Abstract
Purpose
DNA methylation is thought to play a role in exercise-induced gene expression. We aimed to examine changes in muscular strength and body composition in elderly patients with end-stage knee osteoarthritis before and after artificial knee arthroplasty and exercise therapy. We aimed to confirm the relationship between DNA methylation and body composition, using the methylation rate of the pyruvate dehydrogenase kinase 4 (PDK4) gene that regulates skeletal muscle and fat metabolism.
Patients and methods
Patients underwent artificial knee arthroplasty between April 2017 and June 2017 at Kansai Medical University Hospital. Six patients (seven knees) were included in the analysis (four males/two females; average age, 75.7 years; body mass index, 25.1 kg/m2). Body composition and knee extension muscle strength were measured before surgery and 5 months after surgery. Rehabilitation was performed for 3 months after surgery. In the remaining 2 months, patients performed resistance training and aerobic exercise using an ergometer for 20 mins, twice a week. A biopsy of the vastus medialis was taken during surgery and 5 months post-surgery. Biopsy samples were treated with bisulfite after DNA extraction, and DNA methylation rate was calculated.
Results
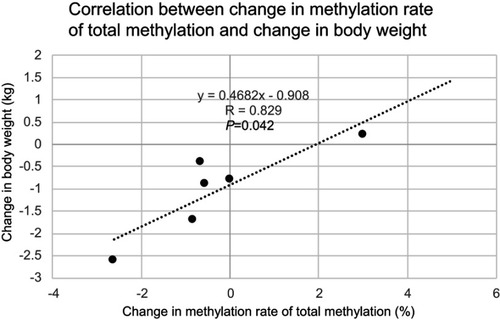
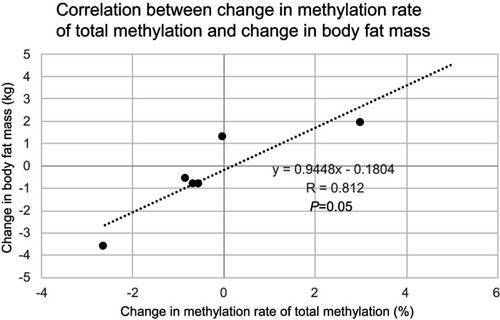
Body weight (P=0.046), total weight (P=0.027), and total fat mass (P=0.028) were significantly lower 5 months postoperatively than preoperatively. Five months post-surgery, the PDK4 gene was significantly more hypomethylated at eight sites in the CpG island, compared to pre-surgery. There was a significant correlation (r=0.88, P=0.02) between promoter region hypomethylation and weight loss. Total methylation rate and weight loss were significantly correlated (r=0.829, P=0.042). Total methylation rate and decrease in total fat mass showed a positive trending relationship (r=0.812, P=0.05).
Conclusion
Rehabilitative exercise resulted in significant decreases in weight and body fat. Hypomethylation of the PDK4 gene promoter region signified the effect of postoperative management focus on exercise therapy on weight and fat loss.
Introduction
Osteoarthritis of the knee (OA knee) is a chronic and degenerative disease of the tissues of the knee joint that negatively affects the quality of daily life of elderly people because of pain and limited range of motion.Citation1 A risk factor for OA knee is obesity, which can lead to the onset of OA knee, as well as contribute to its worsening.Citation2–Citation4 In addition, it is reported that steatosis and disused muscle atrophy occur in the quadriceps muscle of OA knee patients because the patient experiences severe pain and cannot exercise.Citation5,Citation6
Furthermore, in obese patients, the long-term success of total knee arthroplasty/unicompartmental knee arthroplasty (TKA/UKA) is poor and revision rates are higher.Citation7,Citation8 Therefore, it is important to reduce weight with diet and to improve lower limb muscle strength by exercising to treat and/or prevent OA knee.
The effects of exercise therapy vary greatly from one individual to another. Two individuals who undertake the same exercise program, matched for intensity and quantity, can show very different results with regard to weight loss and improvements in muscle strength. Some studies have shown that when rehabilitation exercises are undertaken by elderly people and there are minimal changes to diet, the degree of body weight and fat mass change varies greatly from individual to individual.Citation9,Citation10 Despite these findings, the reasons for these differences are not fully understood.
One of the factors influencing these different exercise effects is an individual’s genetic makeup.Citation11 Another influencing factor is “epigenetics”, which is expressed by the addition or removal of methyl groups to skeletal muscle-related genes, or the modification of histones.Citation12 Acquired environmental factors may also play a role.Citation13 “Epigenetics”, which is the control and maintenance of gene expression by acquired modifications without accompanying DNA base sequence alteration, is a concept proposed by Waddington in 1956.Citation14
Even though all cells have the same original base sequence, it is thought that they have high tissue specificity because they mature into cells with different functions in each organ. Barres et al demonstrated the possibility that epigenetics may be involved in the phenotype of skeletal muscle.Citation15
The pyruvate dehydrogenase kinase 4 (PDK4) gene is associated with lipid metabolism in the skeletal muscle.Citation16 PDK4 plays the role of inactivation via phosphorylation of pyruvate dehydrogenase complex (PDC). PDC is inactivated, thereby inhibiting the conversion from pyruvic acid to acetyl-CoA, and the role of energy substrate utilization is shifted from carbohydrate to lipid.Citation17,Citation18 Previous studies have reported that DNA methylation in skeletal muscle has an important role in gene regulation that depends on CpG methylation in promoter regions.Citation19 Other studies have reported that in the short-term, hypomethylation of DNA occurs in the CpG island in the promoter region of the PDK4 gene, which is a skeletal muscle-related gene, by providing exercise load to healthy people and improving skeletal muscle metabolism.Citation20–Citation24 This suggests that the presence of “DNA methylation” may be an important factor in skeletal muscle metabolism. We theorized that epigenetics can bring about changes in weight and body composition via exercise therapy, not only via adjustments in energy balance, but also changes in metabolic effectiveness. Specifically, OA knee patients can only participate in low levels of physical activity due to pain. Physical activity increases after exercise therapy following TKA/UKA, triggering DNA hypomethylation and changes in body composition. We believe that confirmation of hypomethylation of DNA will be essential in the future guidelines for exercise therapy.
Hence, we hypothesized that the PDK4 gene would be released in the methyl group (triggered by exercise therapy), and fat metabolism activity would occur, resulting in reduction of body weight and fat mass.
The purpose of this study was to calculate for the first time the “methylation rate” of the PDK4 gene that regulates skeletal muscle and fat metabolism in the elderly using a next-generation sequencer (NGS) and elucidate the relationship between body composition and exercise therapy after OA knee surgery. We also considered the possibility that the derived methylation rate could be one of the indexes of exercise therapy.
Materials and methods
Patients
The enrolled patients attended the Department of Orthopedics Clinic at Kansai Medical University Hospital and underwent artificial knee arthroplasty between April 2017 and June 2017 for osteoarthritis and osteonecrosis. There were nine patients (10 knees), comprising five males and four females with an average age of 74.8 years and average body mass index (BMI) of 26.6 kg/m2. Exclusion criteria were rheumatoid arthritis (RA), mental disorders including dementia, inability to tolerate the exercise regimen, or a history of difficulty performing exercise therapy.
This study was conducted according to the Ethics Regulations of Kansai Medical University. After explaining the purpose, contents, and precautions of the study to all subjects, written informed consent regarding study participation was obtained (Approval no. 2,016,709, date of approval: December 26, 2016), and conformed to the standards set by the latest revision of the Declaration of Helsinki.
Body composition
The body composition of all patients was measured, including total weight, total body lean mass, lower limb lean mass, total body fat mass, and lower limb fat mass, using the Lunar Prodigy Advance DXA Ver. 13.6 (GE Healthcare Co., Ltd., Waukesha, WI), and body weight, skeletal muscle mass, and body fat mass, using InBody720 (Biospace Corp., Paris, France) pre-operatively and 5 months after surgery.
Lower limb muscle strength
The knee extensor muscular strength of subjects was measured in a sitting position and with the affected limb suspended at a knee flexion of 90° using a knee muscle strength device, the μTasMF-01 (Anima Corp., Tokyo, Japan). This device was worn 5 cm proximal to the ankle joint and knee extensor muscular strength was measured pre-surgery and 5 months after surgery. Muscular strength of the affected lower limb was measured twice on the left and right by actively extending the knee joint and performing isometric contraction. The measurements were taken after several practices to familiarize patients with the activity. The maximum value of the two was adopted as the measured value.
Muscle biopsy
Muscle biopsy of the affected limb was performed using a mid vastus approach during surgery. Approximately 1×1 cm2 of the vastus medialis muscle was collected. The collected specimen was frozen in liquid nitrogen and stored in a freezer at −80 ºC.
At 5 months after surgery, the vastus medialis muscle was visualized under echo guidance at the same site where the biopsy was collected during surgery.
After local anesthesia with 1% xylocaine® (Astra-Zeneca, Mölndal, Sweden), a needle biopsy was performed on each affected knee with a 14G biopsy needle (Medicon, Seoul, Korea). The obtained sample was also frozen in liquid nitrogen and stored in a freezer at −80 ºC.
Postoperative exercise therapy
Postoperative rehabilitative exercise therapy was performed according to the post-TKA/UKA protocol of our institution until 3 months after surgery. After surgery, continuous passive motion (CPM) was performed until the knee joint movement exceeded 120º. Passive joint movement range exercises, lower limb strength training using about 3 metabolic equivalents (METs), and walking training for about 10 mins were carried out in the presence of a physical therapist (PT). From the third month after surgery, patients performed resistance training, mainly involving the quadriceps muscle using about 3 METs. The resistance training regime was set at 10 repetitions of slow training (afferent contraction for 3 seconds, isometric contraction for 1 second, and isometric contraction for 3 seconds, performed at 50% of 1RM) and an aerobic exercise using a bicycle ergometer at 40–50% strength according to the Karvonen method calculated from the predicted maximum heart rate.Citation25 This aerobic exercise was performed for 20 mins, twice a week.
DNA methylation measurement
Skeletal muscle samples were obtained from the affected knees using a QlAamp DNA Mini Kit (QIAGEN, Hilden, Germany). Bisulfite treatment was carried out according to the MethylEasyTM Xceed (TaKaRa, New Delhi, India) protocol. In order to analyze the targeted PDK4 gene, we designed a primer group (Eurofins Genomics, Tokyo, Japan) covering the region of about 1400 bases, from 711 bases upstream to 692 bases downstream of the transcription start codon, with 13 fragments. Polymerase chain reaction (PCR) amplification () was performed with the Epi-Taq (TaKaRa) using a primer group specific to the sequence after bisulfite treatment. The PCR conditions were 40 cycles of 98 ºC for 10 seconds, 55 ºC for 30 seconds, and 72 ºC for 30 seconds using 1 ng of genomic DNA after bisulfite treatment as a template. After amplification, the library was adjusted using the Ion XpressTM Plus Fragment Library Kit (Thermo Fisher Scientific, Waltham, MA), and the template was prepared by emulsion PCR using a Chef instrument kit (Fisher Scientific, Pittsburgh, PA). Then, sequencing was carried out using a Personal Genome Machine (PGM) sequencer and 318 Ion chips (Ion Torrent, Life Technologies, Carlsbad CA). Data analysis determined the methylation rate of each cytosine treated with bisulfite using a plug-in methylation analysis.Citation26
Figure 1 Designed primer.
Abbreviations: PDK4, pyruvate dehydrogenase kinase 4; BSP, bisulfite sequencing polymerase chain reaction.

We determined all the base sequences of each DNA strand, which represented an amplification product amplified by PCR. The analyzed results were depicted in an Excel table, and each mixed methylation site included methylated cytosine (C) and unmethylated cytosine (T). The methylated cytosine (C) count and unmethylated cytosine (T) count were assessed. The value obtained by dividing the counted C number by the total number (C + T), and (C/C + T) was evaluated as the methylation rate (%) at the methylation site.Citation27,Citation28 In addition, in bilateral knee surgery cases, the skeletal muscle obtained from bilateral knee muscles was evaluated using the methylation rate (%), calculated from the C number and the T number of the right and left sides.
Statistical analysis
All data were expressed as mean ± standard deviation. We compared preoperative and 5-month postoperative measures, including body composition (10 items: body weight, skeletal muscle mass, body fat mass, total weight, total lean mass, lower limb lean mass, total fat mass, lower limb fat mass, and knee extension muscle strength), the methylation rate at each position in the CpG island of the muscle (methylated C/C+T ratio from 744 bp upstream to 890 bp downstream from the transcription initiation codon), and the C/C+T ratio at all positions (the total methylation rate) using the Wilcoxon signed rank test. In addition, the association between the change rate of PDK4 methylation in the CpG island in skeletal muscle and the change in body composition before and after surgery was assessed using a Spearman’s rank correlation coefficient.
Preoperative and 5-month postoperative comparisons between the two groups with regard to body composition (seven items: body weight, BMI, body fat mass, body fat percentage, lean fat mass, lean free rate, and leg muscle strength per affected limb) and the methylation rates of the CpG island cytosines in muscle were evaluated using a Wilcoxon’s signed rank test. In addition, the correlation between body composition and muscle methylation changes of each cytosine in the CpG island before and after surgery was assessed using a Spearman’s rank correlation coefficient. All statistical analyses were performed using SPSS software version 25.0 for Mac OS (IBM Corp., Armonk, NY). P<0.05 was considered significant for all analyses.
Results
One male was treated postoperatively for cervical myelopathy and two females experienced pain to a degree that they could not complete the prescribed rehabilitation, so six patients (seven knees; four males and two females) with an average age of 75.7 years and BMI of 25.1 kg/m2 were analyzed ().
Table 1 General characteristics of the sample (six patients) at the time of admission to hospital
Changes in body composition and lower limb muscle strength
Body weight, as measured by InBody720 (Biospace Corp.), changed from 58.4±7.7 kg preoperatively to 57.4±7.9 kg 5 months post-surgery (P=0.046). Total weight changed from 58.5±7.8 kg to 56.5±8.8 kg (P=0.027). Total body fat mass, as measured by DXA (GE Healthcare Co., Ltd.), changed from 17.1±3.2 kg to 15.6±3.3 kg (P=0.028). These three parameters were significantly lower at 5 months post-surgery. Skeletal muscle mass (20.7±3.1 kg preoperatively vs 20.5±3.1 kg 5 months post-surgery) (P=0.916), body fat mass (19.3±5.3 kg preoperatively vs 18.9±5.2 kg 5 months post-surgery (P=0.752), total lean body mass (39.4±5.2 kg preoperatively vs 39.0±6.1 kg 5 months post-surgery (P=0.600), lower limb lean mass (11.7±1.9 kg preoperatively vs 11.5±2.1 kg 5 months post-surgery (P=0.463), lower limb fat mass (5.0±1.0 kg preoperatively vs 4.6±0.9 kg 5 months post-surgery (P=0.116), and knee extension muscle strength (21.1±4.7 kg preoperatively vs 24.4±12.4 kg 5 months post-surgery(P=0.893) were not significantly different ().
Table 2 Changes in body composition and knee extension muscle strength pre- and post-surgery (TKA or UKA) and exercise therapy in elderly patients with end-stage knee osteoarthritis
PDK4 methylation results
The CpG island region of PDK4 is shown in . A total of 77 CpG sites were sequenced. The promoter region of the PDK4 gene from the Swiss Institute of Bioinformatics (SIB, Lausanne, Switzerland) comprises 60 bp (5ʹ-GAGAGTGCGGGGAGACAAAACCTCGGGCGGCGGCGGCTGGGAAGACTTGAACT-3ʹ) from 49 bp to 108 bp downstream of the transcription initiation codon. The transcription initiation codon is also shown in .
Figure 2 Total base sequence of PDK4 gene CpG island.
Abbreviation: PDK4, pyruvate dehydrogenase kinase 4.

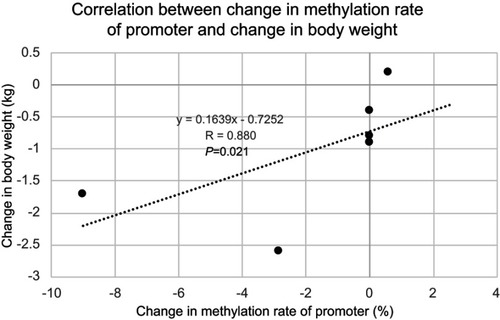
Results of the PDK4 gene methylation rate in skeletal muscle were: 185 bp (83.67±2.23% preoperatively vs 78.83±1.26% 5 months post-surgery (P=0.028) upstream of the CpG island transcription initiation codon, and 188 bp (18.55±1.05% vs 15.94±0.95%, P=0.046), 205 bp (26.80±0.34% vs 24.22±0.74%, P=0.027), 228 bp (9.23±0.28% vs 8.07±0.42%, P=0.028), 261 bp (4.88±0.51% vs 4.07±0.66%, P=0.046), 317 bp (8.21±0.61% vs 7.67±0.24%, P=0.046), 366 bp (6.68±0.66% vs 4.29±0.72%, P=0.028), 420 bp (70.74±2.03% vs 65.84±1.70%, P=0.028) downstream of the CpG island transcription initiation codon. Significant hypomethylation was observed at eight out of 77 sites. No significant hypomethylation was observed in the promoter region (2.19±3.52% preoperatively vs 0.31±0.75% 5 months post-surgery (P=0.285). No significant hypomethylation was observed in the total methylation rate (85.93±0.45% preoperatively vs 85.66±1.81% 5-months post-surgery (P=0.463) (). However, the change in methylation rate of the promoter region showed a significant positive correlation with change in body weight (r=0.880, P=0.021). In addition, the change in methylation rate of the total methylation showed a significant positive correlation with change in body weight (r=0.829, P=0.042), and a positive tendency with change in body fat mass (r=0.829, P=0.050) (, , and ).
Table 3 Changes in PDK4 gene hypomethylation before and after TKA/UKA surgery and exercise therapy in elderly patients with end-stage knee osteoarthritis
Figure 3 Correlation chart between promoter change in the PDK4 gene and body weight change. A positive correlation is found between hypomethylation change at the promoter region in the PDK4 gene and body weight change.
Discussion
In this study, body weight, as measured by InBody720 (Biospace Corp.), total weight, and total body fat mass, as measured by DXA (GE Healthcare Co., Ltd.), were all significantly decreased following surgery and exercise therapy. Significant hypomethylation of the skeletal muscle PDK4 gene was found at eight sites of the non-promoter region (185 bp upstream of the CpG island transcription initiation codon, and 188 bp, 205 bp, 228 bp, 261 bp, 317 bp, 366 bp, and 420 bp downstream of the CpG island transcription initiation codon). However, the promoter region and total methylation rate did not change significantly. Barres et al previously reported that exercise causes hypomethylation in the promoter region of the PDK4 gene.Citation20 Methylation of the promoter region is known to be involved in gene expression,Citation29 but the role of DNA methylation in the non-promoter region is not yet fully elucidated.Citation30,Citation31 However, Carmen et al reported that the non-promoter region also plays an important role, and methylation of the non-promoter region indicates the existence of alternative promoters, RNA processing, and transposable elements.Citation31 Given the above, it was considered to be clinically important to examine the influence of hypomethylation on body composition, not only in the conventional promoter region, but also in the non-promoter region.
Although one of the causes of onset and progression of OA knee has been reported to be the increased knee loading that results from being overweight or obese,Citation32 recent reports suggest that OA knee progression may also be influenced by the cytokines released from adipocytes.Citation33,Citation34 Therefore, reduction of body fat and body weight is clinically important in the prevention of OA knee.Citation35 In addition, after TKA/UKA, obese patients have a high complication rate, and although there is no apparent short-term difference in knee function after surgery,Citation36,Citation37 knee joint function in obese patients is significantly worse in the long-term.Citation7 The results of this study show a favorable postoperative course for TKA/UKA patients, supporting previous studies and suggesting that improvements in long-term prognosis can be expected.
Interestingly, there was no hypomethylation in the promoter region pre- and post-operatively, but there was a significant positive correlation between the methylation change in the promoter region and changes in body weight. There was a significant positive correlation between the total methylation rate and changes in body weight, and there was also a significant positive correlation tendency between the total methylation rate and changes in body fat. In fact, it is unclear whether energy consumption is enough to cause a reduction in body weight and body fat, because energy consumption during exercise was minor. In this study, although the energy intake was not investigated, it was difficult to simply explain the changes in body weight and body fat by the energy balance, and it is speculated that other metabolic factors may be influential. In a previous study, Constantin et al stated that transcription of the PDK4 gene was activated, and PDC suppressed the metabolism of pyruvate to acetyl-CoA. It is possible that this process contributed to the reduction of body fat mass and weight loss through activation of fatty acid oxidation by β-oxidation.Citation38 Exercise has also been reported to induce DNA methylation in previous studies.Citation37–Citation39 In other words, exercise therapy may have involved epigenetics to activate mitochondrial function and affect body weight and body fat.Citation16,Citation39,Citation40 In the future, confirmation and verification of the role of PDK4 methylation in the control of fat metabolism and gene expression is necessary in animal experiments. On the other hand, changes in body composition may cause hypomethylation. Both causal relationships are yet to be elucidated. In any case, it has been shown that changes in body composition are associated with the promoter region and total methylation of PDK4.Citation20 These facts also indicate that hypomethylation in the promoter region may be an indicator of the new effects of exercise therapy on an individual basis. Furthermore, even if the same exercise regime is performed, it is possible that there may be individual differences in the threshold that causes hypomethylation.Citation41
In this study, we verified that the actual skeletal muscle of elderly patients underwent DNA methylation. We measured the methylation rate of PDK4 gene comprehensively on the CpG island using the NGS. To the best of our knowledge, no previous studies have evaluated muscle biopsy samples from elderly people. Unlike conventional Sanger capillary sequences, NGS can process a large amount of data at high speed, so it may become possible to study epigenetic change, not only in part of the genome but also the whole genome region.Citation42 Although there are still few clinical applications of NGS, its use is progressing steadily. We concluded that the “methylation rate” evaluation calculated from the NGS has high clinical significance.
There were several limitations to this study. The first limitation was that the number of samples was small and we did not include a control group. NGS is an expensive test and a complicated procedure.Citation40 It is necessary to reduce the cost and simplify the procedure by the widespread use of this medical device. In this study, post-operative rehabilitation and exercise therapy were absolutely necessary treatments for all patients. Furthermore, it was extremely invasive and ethically impossible to perform similar tests, including muscle biopsy, on healthy individuals. In other words, the result of this study may be that the change was only related to time or due to the dispersion of the measured value, and it cannot be denied that the result may be changed by increasing the number of samples. This study is the first pilot study to evaluate body composition using the “methylation rate” of the PDK4 gene. The second limitation was that it was unclear which part of the CpG island was involved in transcription. We could not evaluate mRNA and miRNA, other than DNA methylation, or the protein involved in the expression of the PDK4 gene. In the future, we should evaluate not only methylation, but also histone modifications. Furthermore, by clarifying the mechanism behind protein expression of the PDK4 gene, it may be possible to understand the fat metabolism of the PDK4 gene in more depth.
Finally, methylation may also affect factors other than exercise. In this study, all patients received similar surgical procedures and rehabilitation programs and pain relief was achieved; however, it was difficult to standardize dietary intake. However, lifestyle intervention only included exercise therapy. The results of this study were probably generated by exercise therapy.
Conclusion
Body weight and total body fat mass decreased significantly 5 months post-surgery, following exercise therapy. The skeletal muscle PDK4 gene was significantly hypomethylated at eight sites in the CpG island. The methylation change rate of in the promoter region and body weight changes were significantly correlated. The changes in methylation rate compared to total methylation was also related to changes in body weight and body fat. These results suggest that postoperative management, with a focus on exercise therapy, affects body weight and body fat via activation of fat metabolism by hypomethylation of the PDK4 gene.
Abbreviation list
PDK4, pyruvate dehydrogenase kinase 4; OA knee, osteoarthritis of the knee; TKA/UKA, total knee arthroplasty/unicompartmental knee arthroplasty; PDC, pyruvate dehydrogenase complex; NGS, Next Generation Sequencer; BMI, body mass index; RA, rheumatoid arthritis; CPM, continuous passive motion; METs, metabolic equivalents; PT, physical therapist; PCR, polymerase chain reaction.
Acknowledgments
We would like to thank the patients who participated in the present study. We express our gratitude for the assistance of staff members of the Health Science Center, Orthopedic and Rehabilitation of Kansai Medical University Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly. The framingham osteoarthritis study. Arthritis Rheum. 1995;38(10):1500–1505. doi:10.1002/art.17803810177575700
- Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the framingham study. Arthritis Rheum. 1997;40(4):728–733. doi:10.1002/1529-0131(199707)40:7<1267::AID-ART11>3.0.CO;2-L9125257
- Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128(1):179–189. doi:10.1093/oxfordjournals.aje.a1149393381825
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The framingham study. Ann Intern Med. 1988;109(1):18–24. doi:10.7326/0003-4819-109-1-183377350
- Slemaenda C, Brandt KD, Heilman DK, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127(2):97–104. doi:10.7326/0003-4819-127-3-199708010-000229230035
- Kumar D, Karampions DC, MacLeod TD, et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):226–234. doi:10.1016/j.joca.2013.12.00524361743
- Griffin FM, Scuderi GR, Insall JN, Colizza W. Total knee arthroplasty in patients who were obese with 10 years followup. Clin Orthop Relat Res. 1998;356:28–33. doi:10.1097/00003086-199811000-00006
- Ritter MA, Davis KE, Meding JB, Pierson JL, Berend ME, Malinzak RA. The effect of alignment and BMI on failure of total knee replacement. J Bone Joint Surg Am. 2011;93(17):1588–1596. doi:10.2106/JBJS.J.0071121915573
- Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33(6 Suppl):446–451. doi:10.1097/00005768-200106001-00013
- American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American college of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi:10.1249/MSS.0b013e3181a0c95c19516148
- Rankinen T, Perusse L, Rauramaa R, Rivera MA, Wolfarth B, Bouchard C. The human gene map for performance and health-related fitness phenotypes. Med Sci Sports Exerc. 2001;33(6):885. doi:10.1097/00005768-200106000-00001
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–638. doi:10.1016/j.cell.2007.02.00617320500
- Lawrence RJ, Earley K, Pontes O, et al. A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell. 2004;13(4):599–609. doi:10.1016/S1097-2765(04)00064-414992728
- Waddington CH. Genetic assimilation of the bithorax phenotype. Evolution. 1956;10(1):1–13. doi:10.1111/evo.1956.10.issue-1
- Baar K. Epigenetic control of skeletal muscle fibre type. Acta Physiol (Oxf). 2010;199(4):477–487. doi:10.1111/j.1748-1716.2010.02121.x20345412
- Pilegaard H, Keller C, Steensberg A, et al. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol. 2002;541(Pt 1):261–271. doi:10.1113/jphysiol.2002.01683212015434
- Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–196. doi:10.1042/bj32901919405293
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284(5):855–862. doi:10.1152/ajpendo.00526.2002
- Carrió E, Suelves M. DNA methylation dynamics in muscle development and disease. Front Aging Neurosci. 2015;7:19. doi:10.3389/fnagi.2015.0001925798107
- Barres R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–411. doi:10.1016/j.cmet.2012.01.00122405075
- Nitert MD, Dayeh T, Volkov P, et al. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61(12):3322–3332. doi:10.2337/db11-129623028138
- Seaborne RA, Strauss J, Cocks M, et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep. 2018;8(1):1898. doi:10.1038/s41598-018-20287-329382913
- Turner DC, Seaborne RA, Sharples AP. Comparative transcriptome and methylome analysis in human skeletal muscle anabolism, hypertrophy and epigenetic memory. Sci Rep. 2019;9(1):4251. doi:10.1038/s41598-019-40787-030862794
- Rowlands DS, Page RA, Sukala WR, et al. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in type 2 diabetic obesity. Physiol Genomics. 2014;46(20):747–765. doi:10.1152/physiolgenomics.00024.201425138607
- Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307–315.13470504
- Bakshi A, Ekram MB, Kim J. Locus-specific DNA methylation analysis of retrotransposons in ES, somatic and cancer cells using high-throughput targeted repeat element bisulfite sequencing. Genom Data. 2015;3:87–89. doi:10.1016/j.gdata.2014.11.01325554740
- Zhao W, Mo Y, Wang S, et al. Quantitation of DNA methylation in epstein-barr virus-associated nasopharyngeal carcinoma by bisulfite amplicon sequencing. BMC Cancer. 2017;17(1):489. doi:10.1186/s12885-017-3482-328716111
- Garinet S, Neou M, de La Villeon B, et al. Calling chromosome alterations, DNA methylation statues, and mutations in tumors by simple targeted next-generation sequencing: a solution for transferring integrated pangenomic studies into routine practice? J Mol Diagn. 2017;19(5):776–787. doi:10.1016/j.jmoldx.2017.06.00528826610
- Maunakea AK, Nagarajan RP, Bilenky M, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466(7303):253–257. doi:10.1038/nature0917220613842
- Willmer T, Johnson R, Louw J, Pheiffer C. Blood-based DNA methylation biomarkers for type2 diabetes: potential for clinical applications. Front Endocrinol (Lausanne). 2018;9:744. doi:10.3389/fendo.2018.0042030564199
- Pheiffer C, Erasmus RT, Kengne AP, Matsha TE. Differential DNA methylation of microRNAs within promoters, intergenic and intragenic regions of type 2 diabetic, pre-diabetic and non-diabetic individuals. Clin Biochem. 2016;49(6):433–438. doi:10.1016/j.clinbiochem.2015.11.02126656639
- Schouten JS, van Den Ouweland FA, Valkenburg HA. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis. 1992;51(8):932–937. doi:10.1136/ard.51.8.9321417116
- Lee S, Kim TN, Kim SH. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum. 2012;64(12):3947–3954. doi:10.1002/art.3769623192792
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship lipoprotein lipase. J Clin Invest. 1995;95(5):2111–2119. doi:10.1172/JCI1178997738178
- Bray GA. Complications of obesity. Ann Intern Med. 1985;103(6):1052–1062. doi:10.7326/0003-4819-103-6-10524062125
- Ersozlu S, Akkaya T, Ozgur AF, Shin O, Senturk I, Tandogan R. Bilateral staged total knee arthroplasty in obese patients. Arch Orthop Trauma Surg. 2008;128(2):143–148. doi:10.1007/s00402-007-0356-117694313
- Mont MA, Mathur SK, Krackow KA, Loewy JW, Hungerford DS. Cementless total knee arthroplasty in obese patients. A comparison with a matched control group. J Arthroplasty. 1996;11(2):153–156. doi:10.1016/S0883-5403(05)80009-98648308
- Constantin-Teodosiu D, Constantin D, Stephens F, Laithwaite D, Greenhaff PL. The role of FOXO and PPAR transcription factors in diet-mediated inhibition of PDC activation and carbohydrate oxidation during exercise in humans and the role of pharmacological activation of PDC in overriding these changes. Diabetes. 2012;61(5):1017–1024. doi:10.2337/db11-129622315317
- Psilander N, Frank P, Flockhart M, Sahlin K. Exercise with low glycogen increases PGC-1α gene expression in human skeletal muscle. Eur J Appl Physiol. 2013;113(4):951–963. doi:10.1007/s00421-012-2504-823053125
- Bartlett JD, Louhelainen J, Iqbal Z, et al. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;304(6):450–458. doi:10.1152/ajpregu.00498.2012
- Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87(1):22–29. doi:10.1152/jappl.1999.87.1.2210409554
- Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26(10):1135–1145. doi:10.1038/nbt148618846087