF. According to the WHO, osteoporosis is defined as a BMD value of -2.5 below the mean value, measured at the femoral neck, total hip, or the lumbar spine (L1–L4).Citation1,Citation33 The WHO suggests use of the fracture risk assessment tool (FRAX), without or with BMD measurement, to predict 10 years probabilities of major osteoporotic fractures and hip fractures.Citation22
However, discrepancies between actual LVF occurrences and risk predicted by DXA and FRAX exist. This may be due to imaging modalities that overlook vertebral height loss. Previous research found that vertebral height loss was an important sign of LVF. Mikula et alCitation36 reported that gradual height loss is a useful measurement tool to identify patients with LVF. Other prospective research, conducted by Moayyeri et al,Citation37 found that height loss was an important independent risk factor of future LVF at any site (>2 cm over a period of 4 years). Another clinical study, conducted by Kaptoge et al,Citation38 found that a 1 cm height loss was an independent risk factor for a LVF. Research by Xu et alCitation39 found that 231 postmenopausal females with LVF lost 1.37 cm more height than normal subjects. Tobias et alCitation40 studied 540 females and demonstrated a difference in height between the control and LVF group of 0.79 cm. However, the study had several weak points. They did not focus on T12 vertebra. T12V body morphology is altered with aging. It is the largest of the thoracic vertebrae and bears the most weight of all vertebrae, making T12V not only the strongest thoracic vertebra but also the most susceptible to mechanical stress-related injuries. In several ways, the T12V is a hybrid vertebra with the anatomical structures of both a lumbar and thoracic vertebra. And previous studies did not separately measure dorsal and ventral T12V body height, even though the ventral surface of a T12V body is slightly convex from side to side, and the dorsal surface a T12V body is almost flat. We found the difference in height between the LVF group and control group to be 1.14 cm in the dorsal height and 1.89 cm in the ventral height. We also evaluated the dorsal and ventral T12V heights by MRI for their value as morphological parameters predicting LVF.
The current study revealed four important new findings. First, the LVF group showed lower dorsal and ventral T12V body heights than the control group. Second, we determined that the best cut-off value of the dorsal T12V body height was 20.74 mm, with 62.5% sensitivity, 60.0% specificity, and AUC of 0.69. Third, the optimal cut-off point of the ventral T12V body height was 18.76 mm, with 68.8% sensitivity, 67.5% specificity (), and AUC of 0.78. Fourth, we found that the ventral T12V body height is a better morphologic predictor of LVF than the dorsal T12V body height.
The ventral T12V body height has a higher sensitivity and specificity for predicting LVF than the dorsal T12V body height and may be more effective in screening for LVF.
Limitations
Our original research has some limitations. First, there were a limited number of participants in each group.Citation41,Citation42 Second, there may be measurement errors associated with measuring the heights on MRI. Even though we tried to measure these morphologic parameters in the sagittal image that best showed T12V, the sagittal images we used to measure the variables could be inhomogeneous due to differences in the MRI cutting angle, resulting from anatomic and technical variations. In addition, a 4.0-mm slice of sagittal T2-weighted MR image is thicker than the ideal slice. Third, the principal methodological limitation was the retrospective data analysis. Fourth, this new tool had not yet been demonstrated to predict LVF. Therefore, larger scale investigations should be performed. Fifth, this research was the use of MRI imaging; it could be interesting to compare with lumbothoracic vertebrae from X-ray images in the future work. Despite these limitations, this is the first objective study to verify the role of the ventral T12V body height in patients with LVF.
Conclusion
From the current study, we conclude that the ventral T12V body height is an important morphological parameter for prediction and diagnosis of LVF in postmenopausal women with osteoporosis. When evaluating patients with LVF, physicians should carefully assess the ventral T12V body height.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- LeeSJGraffyPMZeaRDZiemlewiczTJPickhardtPJFuture osteoporotic fracture risk related to lumbar vertebral trabecular attenuation measured at routine body CTJ Bone Miner Res201833586086729314261
- MatakiKFukushimaMKaneokaKVertebral fracture after removing pedicle screws used for posterior lumbar interbody fusion: a case reportJ Clin Neurosci20185718218430243598
- NakaoSIshikawaKOnoHRadiological classification of retroperitoneal hematoma resulting from lumbar vertebral fractureEur J Trauma Emerg Surg20185910 Suppl
- WangHDingWPosterior vertebral column resection through unilateral osteotomy approach for old lumbar fracture combined with Kummell diseaseWorld Neurosurg201810914715128974411
- ZhangYGuoJDuanmuYQuantitative analysis of modified functional muscle–bone unit and back muscle density in patients with lumbar vertebral fracture in Chinese elderly men: a case–control studyAging Clin Exp Res201825
- KadamGNarsinghpuraKDeshmukhSDesaiSTraumatic lumbar vertebral ring apophysis fracture with disk herniation in an adolescentRadiol Case Rep201712242743028491203
- KumarSAnijarLAgarwalRKyphoplasty for the treatment of an atypical osteoporotic vertebral compression fracture of the lumbar spine: a case reportSAGE Open Med Case Rep201752050313 X1774498
- LiQSunJCuiXJiangZLiTAnalysis of correlation between degeneration of lower lumbar paraspinal muscles and spinopelvic alignment in patients with osteoporotic vertebral compression fractureJ Back Musculoskelet Rehabil20173061209121429154265
- UshikuCSudaKMatsumotoSDural penetration caused by a vertebral bone fragment in a lumbar burst fracture: a case reportSpinal Cord Ser Cases2017316040
- VanniDPantaloneADi CarloSMaglianiVBerjanoPSaliniVSpontaneous corpectomy and anterior arthrodesis in lumbar spine: how ankylosing spondylitis can resolve a vertebral fractureJ Spine Surg201731737528435922
- ChunEHKimJEKimDYUltrasound measurement of the vertebral level of Tuffier’s line in elderly womenKorean J Anesthesiol201669547447927703628
- GuptaBAgarwalMSharmaRSaithVAnesthesia management in a case of Turner syndrome with anti-NMDA limbic encephalitis and multiple co-morbidities for repair of fracture femurKorean J Anesthesiol201871433033130071714
- HongJHOhJHParkKBAnalysis of thoracic epidurography and correlating factors affecting the extent of contrast medium spreadKorean J Pain201629425526127738504
- InCB Cho SALeeSJTySChoCKClinical performance of airway management with the i-gel and laryngeal mask airway Supreme in geriatric patients: a prospective and randomized studyKorean J Anesthesiol2018
- KimJHLeeYCLeeSIEffective doses of cisatracurium in the adult and the elderlyKorean J Anesthesiol201669545345927703625
- KimYDYuJYShimJHeoHJKimHRisk of encountering dorsal scapular and long thoracic nerves during ultrasound-guided Interscalene brachial plexus block with nerve stimulatorKorean J Pain201629317918427413483
- LeeCWKimMEffects of preanesthetic dexmedetomidine on hemodynamic responses to endotracheal intubation in elderly patients undergoing treatment for hypertension: a randomized, double-blinded trialKorean J Anesthesiol2017701394528184265
- PiracciniEMusettiGByrneHMaitanSThe use of sonographic guidance in caudal epidural steroid injectionsKorean J Pain201831430530630310556
- RyuTSongSYPerioperative management of left ventricular diastolic dysfunction and heart failure: an anesthesiologist’s perspectiveKorean J Anesthesiol201770131228184260
- SchwenkESMarianoERDesigning the ideal perioperative pain management plan starts with multimodal analgesiaKorean J Anesthesiol201871534535230139215
- Al-AntariMAAl-MasniMAMetwallyMKDenoising images of dual energy X-ray absorptiometry using non-local means filtersJ Xray Sci Technol201826339541229562584
- BerghCSöderpalmACBrisbyHPreoperative dual-energy X-ray absorptiometry and FRAX in patients with lumbar spinal stenosisJ Orthop Surg Res201813125330326950
- KruegerDBinkleyNMorganSDual-energy X-ray absorptiometry quality mattersJ Clin Densitom201821215515629153384
- LeeKSamiNSweeneyFCDieli-ConwrightCMBody composition with dual-energy X-ray absorptiometry and bioelectrical impedance analysis in breast cancer survivorsNutr Clin Pract2018285
- RouxJPBelghaliSWegrzynJRenduESChapurlatRVertebral body morphology is associated with incident lumbar vertebral fracture in postmenopausal women. The OFELY studyOsteoporos Int20162782507251326957288
- RouxJPWegrzynJArlotMEContribution of trabecular and cortical components to biomechanical behavior of human vertebrae: an ex vivo studyJ Bone Miner Res201025235636119653808
- WegrzynJRouxJPArlotMEDeterminants of the mechanical behavior of human lumbar vertebrae after simulated mild fractureJ Bone Miner Res201126473974620928886
- KanisJAJohnellOOdenALong-term risk of osteoporotic fracture in MalmöOsteoporos Int200011866967411095169
- DiacintiDPisaniDBarone-AdesiFA new predictive index for vertebral fractures: the sum of the anterior vertebral body heightsBone201046376877319895914
- AlanayAPekmezciMKaraeminogullarıORadiographic measurement of the sagittal plane deformity in patients with osteoporotic spinal fractures evaluation of intrinsic errorEur Spine J200716122126213217912558
- JangHDBangCLeeJCRisk factor analysis for predicting vertebral body re-collapse after posterior instrumented fusion in thoracolumbar burst fractureSpine J201818228529328735766
- HernandezCJLoomisDACotterMMBiomechanical allometry in hominoid thoracic vertebraeJ Hum Evol200956546247019427672
- TuKNLieJDWanCKVOsteoporosis: a review of treatment optionsP T20184329210429386866
- WattsNBPostmenopausal osteoporosis: a clinical reviewJ Womens Health201827910931096
- ZhaoWTQinDPZhangXGWangZPTongZBiomechanical effects of different vertebral heights after augmentation of osteoporotic vertebral compression fracture: a three-dimensional finite element analysisJ Orthop Surg Res20181313229422073
- MikulaALHetzelSJBinkleyNAndersonPAValidity of height loss as a predictor for prevalent vertebral fractures, low bone mineral density, and vitamin D deficiencyOsteoporos Int20172851659166528154943
- MoayyeriALubenRNBinghamSAWelchAAWarehamNJKhawKTMeasured height loss predicts fractures in middle-aged and older men and women: the EPIC-Norfolk prospective population studyJ Bone Miner Res200823342543217997714
- KaptogeSArmbrechtGFelsenbergDWhom to treat? The contribution of vertebral X-rays to risk-based algorithms for fracture prediction. Results from the European prospective Osteoporosis StudyOsteoporos Int20061791369138116821002
- XuWPereraSMedichDHeight loss, vertebral fractures, and the misclassification of osteoporosisBone201148230731120870048
- TobiasJHHutchinsonAPHuntLPUse of clinical risk factors to identify postmenopausal women with vertebral fracturesOsteoporos Int2007181354316951907
- ShinYHYoonJSJeonHJKimYBKimYSParkJYPostoperative delirium in elderly patients with critical limb ischemia undergoing major leg amputation: a retrospective studyKorean J Anesthesiol201871431131629684991
- WegenerVStäblerAJanssonVBirkenmaierCWegenerBLumbar burner and stinger syndrome in an elderly athleteKorean J Pain2018311545729372027