Abstract
Purpose
Abdominal aortic aneurysm (AAA) demonstrates many features of autoimmune diseases. Y chromosome, sex-determining region of the Y chromosome (SRY) gene, androgen receptor (AR) gene, and androgen appear as potential candidates for influence of the male immune function. This study investigated Y chromosome numbers, SRY gene, AR gene, and androgen levels in male AAAs. We also investigated the correlation between Y chromosome loss (LOY) ratio, SRY expression, androgen levels, and age.
Patients and methods
We investigated LOY by fluorescence in situ hybridization (FISH) in 37 AAAs and compared with 12 patients with abdominal aortic atherosclerotic occlusive disease (AOD) and 91 healthy controls (HC). We investigated SRY and AR expression at mRNA level by real-time PCR in peripheral T lymphocytes in AAA compared with AOD and HC, and AR protein levels by immunohistochemistry (IHC) in AAA. LOY, SRY expression, androgen levels, and age were examined for correlations using the Spearman’s rank correlation coefficient.
Results
LOY ratio in peripheral T lymphocytes was significantly higher in the AAA group compared with the HC (9.11% vs 5.56%, P<0.001) and AOD groups (9.11% vs 6.42%, P=0.029). The SRY mRNA expression in peripheral T lymphocytes was 4.7-fold lower expressed in the AAA group than in the HC group (P<0.001). Free plasma testosterone levels were lower in the AAA group compared with the HC group (P=0.036), whereas sex hormone-binding globulin levels were higher (P=0.020). LOY ratio and expression of SRY mRNA level increased with age in the AAA group (R=0.402 and, R=0.366, respectively). A significant correlation between AR mRNA level (R=0.692) and aortic diameter was detected. Simultaneously, in AAA tissue, the rate of LOY increased with age (R=0.547) and also positively associated with LOY in peripheral blood T lymphocytes (R=0.661).
Conclusion
This study identified a prominent Y chromosome loss in male AAAs, which is correlated to age, lower level of SRY expression and free testosterone, providing a new clue for the mechanisms of AAA.
Introduction
Abdominal aortic aneurysm (AAA) is a major cause of morbidity and mortality in western countries,Citation1,Citation2 the incidence and prevalence of which in China have increased significantly during the last two decades.Citation3 Despite surgical advances, the recent interest in developing new therapies for treating small asymptomatic, nonoperation-indicated AAA has led to greater efforts to investigate and define its cellular and molecular nature. Increasing evidence suggests that the immune response contributes to the development of aneurysmal diseases, and AAAs demonstrate many features of autoimmune diseases, including genetic predisposition, organ specificity, and chronic inflammation.Citation4,Citation5 It is well documented that infiltrating T cells are essentially always present in AAA lesions, and AAA is likely a specific autoimmune T-cell disease.Citation6 Besides, a striking gender difference exhibited in AAA patients revealed a male: female ratio of up to 4:1, and male sex is one of the strongest risk factors for AAA.Citation7 Pathogenetic hypotheses include possible roles for androgen, environmental triggers, and genetic susceptibility.
The genetic influence on AAA initially focused on the relevance of autosomal genes, and possible susceptibility gene(s) have been identified in rare cases of AAAs with familial tendencies.Citation8,Citation9 However, most AAAs are sporadic, and no relevant genetic factors have yet been identified in these cases. Sex chromosomes have been recently investigated in terms of loss of mosaicism, reactivation, and haploinsufficiency in numerous autoimmune diseases and cancers, and other diseases demonstrating sexual dimorphism.Citation10–Citation14 The Y chromosome contains many genes related to sex determination, hormone generation, and fertility, but also harbors several X homologs, which might exert relevant roles in immune function.Citation15 The sex-determining region of the Y chromosome (SRY) gene includes a high-mobility group domain, a conserved motif present in many DNA-binding proteins, which could be involved in the regulation of multiple functions such as postnatal steroidogenesis and spermatogenesis.Citation16
A recent large population-based study of Australian men showed that patients with AAA had lower free and total testosterone levels than men without AAA.Citation17 Furthermore, levels of free testosterone were inversely correlated with AAA, suggesting that decreased levels of testosterone were associated with aortic dilation. These results challenge previous ideas that androgens play a pathogenetic role in vascular physiology and pathophysiology and promote the development of AAA,Citation17,Citation18 indicating the need for more studies examining the role of the male sex chromosome in AAA.
In this study, we investigated Y chromosome numbers in T lymphocytes and aneurysmal tissue in men with AAA. We also detected mRNA expression of SRY and androgen levels anf investigated the correlation between SRY, AR expression, and age.
Materials and methods
AAA population selection
Between January 2009 and December 2018, a total of 364 AAA patients registered in China Medical University aneurysm Biobank (CMUaB) and China Medical University aneurysm blood Biobank (CMUabB) were selected to this study, which were obtained from the Department of Vascular Surgery of the First Hospital of China Medical University (CMU) and Department of General Surgery of Shengjing Hospital of CMU. The AAA tissue specimen, peripheral blood samples, and corresponding clinical data were collected from these patients consecutively, after aneurymal open surgery (OSR). Simultaneously, peripheral blood samples and related clinical data were extracted within these patients consecutively, after endovascular repair (EVAR).
Inclusion criteria for AAA patients in this study were: 1) male patients with an infrarenal abdominal aorta diameter above 30 mm or larger than 1.5–2 times the diameter of the abdominal aorta in their normal segment diagnosed by three-dimensional computed tomography angiography (3D-CTA);Citation19 2) availability of corresponding patient history and clinical data, including history of ruptured, history of coronary and peripheral artery disease, medication history, and the presence of risk factors of atherosclerosis, such as smoking habits, hypertension, diabetes mellitus, or hyperlipidemia. Exclusive criterion: male AAA patients with Ehlers–Danlos syndrome, Marfan syndrome, and other known vascular disorders were excluded. None of the patients had any evidence or medical history of other autoimmune diseases.
AAA tissue specimen
126 patients from CMUaB were examined by an experienced vascular surgeon within 2 days before and after the OSR. Until December 2018, nine patients not willingly and openly reveal their inpatients of information via publication, whether their clinical data, aneurymal tissue, or blood sample. Finally, aneurysm specimens from 96 male patients were eligible for further analyses. Here, AAA tissue specimen (n=17) and corresponding peripheral blood samples (n=17) were obtained who underwent OSR. All samples were harvested at the most dilated place of aortaventralis, by which tissues in vitro were washed in PBS (Boster, Wuhan, China), opened transversely, and fixed in 4% paraformaldehyde (Beijing Chemical Works, Beijing, China) for 24 hrs. The samples were then embedded in paraffin and sectioned transversely at 2–3 µm continuously to make parallel sections. Immunofluorescence was stained by anti-CD3 monoclonal antibody (BD Biosciences, San José, CA, USA) to identify T lymphocytes on one section for cell type analysis and localization and the other was prepared for fluorescence in situ hybridization (FISH).
AAA peripheral blood samples
A total of 338 patients from CMUabB were examined by an experienced vascular surgeon within 2 days before and after the EVAR. Until December 2018, 29 patients discarded reveal their inpatients' information, eg, clinical data, blood laboratory results, and blood sample. Finally, aneurysm specimens from 254 male AAA patients were eligible for further analyses. The androgen level in serum sample, total RNA extraction and chromosome preparations in peripheral blood mononuclear cell (PBMC) we need to performed, both serum and PMBC samples were obtained successfully in 20 male AAA patients who underwent EVAR shown in . Blood was obtained preoperatively from patients and controls between 5 and 6 o’clock in the early morning in 10-mL anticoagulant tubes, with three tubes per sample.
Normal control population selection
The normal control group comprised age-matched healthy control males (HCs) (n=91, 50–84 years old, aorta diameter 16.9–25.8 mm), selected from a healthy population who received annual regular physical examinations. HCs had a relatively healthy peripheral vascular system determined by ultrasonography, and there was no evidence or medical history of aneurysm, abdominal aortic atherosclerotic occlusive disease (AOD), or other vascular disorders. Other exclusion criteria were cancer, infection, and any other immune-mediated disease. We collected clinical data, blood laboratory results, and blood samples from the HC group as controls.
AOD population selection
Because AAA patients also had prominent atherosclerosis, we selected a second control group of age-matched men with infrarenal AOD from our hospital (n=12, 49–79 years, aorta diameter 14.8–25.6 mm), with no medical history of aneurysmal disease, other vascular disorders, connective tissue disorders, or known autoimmune diseases. AOD was diagnosed by enhanced CT scanning or 3D-CTA. This group was used to control for any potential involvement of atherosclerosis in the possible autoimmune mechanisms, especially in early lesions. We collected clinical data, blood laboratory results, and blood samples from the AOD group as another control.
Ethics approval and consent to participate
Written informed consent was obtained from AAA patients, AOD patients, and controls in the study, before tissue and blood sampling. This study was performed according to the Guidelines of the World Medical Association Declaration of Helsinki and was approved by the local ethics committee of the China Medical University. The selection process of the corresponding patients is summarized in .
Figure 1 Flowchart of AAA patient selection and biopsy tissue from China Medical University Aneurysm Biobank (CMU-aB) or peripheral blood sample recovered from China Medical University Aneurysm blood Biobank (CMU-abB).
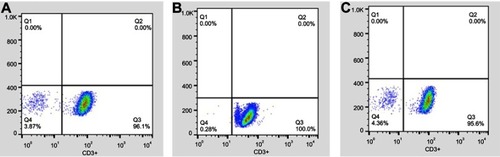
Peripheral blood mononuclear cell (PBMC) extraction and T-cell preparation
PBMCs were extracted by Ficoll-sodium diatrizoate (Ficoll-Hypaque, Haoyang TBD, Co, Tianjin, China) density-gradient centrifugation. After several washing steps with PBS, PBMCs were allowed to adhere to the plastic for 2 hrs at 37°C to deplete monocytes. Floating cells were collected and expression of CD3 in fresh peripheral blood lymphocytes was evaluated by flow cytometry (FACSCalibur; BD Biosciences) using PerCP-conjugated anti-CD3 monoclonal antibody (BD Biosciences) to identify the population of T lymphocytes. Each sample contained >90% CD3+ T lymphocytes at the time of assessment ().
Serum preparation
Serum was prepared after centrifuge of blood and stored at −80°C until assayed. Total serum levels of testosterone, dehydroepiandrosterone (DHS), and sex hormone-binding globulin (SHBG) were determined by chemiluminescent immunoassays using Immulite 2000 total testosterone, Immulite 2000 DHEA-SO4, and Immulite 2000 SHBG assays (Siemens Healthcare Diagnostics Products Limited, Llanberis, UK) on an Immulite 2000 analyzer (Siemens Healthcare Diagnostics, Flanders, USA). Free testosterone was determined by chemiluminescent immunoassay using free testosterone in vitro diagnostic kits (Snibe, Shenzhen, China) on a Maglumi 4000 analyzer (Snibe). The working ranges for total testosterone, DHS, SHBG, and free testosterone were 0.7–55 nmol/L, 0.41–27 µmol/L, ≤180 nmol/L, and 0.50–150.0 pg/mL, respectively. The sensitivities were 0.5 nmol/L, 0.08 µmol/L, 0.02 nmol/L, and 0.5 pg/mL, respectively, and the reference values were 6.27–26.28 nmol/L, 2.17–15.20 µmol/L, 13.00–71.00 nmol/L, and 55.05–183.50 pmol/L, respectively, according to the Institute of Endocrinology, the First Affiliated Hospital of China Medical University.
Chromosome preparations and FISH analysis
T lymphocytes were separated from peripheral blood (AAA, n=37; AOD, n=12; HC, n=91) and prepared as follows. Cytology Pepsin Solution (Zytovision, Bremen, Germany) was applied to the cytology specimens and incubated for 5 mins at 37°C in a humidity chamber. The slides were then incubated in 1% wash buffer Tris-HCl buffer solution (TBS) 1% formaldehyde solution, and again in 1× wash buffer TBS (Zytovision) for 5 mins each, dehydrated in 70%, 90%, and 100% ethanols for 1 min each, and then air dried. Ten microliters of CENX/Yq12 dual-color probe (Zytovision) was pipetted onto each individual sample, covered with a coverslip (22 mm×22 mm), and sealed with rubber cement (Zytovision). The slides were denatured at 72±1°C for 2 mins on a hot plate, transferred to a humidity chamber (Abbott, Chicago, USA), and hybridized overnight at 37°C. The cement and coverslip were removed the following day using Cytology Stringency Wash Buffer SSC (Zytovision) for 2 minss at 72±1°C for 1 min at room temperature. Thirty microliters of DAPI (Zytovision) was then pipetted onto the slides, covered with a coverslip (24 mm×60 mm), avoiding trapped bubbles, and incubated in the dark for 15 mins.
T lymphocytes of AAA tissue specimen (n=17) were prepared as follows. Cytology Pepsin Solution (Zytovision) was applied to the cytology specimens and incubated for 10 mins at 37°C in a humidity chamber, followed by incubation in 1× wash buffer TBS, 1% formaldehyde solution, and 1× wash buffer TBS (Zytovision) for 5 mins each; dehydrated in 70%, 90%, and 100% ethanols for 1 min each, and then air dried. Ten microliters of CENX/Yq12 dual-color probe (Zytovision) was pipetted onto each individual sample, covered with a coverslip (22 mm ×22 mm), and sealed with rubber cement (Zytovision). The slides were denatured at 83±1°C for 2 min on a hot plate, transferred to a humidity chamber (Abbott), and hybridized overnight at 37°C. The rubber cement and coverslip were removed carefully the following day using Cytology Stringency Wash Buffer SSC (Zytovision) for 2 mins at 72±1°C for 1 min at room temperature. Thirty microliters of DAPI (Zytovision) was then pipetted onto the slides, covered with a coverslip (24 mm×60 mm) avoiding trapped bubbles, and incubated in the dark for 15 mins.
All samples were analyzed under an Olympus Bx51 microscope equipped with DAPI and Fluorescein isothiocyanate-Tetramethyl isothiocyanate (FITC-TRITC) epifluorescence optics and a digital camera by two independent observers, who were blinded to the patient’s characteristics. Hybridization signals were captured using a CytoVision system (Leica Biosystems, Nussloch, Germany). At least 500 nuclei were scored for each sample, and the Y chromosome loss rate was expressed as a percentage (%) of the total number of nuclei counted.
RNA extraction and real-time PCR
To study SRY and androgen receptor (AR), total RNA was isolated from peripheral T lymphocytes (AAA, n=32; AOD, n=12; HC, n=54) using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. RNA quality and quantity were assessed by NanoDrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA) analysis of 260/280 ratios. High-quality RNA samples were obtained with an A260/A280 ratio >1.8. RNA was treated with DNase I (Takara, Dalian, China) before reverse transcription to eliminate contaminating genomic DNA. Total RNA was reverse transcribed with reverse transcriptase (PrimeScript RT Reagent Kit; Takara) and oligo-dT primer, according to the manufacturer’s instructions. Real-time fluorescent quantitative PCR was performed using SYBR-green I Premix Ex Taq (Takara) using the Thermo 9700 Real-Time PCR System (Thermo Fisher), following the manufacturer’s instructions, with ddH2O as a negative control. The primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were 5ʹ-CCACCCATGGCAAATTCCCATGGCA-3ʹ (sense) and 5ʹ-TCTAGACGGCAGGTCAGGTCCACC-3ʹ (anti-sense), which was used as an endogenous control. The primers for SRY were 5ʹ-CGCATTCATCGTGTGGTCT-3ʹ (sense) and 5ʹ-GCCATTTTTCGGCTTCAGTA-3ʹ (anti-sense), primers for AR were 5ʹ-GCAGGAAGCAGTATCCGAAG-3ʹ (sense) and 5ʹ-TTGGCGTTGTCAGAAATGG-3ʹ (anti-sense). Thermal cycle parameters were: 95°C for 30 s for 1 cycle, then 95°C for 5 s, and 60°C for 30 s for 40 cycles. All PCR assays were carried out in duplicate, and data were analyzed with the Thermo 9700 Real-Time PCR System (Thermo Fisher) using the comparative threshold cycle (2−ΔΔCT) method.
Histological, immunohistochemistry (IHC), and immunofluorescence analysis
The AAA fragments were prepared as aforesaid and samples were embedded in paraffin to obtain transverse sections. Serial sections were cut at 2–3 μm and prepared for H&E, immunochemical, and immunofluorescence staining.
Histological evaluation: The quality of each slide was assessed twice independently as follows: no staining (-), weak positive staining (±), scattered positive staining (+), majority positive staining (++), and strong overall positive staining (+++). These score analyses were performed for all cells within the vessel wall and for all specimens. In addition, for interrelated quality evaluation between the abovementioned quality evaluation following scoring was used: +/++ and ++/+++.
IHC analysis: The sections were treated with 3% hydrogen peroxide/methanol (Mai Xin_Bio, Fuzhou, China), followed by incubating with rabbit monoclonal antibody against AR (ab133273,1:100; Abcam, Cambridge, UK) or mouse monoclonal antibody against CD3 (sc-20047, 1:500; Santa Cruz Biotechnology, Shanghai, China) in 2% BSA PBS overnight at 4°C. After being washed with PBS, the sections were incubated with anti-rabbit/mouse IgG-HRP antibody (Boster) for 0.5 hrs at 37°C. Finally, 3,3ʹ-diaminobenzidine (DAB) (Mai Xin_Bio) was used as a chromogen for 10 mins until the brown yellow color appeared. Four visual fields were randomly selected on each slide. Light microscopic results were graded semiquantitatively from 0 (to the corresponding no staining change) to +3 (to the corresponding strong staining change: +++). This histopathological grading was performed for endothelial cells, inflammatory infiltrate, and macrophages in the AAA tissue samples.
Immunofluorescence analysis: The sections were treated with 3% hydrogen peroxide/methanol (Mai Xin_Bio), followed by incubating with rabbit monoclonal antibody against AR (ab133273,1:100; Abcam) or mouse monoclonal antibody against CD3 (sc-20047, 1:500; Santa Cruz Biotechnology) in 2% BSA PBS overnight at 4°C. After being washed with PBS, the sections were incubated with FITC-labelled secondary anti-rabbit/mouse IgG-HRP antibody (Boster) for 0.5 hrs at room temperature in 0.5% BSA/PBS. Coverslips were mounted in Prolong Gold with (DAPI) (Zytovision), then pipetted onto the slides, covered with a coverslip (24 mm×60 mm), avoiding trapped bubbles, and incubated in the dark for 15 mins. All samples were analyzed under an Olympus Bx51 microscope equipped with DAPI and FITC-TRITC epifluorescence optics and a digital camera.
Statistical analysis
Data were tested by the Shapiro–Wilk’s test and followed a normal distribution. Independent t-test was used for comparisons of normally distributed data and Mann–Whitney U test for not normally distributed data. Pearson’s Chi-square test and Fischer’s exact test were used for normally and not normally distributed categorical variables, respectively. For correlational analyses, correlations between continuous variables were analyzed first through Pearson’s correlation coefficient for normally distributed values or through Spearman’s rank correlation coefficient for nonparametric samples. The correlations between plasma TES, androstenedione (AND), SHBG, DHS, free plasma testosterone (F-TES), diameter, LOY%, and mRNA of SRY were analyzed through partial correlation analysis adjusted by age. Statistical comparisons were performed using SPSS 20.0 (IBM, Chicago, IL, USA). Statistical significance was defined as P<0.05.
Results
Characterization of AAA and AOD patients analysis
The experimental study group included 37 male patients (17 underwent OSR, 20 underwent EVAR) of infrarenal AAA. The youngest AAA subject was 49 years and the oldest 81 years old. Regarding the comparison of the study groups (AAA group vs AOD group vs HC group) as shown in , no significant differences were observed in age, hypertension, and other indices (all P-value >0.05). As respected, the mean maximum diameter of AAA was 56.6±15.3 mm which was significantly higher than AOD and HC group.
Table 1 Clinical features in patients with AAA, AOD, and healthy controls
As reported, tobacco smoking was a possible risk factor for Y chromosome loss.Citation20 Therefore, a corresponding number of smoking individuals have been selected in control groups to eliminate the possible interference. The individuals were selected to ensure that there was no significant difference in the rate of smokers and smoking index (pack-years) in AAA, AOD, and HC groups (also in ). Smoker was defined in this study as continuous smoking for more than half a year and at least 10 cigarettes per day (WHO1997).
Incidence of LOY and SRY expression in AAA peripheral T lymphocytes
A total of 70,000 interphase nuclei from peripheral T lymphocyte of the 37 male AAA patients, 12 male AOD patients, and 91 male donors were analyzed by in situ hybridization for X and Y hypoploidy, respectively (). Y chromosome loss in peripheral T lymphocytes detected by FISH was significantly higher in the AAA group compared with the HC (9.11±3.96% vs 5.56±3.50%, P<0.001) and AOD groups (9.11±3.96% vs 6.42±1.93%, P=0.029). There was no significant difference in Y chromosome loss between AOD groups and HC groups (P=0.253) (). Furthermore, we investigate the correlation between the rate of Y chromosome loss correlate and other baseline factors in AAAs. After logistic regression analysis, there were no significant correlations between LOY and comorbidity such as hypertension, diabetes mellitus, coronary artery disease, and smoking history in patients with AAA ().
Figure 3 FISH image of X/Y chromosome in peripheral blood T lymphocytes from AAA group (A–C), AOD group (D–F), and HC group (G–I) individuals (×1,000). The X chromosome probe was green labeled and Y chromosome probe was pink labeled. Y chromosome loss can be detected by this image.
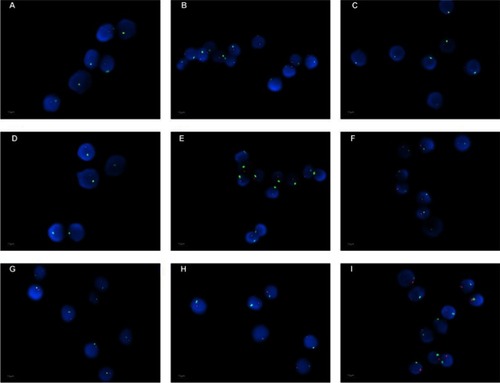
Figure 4 Y chromosome loss ratio (A), mRNA level of SRY (B), and mRNA level of AR (C) in peripheral T cells in AAA, AOD, and HC groups. Note, AAA (n=37), AOD (n=12), and HC (n=91) groups. Shapiro–Wilk’s test for three groups achieved P-value of 0.418, 0.821, and <0.001, respectively. Thus, the sample was not normal distributed and Mann–Whitney U test was used for the HC group compared with AAA and AOD groups. Relative to AOD and HC groups, AAA group showed a significant increase of Y chromosome loss; (B) AAA (n=32), AOD (n=12) and HC (n=54) groups. Shapiro–Wilk’s test for three groups achieved P-value of 0.001, 0.465, and <0.001, respectively. Thus, the sample was not normal distributed and Mann–Whitney U test was used for the individual comparison group. Compared with HC group, AAA and AOD groups showed a significant decrease of SRY mRNA expression; (C) AAA (n=32), AOD (n=12), and HC (n=54) groups. Shapiro–Wilk’s test for three groups achieved P-value of <0.001, 0.001, and <0.001, respectively. Thus, the sample was not normally distributed and Mann–Whitney U test was used for individual comparison group.

The SRY mRNA expression in peripheral T lymphocytes was 4.7-fold lower expressed in the AAA group than in the HC group (P<0.001). Moreover, SRY mRNA expression in peripheral T lymphocytes was significantly lower in AOD group than in HC group (0.19±0.12 vs 3.94±2.68, P<0.001), but there was no significant difference between the AAA and AOD group (P=0.328) in .
Plasma sex hormone level and androgen receptor level in AAA patients and controls
F-TES levels were lower in the AAA group compared with the HC group (P=0.036), whereas SHBG levels were higher (P=0.020). There was no significant difference between the AAA and HC groups in terms of total testosterone (TEST), DHS, and AND level (). However, there was no significant difference in the expression of androgen receptor (AR) mRNA level between the AAA group and HC group (P=0.083), AOD group (P=0.140) in .
Table 2 Plasma sex hormone level in AAA patients and controls
Correlations between peripheral LOY and age-related AAA or sex hormone level
In , peripheral LOY ratio and expression of SRY mRNA level were examined for correlations using the Spearman’s rank correlation coefficient due to the heterogeneous distribution of the experimental data. As respected, the result demonstrated that peripheral LOY ratio was positive significantly correlated with expression of SRY mRNA level (R=0.709, P<0.001) adjusted by age. Second, we analyzed the rate of LOY in peripheral T lymphocytes in relation to continuous age. Y chromosome loss and expression of SRY mRNA level increased with age in the AAA group (R=0.402, P=0.014 and R=0.366, P=0.039S, respectively). In addition, plasma SHBG level correlated significantly with SRY mRNA level (R=0.433, P=0.039) and continuous age in AAA patients (R=0.456, P=0.007). A significant correlation between AR mRNA level (R=0.692, P<0.001) and aortic diameter was detected adjusted by age.
Table 3 Partial correlation analysis of the peripheral T lymphocytes LOY ratio, mRNA expression of SRY and AR, plasma sex hormone level within AAA using partial correlation coefficient
Incidence of LOY and correlations within AR protein level in AAA tissue
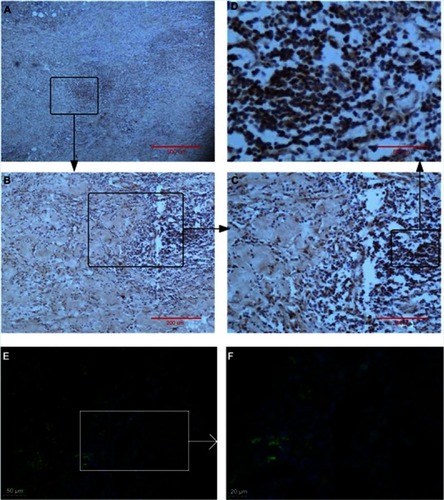
To assess the extension of the individual histopathological features in AAA wall (n=17), a semiquantitative histological characterization of all AAA tissue samples was performed (). There was no significant difference in Y chromosome loss between AAA peripheral blood T lymphocytes and AAA tissues (9.14±3.93% for peripheral T lymphocytes vs 8.08±2.42% for AAA tissue, P=0.460). Regarding the AR protein level, using IHC and immunofluorescence, inflammation cells were strongly positive for AR expression, particularly in T lymphocytes (). Furthermore, the semiquantitative histological characterization and the results of LOY ratio were analyzed again by calculating correlation coefficient in order to explore any relationships between aneurymal LOY ratio and AR protein level within AAA tissue. There is no significant correlation between LOY ratio and AR protein level (R=0.052, P=0.844). Interestingly, in AAA tissue, the rate of LOY increased with age (R=0.547, P=0.023) and also positively associated with LOY in peripheral blood T lymphocytes (R=0.661, P=0.004) ().
Table 4 Overview of the LOY ratio and classification of semiquantitative IHC analysis in AAA tissue (n=17)
Table 5 Correlation analysis of the LOY ratio, mRNA expression of AR in AAA tissue, and peripheral T lymphocytes LOY and peripheral blood parameters using Spearman rank correlation coefficient
Figure 5 Selected AAA example of IHC for cellular localization and expression of AR. (A) Overview of secreted AAA tissue, scale bar represents 500 μm; (B) AR seems to be expressed mainly in inflammatory cells and macrophages, scale bar represents 200 μm; (C and D) AR expression, scale bar represents 100 μm and 50 μm, respectively. Selected AAA example of IF for cellular localization and expression of AR. (E and F) AR expression, scale bar represents 50 μm and 20 μm, respectively.
Discussion
The results of the present study provide the first evidence for an association between Y chromosome loss in T lymphocytes and AAA in male patients. Previous studies demonstrated an increased incidence of X monosomy in PBMC of female patients,Citation11,Citation21 and Y chromosome loss in PBMC was investigated in male patients with autoimmune thyroiditis and primary biliary cirrhosis.Citation15,Citation22 Sex chromosome loss is not an exclusively female phenomenon, but it is also associated with disease in the male population. Y chromosome appears as a potential candidate for influence of the immune function in men. Y chromosome loss has been shown to increase with age,Citation23,Citation24 but our findings indicated that the degree of Y chromosome loss in AAA patients exceeded that in HCs, similar to the situation for X chromosome loss in female primary biliary cirrhosis patient populations.Citation11
The reasons for Y chromosome loss remain unknown, though the rate of cellular turnover and a potential increase in Y-loss susceptibility with aging may be involved.Citation6,Citation22 Y chromosome loss has been associated with phenotype in several malignant diseases and has been suggested as a marker predicting response to therapy.Citation24–Citation27 The intrinsic pathophysiologic mechanism is largely unclear. However, loss of one X chromosome could potentially lead to haploinsufficiency of X-linked loci, escaping X chromosome inactivation processes,Citation21,Citation28 and Y chromosome loss may similarly result in an imbalance of alleles shared with the X chromosome.
The Y chromosome mainly harbors genes involved in male sexual development and spermatogenesis.Citation16 However, a number of X chromosome homologs potentially relevant to immune function have recently been identified in pseudoautosomal regions, including the IL-9 receptor (IL-9R) and IL-3 receptor subunit alpha (IL-3RA, CD123) (www.genecards.org). IL-9R is expressed by a series of T lymphocytes, including Th2 cells, Th17, and T-regs,Citation29 and imbalances in this gene might thus affect the suppressive potential of the T-cell compartment. We previously identified deficient Treg function in patients with AAAs,Citation30 which could partly be explained by the present findings. Moreover, the IL-9R gene has also been suggested to be associated with the development of some autoimmune conditions such as asthma.Citation31 IL-3RA is an important marker during maturation of myeloid dendritic cells, which have recently been shown to play an important role in maintaining autoimmune tolerance.Citation32 However, the influence of loss of this gene to on immunoregulation remains to be determined.
The SRY locus is an evolutionarily conserved locus on the mammalian Y chromosome responsible for testis determination and development.Citation33 SRY encodes a transcription factor that acts by binding to and activating the testis-specific enhancer of the related gene SOX9.Citation34 SOX9 is tightly associated with Sertoli cell development.Citation35–Citation37 It is also expressed in postnatal testicular Leydig cells, targeting the proteins involved in steroidogenesis,Citation38 and has been considered to regulate testosterone production.Citation39 We found that SRY expression was significantly decreased as a result of Y chromosome loss in T lymphocytes of AAA patients compared with control groups, which may contribute to the lower total and free testosterone levels in men with AAA identified in the current and previous studies.Citation17
Lower free or total testosterone levels have been associated with higher overall and cardiovascular mortalities in middle-aged and older men. It has previously been linked with atherosclerotic plaque formation. One possible mechanism may be that testosterone depletion may predispose to AAA through loss of the beneficial effects of testosterone on endothelial function, and adverse influences on circulating lipids and vascular inflammation.Citation17,Citation40–Citation42 Emerging evidenceCitation43 suggests that testosterone protects against the development of autoimmune conditions such as arthritis and lung disease in male SKG mice, and removal of testosterone increased both lung and joint diseases in male mice. This mechanism may involve the ability of testosterone to modulate the development of antibodies against citrullinated proteins. Occurrence of AAA in female patients is probably involved in a different pathway in which androgen does not take a major role. Accumulating data indicate the protective effect of estrogen in the mechanism of vascular disease including aneurysms.Citation44,Citation45 However, it would be expected that after aging this effect has been largely weakened due to a lower level of female hormone. This may explain the increased occurrence of AAA in aged women.
Our observations establish that, in addition to the immune effects of Y chromosome loss, which may contribute autoimmune injury in AAA, lower SRY expression may downregulate testosterone production, thus weakening its protective role and further contributing to the formation and development of AAA.
There are limitations to this study such as the limited sample size, lack of healthy aorta tissue, and lack of vivo experiment. First, our study comprises a relatively small sample size. As in all studies, especially those with a small sample size, both beta errors (due to small cohorts) and alpha errors (due to multiplicity of comparisons) should be considered. Second, the present study illustrates a correlation between the Y chromosome loss and age-related AAA; temporal relations between LOY and the age within AAA cannot be discerned. Still, as it is the first of its kind in humans, the data do provide important information. The lack of appropriate aortic wall tissue from controls limited the protein expression analysis. Third, this research mainly exploded the difference between AAA, AOD, and HC in LOY. Therefore, the residual blood samples could not be obtained abundantly from these AAA patients or control group, which is insufficient to perform the expression of SRY and AR at mRNA level or protein level after the FISH study. Additional studies are also needed to clarify the relationship between exogenous androgen treatment and the dilation of aortic aneurysm. Even with these potential limitations, our study results are good, providing a new clue for the mechanisms of AAA.
Conclusion
In conclusion, this study demonstrated a significant increase of Y chromosome loss in peripheral T lymphocytes and aneurysm sac wall tissue in male patients with AAAs, accompanied by decreased SRY expression in peripheral T lymphocytes and lower F-TES. Y chromosome loss ratio and expression of SRY mRNA level increased with age in AAA peripheral T lymphocytes. Furthermore, in AAA tissue, the rate of LOY increased with age and also positively associated with LOY in peripheral blood T lymphocytes. These findings indicate a novel clue in AAA mechanisms, and possibly a novel target for personalized treatment of this disease.
Acknowledgments
This work was supported by National Nature Science Foundation of China (Grant No. 304717076 and 81600370), Nature Science Foundation of Liaoning Province, China (Grant No. 2014021028), and Basic research program of Liaoning provincial institutions of 550 higher learning 2017 (LZDK201701), and China Postdoctoral Science Foundation (Grant No. 2018M640270).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
Table S1 The adjusted logistic regression analysis of peripheral T lymphocytes LOY ratio and association with AAA clinical comorbidity
References
- Sampson UK, Norman PE, Fowkes FG, et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart. 2014;9(1):159–170. doi:10.1016/j.gheart.2013.12.00925432125
- Barba A, Vega De Ceniga M, Estallo L, de la Fuente N, Viviens B, Izagirre M. Prevalence of abdominal aortic aneurysm is still high in certain areas of southern Europe. Ann Vasc Surg. 2013;27(8):1068–1073. doi:10.1016/j.avsg.2013.01.01724011812
- Song JQ, Zhang J, Yin MD, et al. Clinico-epidemiological features of infrarenal abdominal aortic aneurysm and relevant prognostic factors. Nat Med J China. 2008;88(23):1613–1617.
- Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26(5):987–994. doi:10.1161/01.ATV.0000214999.12921.4f16497993
- Kuivaniemi H, Platsoucas CD, Tilson MD 3rd. Aorticaneurysms: an immune disease with a strong genetic component. Circulation. 2008;117(2):242–252. doi:10.1161/CIRCULATIONAHA.107.69098218195185
- Lu S, White JV, Lin WL, et al. Aneurysmal lesions of patients with abdominal aortic aneurysm contain clonally expanded T cells. J Autoimmun. 2014;192(10):4897–4912.
- Hannawa KK, Eliason JL, Upchurch GR Jr. Gender differences in abdominal aortic aneurysms. Vascular. 2009;17(Suppl 1):S30–S90.19426607
- Sakalihasan N, Defraigne JO, Kerstenne MA, et al. Family members of patients with abdominal aortic aneurysms are at increased risk for aneurysms: analysis of 618 probands and their families from the Liege AAA Family Study. Ann Vasc Surg. 2014;28(4):787–797.24365082
- Hinterseher I, Tromp G, Kuivaniemi H. Genes and abdominal aortic aneurysm. Ann Vasc Surg. 2011;25(3):388–412.21146954
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594–604. doi:10.1038/nri281520651746
- Invernizzi P, Miozzo M, Battezzati PM, et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363(9408):533–535. doi:10.1016/S0140-6736(04)15541-414975617
- Miozzo M, Selmi C, Gentilin B, et al. Preferential X chromosome loss but random inactivation characterize primary biliary cirrhosis. Hepatology. 2007;46(2):456–462. doi:10.1002/hep.2169617659578
- Jeong SY, Park SJ, Lee SJ, Park HJ, Kim HJ. Loss of Y chromosome in the malignant peripheral nerve sheet tumor of a patient with neurofibromatosis type 1. J Korean Med Sci. 2010;25(5):804–808. doi:10.3346/jkms.2010.25.5.80420436723
- Park SJ, Jeong SY, Kim HJ. Y chromosome loss and other genomic alterations in hepatocellular carcinoma cell lines analyzed by CGH and CGH array. Cancer Genet Cytogenet. 2006;166(1):56–64. doi:10.1016/j.cancergencyto.2005.08.02216616112
- Lleo A, Oertelt-Prigione S, Bianchi I, et al. Y chromosome loss in male patients with primary biliary cirrhosis. J Autoimmun. 2013;41:87–91. doi:10.1016/j.jaut.2012.12.00823375847
- Kido T, Lau YF. Roles of the Y chromosome genes in human cancers. Asian J Androl. 2015;17(3):373–380. doi:10.4103/1008-682X.15084225814157
- Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J. Associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Meta B. 2010;95(3):1123–1130. doi:10.1210/jc.2009-1696
- Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28(7):1251–1256. doi:10.1161/ATVBAHA.107.16038218451329
- Klink A, Hyafil F, Rudd J, et al. Diagnostic and therapeutic strategies for small abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(6):338–347. doi:10.1038/nrcardio.2011.121304473
- Dumanski JP, Rasi C, Lönn M, et al. Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science. 2015;347(6217):81–83. doi:10.1126/science.126209225477213
- Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M. Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun. 2009;33(1):12–16. doi:10.1016/j.jaut.2009.03.00519356902
- Persani L, Bonomi M, Lleo A, et al. Increased loss of the Y chromosome in peripheral blood cells in male patients with autoimmune thyroiditis. J Autoimmun. 2012;38(2–3):J193–J196. doi:10.1016/j.jaut.2011.11.01122196921
- Guttenbach M, Koschorz B, Bernthaler U, Grimm T, Schmid M. Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet. 1995;57(5):1143–1150.7485166
- Wong AK, Fang B, Zhang L, Guo X, Lee S, Schreck R. Loss of the Y chromosome: an age-related or clonal phenomenon in acute myelogenous leukemia/myelodysplastic syndrome? Arch Pathol Lab Med. 2008;132(8):1329–1332. doi:10.1043/1543-2165(2008)132[1329:LOTYCA]2.0.CO;218684036
- Minner S, Kilgue A, Stahl P, et al. Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology. 2010;42(4):356–359. doi:10.3109/0031302100376729820438408
- Forsberg LA, Rasi C, Malmqvist N, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46(6):624–628. doi:10.1038/ng.296624777449
- Kujawski M, Jarmuz M, Rydzanicz M, et al. Frequent chromosome Y loss in primary, second primary and metastatic squamous cell carcinomas of the head and neck region. Cancer Lett. 2004;208(1):95–101. doi:10.1016/j.canlet.2003.11.00615105051
- Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M. Skewing of X chromosome inactivation in autoimmunity. Autoimmunity. 2008;41(4):272–277. doi:10.1080/0891693080202457418432407
- Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9.Nat. Rev Immunol. 2010;10(10):683–687. doi:10.1038/nri2848
- Yin M, Zhang J, Wang Y, et al. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2010;30(9):1825–1831. doi:10.1161/ATVBAHA.109.20030320448211
- Shimbara A, Christodoulopoulos P, Soussi-Gounni A, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000;105(1 pt 1):108–115. doi:10.1016/S0091-6749(00)90185-410629460
- TestaU S, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2(1):4. doi:10.1186/2050-7771-2-424513123
- Larney C, Bailey TL, Koopman P. Switching on sex: transcriptional regulation of the testis-determining gene Sry. Development. 2014;141(11):2195–2205. doi:10.1242/dev.10705224866114
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453(7197):930–934. doi:10.1038/nature0694418454134
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122(9):2813–2822.8787755
- Morais Da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14(1):62–68. doi:10.1038/ng0996-628782821
- Vidal VP, Chaboissier MC, De Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28(3):216–217. doi:10.1038/9004611431689
- Bhandari RK, Haque MM, Skinner MK. Global genome analysis of the downstream binding targets of testis determining factor SRY and SOX9. PLoS One. 2012;7(9):e43380. doi:10.1371/journal.pone.004338022984422
- Daigle M, Roumaud P, Martin LJ. Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development. Mol Cell Biochem. 2015;407(1–2):209–221. doi:10.1007/s11010-015-2470-726045173
- F C W, Von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24(2):183–217. doi:10.1210/er.2001-002512700179
- Tivesten A, Mellstrom D, Jutberger H, et al. Low serum testosterone and high serum estradiol associate with lower extremity peripheral arterial disease in elderly men. The MrOS study in Sweden. J Am Coll Cardiol. 2007;50(11):1070–1076. doi:10.1016/j.jacc.2007.04.08817825717
- Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91(2):546–554. doi:10.1210/jc.2005-166416317058
- Keith RC, Sokolove J, Edelman BL, et al. Testosterone is protective in thesexually dimorphic development of arthritis and lung disease in SKG mice. Arthritis. 2013;65(6):1487–1493. doi:10.1002/art.37943
- Wu XF, Zhang J, Paskauskas S, Xin SJ, Duan ZQ. The role of estrogen in the formation ofexperimental abdominal aortic aneurysm. Am J Surg. 2009;197(1):49–54. doi:10.1016/j.amjsurg.2007.11.02218585678
- Sinha I, Cho BS, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR Jr. Female gender attenuatescytokine and chemokine expression and leukocyte recruitment in experimental rodent abdominal aortic aneurysms. Ann N Y AcadSci. 2006;1085:367–379. doi:10.1196/annals.1383.027