Abstract
Purpose
Behavioral and psychiatric symptoms of dementia (BPSD) are common in Alzheimer’s disease (AD) and disrupt the effective management of AD patients. The present study explores the use of radio electric asymmetric brain stimulation (REAC) in patients who have had a poor response to pharmacological treatment.
Patients and methods
Eight patients (five females and three males; mean [±standard deviation] age at study baseline: 69.9 ± 3.0 years) diagnosed with AD according to the DSM-IV-TR criteria (mean onset age of AD: 65.4 ± 3.5 years) were cognitively and psychometrically assessed with the Mini-Mental State Examination (MMSE), the Activity of Daily Living (ADL), the Instrumental Activity of Daily Living (IADL), and the Neuropsychiatric Inventory (NPI), prior to and after each of 2 REAC treatment cycles.
Results
Scores on the MMSE and all subscales of the NPI (frequency, severity, and distress), the ADL, and the IADL were significantly improved following the initial REAC treatment. There was further significant improvement in all measurements (with a tendency for improvement in the IADL) after the second REAC treatment cycle.
Conclusion
The improvement of cognitive and behavioral/psychiatric functioning following REAC treatment suggests that this innovative approach may be an effective, safe, and tolerable alternative to pharmacological treatment of AD patients, especially in the area of BPSD. Elderly patients suffering from other types of dementia may also benefit from REAC treatment.
Introduction
The progressively increasing number of the elderly in the general population and, consequently, the growing prevalence of Alzheimer diseaseCitation1,Citation2 (AD) highlight the need for new specifically targeted therapeutic options.Citation3–Citation6 The currently available psychologicalCitation7–Citation10 and psychopharmacologicalCitation11–Citation15 treatmentsCitation16 for AD provide relatively poor results. The inadequate and/or insufficient safety and tolerability profile of ‘typical’ AD therapies produce familiar social and economic difficulties.Citation17,Citation18 According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), AD includes a disruption in attention,Citation19,Citation20 concentration, memory,Citation21,Citation22 and cognition.Citation23 While the DSM-IV-TR instructs diagnosing clinicians to specify whether cognitive symptoms of AD are accompanied by behavioral abnormalities (eg, delusions, depression, anxiety, agitation, wandering, sexual behavior alterations, sleep dysfunctions, disorientation), behavioral symptoms of AD are not included in the working definition.
The relationship between AD and the behavioral and psychiatric symptoms of dementiaCitation24 (BPSD) is becoming more prevalent. Symptoms of dementia accompany AD in about 90% of cases,Citation25 typically arising early in the course of the disease and persisting. Unlike the steady loss of “global” cognition, throughout the course of AD, behavioral symptoms are more variable, with different types of BPSD seen among patients.Citation26 Despite the observed inter-patient variability in number and type of behavioral symptoms encountered in AD, patients with advanced illness tend to have more behavioral symptoms than those in earlier phases of the disorder. Several long-term studies indicate that once a specific symptom occurs in a given patient, it is likely to persist or recur thereafter.
Radio electric asymmetric brain stimulationCitation27,Citation28 (REAC) treatment has proven efficacy in ameliorating several stress-related disorders, depression, and anxiety,Citation29–Citation35 and our unpublished data have shown promising results for some forms of dementias. REAC treatments are painless, noninvasive, and have a high safety and tolerability profile. On the basis these we can hypothesize that the treatments with REAC technology may be helpful in AD and, in general, in several forms of cognitive-impairment disorders.
Aim of the present study
REAC has demonstrated efficacy in improving certain psychiatric disorders such as anxiety, depression and bipolar disorder. The main goal of the present study was to investigate the efficacy of REAC in the treatment of behavioral symptoms in AD patients. In addition, effects on cognitive functioning were also investigated.
Materials and methods
The data for the current study were collected during routine therapy sessions at the Rinaldi Fontani Institute, Behavioral Disorder Department in Florence, Italy. Most patients referred to the clinic were nonresponders to typical pharmacological strategies. Eight patients (five females and three males; mean [±SD] age at study baseline: 69.9 ± 3.0 years) diagnosed with AD according to the DSM-IV-TR criteria (mean onset age of AD: 65.4 ± 3.5 years), presenting with behavioral and/or psychiatric disturbances were psychometrically assessed using the Mini-Mental State ExaminationCitation36 (MMSE), the Activity of Daily LivingCitation37 (ADL), the Instrumental Activity of Daily LivingCitation38 (IADL), and the Neuropsychiatric InventoryCitation39 (NPI), before and after 2 treatment cycles of REAC.
REAC is applied by a medical device that is based on an innovative biostimulation technology.Citation27,Citation28 REAC works within a typical frequency range of 2.4, 5.8, or 10.5 GHz, selected by the operator for each specific protocol. For the current study, a frequency of 10.5 GHz, with a specific absorption rate (SAR) of 7 μW/kg, was used. The neuro postural optimization (NPO) protocol consisting of a single radiofrequency burst of 500 milliseconds (ms) applied by touching the metallic tip of the REAC probe (Convogliatore di Radianza Modulante – CRM; ASMED, Italy) to the ear pavilion was performed as an initial treatment. NPO was followed by another treatment protocol, named neuro psycho physical optimization (NPPO). This protocol consisting of seven REAC radiofrequency bursts of 500 ms applied by touching the metallic tip of the REAC probe at precise points of the ear. Each therapy session lasts about 5 seconds. An NPPO treatment cycle consists of 18 therapy sessions, administered on alternate days. The mean interval between the first and the second NPPO treatment cycle is about 6 months.
ANOVA for repeated measures was performed for statistical analysis of differences in MMSE, ADL, IADL, and NPI scores prior to and following REAC treatments. P values <0.05 were considered significant.
Results
After the first REAC treatment cycle, patients showed great improvement in cognitive performance as demonstrated by a significant increase in average MMSE total score from 18.5 (±2.4) to 23.4 (±2.3) (ANOVA P < 0.01) ( and ). In addition, BPSD was improved as demonstrated by a decrease in average NPI frequency score ( and ) from 32.2 (±2.5) to 26.4 (±2.4) (ANOVA P = 0.000), NPI severity score ( and ) from 28.6 (±3.4) to 22.1 (±3.4) (ANOVA P = 0.000), and NPI distress score from ( and ) 49.5 (±3.6) to 41.0 (±2.1) (ANOVA P = 0.000). In addition, average ADL score ( and ) increased from 3.8 (±1.0) to 4.9 (±0.5) (ANOVA P = NS) and average IADL score ( and ) increased from 10.0 (±2.4) to 13.0 (±1.1) (ANOVA P = 0.000).
Table 1 Total score variation before and after first REAC treatment cycle
Figure 1 Mini-Mental State Examination (MMSE) before, after the first and second REAC treatment cycle.
Abbreviations: REAC, radio electric asymmetric brain stimulation; TSV, total score variation.
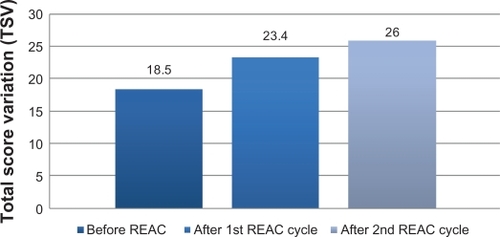
Figure 2 Neuro Psychiatric Inventory (NPI) before after first and second REAC treatment cycle.
Abbreviation: REAC, radio electric asymmetric brain stimulation.
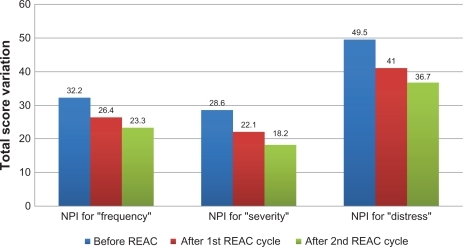
Figure 3 Activity of Daily Living (ADL), before, after first and second REAC treatment cycle.
Abbreviation: REAC, radio electric asymmetric brain stimulation.
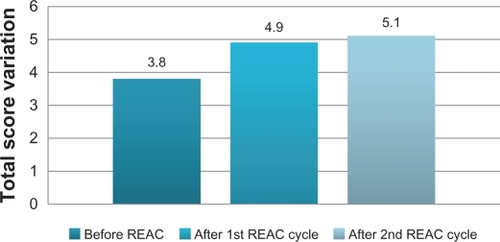
Figure 4 Instrumental Activity of Daily Living (IADL), before, after first and second REAC treatment cycle.
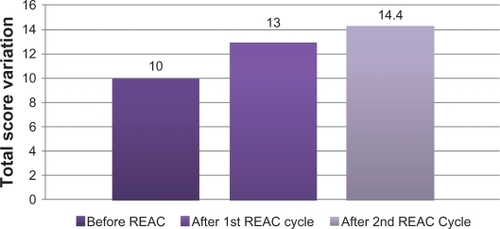
After the second REAC treatment cycle, there were further significant ameliorations in both cognitive and behavioral performance (). There was a significant increase in average MMSE total score ( and ) to 26.0 (±3.4) (ANOVA P < 0.001), and a significant decrease in NPI frequency score ( and ) to 23.3 (±2.3) (ANOVA P < 0.001), NPI severity score ( and ) to 18.2 (± 3.7) (ANOVA P = 0.000), and NPI distress score ( and ) to 36.7 (±4.3) (ANOVA P = 0.000). IADL total score was significantly increased after the second REAC treatment to 14.4 (±0.7) (ANOVA P = NS) ( and ). The increase in ADL total score to 5.1 ± 0.4 ( and ) was not statistically significant (ANOVA P = NS).
Table 2 Total score value of first versus second REAC treatment cycle
Discussion
REAC treatment enhanced cognitive and behavioral functioning in patients with AD. All measures of cognitive functioning were significantly improved after the initial REAC treatment and continued to improve after the second REAC cycle. Similarly, all behavioral measurements were positively affected after the first REAC treatment and most continued to improve after the second cycle.
It is likely that the effects on cognition and behavior are due to a process of synchronization of brain function caused by the microelectric stimulation of the REAC.Citation29
While cognitive functioning benefitted from REAC treatment, the positive effects on BPSD may be of more importance in the management of AD patients. The improvement in these clinical parameters is reflected in functional terms by an increase in the quality of life of patients as reported by relatives, physicians, and care-givers and is psychometrically demonstrated by the ADL and IADL total scores. The use of REAC treatment may decrease the need to continually alter pharmacological treatments to achieve optimal results, leading to significant savings in economic resources of rehabilitation programs for AD patients.
REAC treatments alone are not likely to manage all deficits associated with neuropsychiatric disorders in the elderly. However, the results of the present study suggest that REAC treatment has a high safety, tolerability, and efficacy profile and may be useful not only in patients who have experienced poor and/or unstable responses to psychotropic drugs, but also as a first-line therapeutic option. REAC treatment may be useful not only for AD patients, but also for other forms of dementia (ie, vascular dementia or mixed conditions).
Acknowledgements
The authors thank Dr Eng Matteo Lotti Margotti for data analysis. Lucia Aravagli MD and Stefania Bini MD of Rinaldi Fontani Institute, Department of Neuro Psycho Physio Pathology, Florence, Italy, for their helpful discussions.
Disclosure
Salvatore Rinaldi and Vania Fontani are the inventors of the Radio Electric Asymmetric Conveyer.
Reference
- ChouliarasLRuttenBPKenisGEpigenetic regulation in the pathophysiology of Alzheimer’s diseaseProg Neurobiol201090449851020097254
- SallowaySIntroduction: the prevalence of Alzheimer’s disease – a growing crisisCNS Spectr2008133 Suppl 3118323759
- LleoACurrent therapeutic options for Alzheimer’s diseaseCurr Genomics20078855055819415128
- MoreiraPIZhuXNunomuraASmithMAPerryGTherapeutic options in Alzheimer’s diseaseExpert Rev Neurother20066689791016784412
- Garcia-Ruiz EspigaPJEcheverriaAGarcia-TorresAContrerasANew therapeutic options in Alzheimer’s diseaseRev Neurol200642847848116625510
- KurzAPerneczkyRNovel insights for the treatment of Alzheimer’s diseaseProg Neuropsychopharmacol Biol Psychiatry201135237337920655969
- BallardCCorbettAChitramohanRAarslandDManagement of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutionsCurr Opin Psychiatry200922653254019696673
- MathiasJLBurkeJCognitive functioning in Alzheimer’s and vascular dementia: a meta-analysisNeuropsychology200923441142319586206
- LogsdonRGMcCurrySMTeriLEvidence-based psychological treatments for disruptive behaviors in individuals with dementiaPsychol Aging2007221283617385980
- O’ConnorDWAmesDGardnerBKingMPsychosocial treatments of psychological symptoms in dementia: a systematic review of reports meeting quality standardsInt Psychogeriatr200921224125119138459
- RoccaPMarinoFMontemagniCPerroneDBogettoFRisperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer’s disease: preliminary findings from a naturalistic, retrospective studyPsychiatry Clin Neurosci200761662262918081622
- LauterbachECVictoroffJCoburnKLShillcuttSDDoonanSMMendezMFPsychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical dataJ Neuropsychiatry Clin Neurosci201022181820160205
- MarksteinerJSchmidtRTreatment strategies in Alzheimer’s disease with a focus on early pharmacological interventionsDrugs Aging200421741542615132710
- MassoudFGauthierSUpdate on the pharmacological treatment of Alzheimer’s diseaseCurr Neuropharmacol201081698020808547
- HoganDBProgress update: pharmacological treatment of Alzheimer’s diseaseNeuropsychiatr Dis Treat20073556957819300586
- ZecRFBurkettNRNon-pharmacological and pharmacological treatment of the cognitive and behavioral symptoms of Alzheimer diseaseNeuro Rehabilitation200823542543818957729
- WimoANorlundACommentary on “health economics and the value of therapy in Alzheimer’s disease.” Cost-effectiveness studiesAlzheimers Dement20073315716119595931
- ZhuCWSanoMEconomic considerations in the management of Alzheimer’s diseaseClin Interv Aging20061214315418044111
- PerettiCSFerreriFBlanchardFNormal and pathological aging of attention in presymptomatic Huntington’s, Huntington’s and Alzheimer’s disease, and nondemented elderly subjectsPsychother Psychosom200877313914618277060
- SolfrizziVPanzaFTorresFSelective attention skills in differentiating between Alzheimer’s disease and normal agingJ Geriatr Psychiatry Neurol20021529910912083601
- DaulatzaiMAEarly stages of pathogenesis in memory impairment during normal senescence and Alzheimer’s diseaseJ Alzheimers Dis201020235536720164576
- ScheffSWPriceDASchmittFAMufsonEJHippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairmentNeurobiol Aging200627101372138416289476
- DumontCVoisinTNourhashemiFAndrieuSKoningMVellasBPredictive factors for rapid loss on the mini-mental state examination in Alzheimer’s diseaseJ Nutr Health Aging20059316316715864396
- ChanDCKasperJDBlackBSRabinsPVPrevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: the memory and medical care studyInt J Geriatr Psychiatry200318217418212571828
- Garcia-AlbercaJMLaraJPBerthierMLCan impairment in memory, language and executive functions predict neuropsychiatric symptoms in Alzheimer’s disease (AD)? Findings from a cross-sectional studyArch Gerontol Geriatr201152326426920570375
- Vilalta-FranchJLopez-PousaSTuron-EstradaASyndromic association of behavioral and psychological symptoms of dementia in Alzheimer disease and patient classificationAm J Geriatr Psychiatry2010185421432
- RinaldiSFontaniVInventorRinaldiSFontaniVassigneeRadioelectric Asymmetric Conveyer for therapeutic use US patent 7,333,8592001
- RinaldiSFontaniV Radioelectric Asymmetric Conveyer for therapeutic use. European Patent Office – World Intellectual Property Organization. October 11, 20062000 (EP20010960475 20010706)
- MannuPRinaldiSFontaniVCastagnaALong-term treatment of bipolar disorder with a radio-electric asymmetric conveyorNeuropsychiatr Dis Treat201171373379
- CastagnaARinaldiSFontaniVAravagliLMannuPMargottiMLDoes osteoarthritis of the knee also have a psychogenic component? Psycho-emotional treatment with a radio-electric device vs intra-articular injection of sodium hyaluronate: an open-label, naturalistic studyAcupunct Electrother Res2010351–211620578643
- CollodelGMorettiEFontaniVEffect of emotional stress on sperm qualityIndian Journal of Medical Research2008128325426119052335
- MannuPRinaldiSFontaniVCastagnaALotti MargottiMRadio electric treatment vs es-citalopram in the treatment of panic disorders associated with major depression: an open-label, naturalistic studyAcupunct Electrother Res20093413514920344882
- RinaldiSFontaniVAravagliLMargottiMLPsychological and symptomatic stress-related disorders with radio-electric treatment: psychometric evaluationStress Health2010265350358
- RinaldiSFontaniVAravagliLMannuPPsychometric evaluation of a radio electric auricular treatment for stress related disorders: a double-blinded, placebo-controlled controlled pilot studyHealth Qual Life Outcomes2010813120302662
- RinaldiSFontaniVMorettiEA new approach on stress-related depression and anxiety: neuro-psycho-physical-optimization with radio electric asymmetric-conveyerIndian Journal of Medical Research201013218919420716819
- SoubeletASalthouseTACorrelates of level and change in the mini-mental state examinationPsychol Assess4112011
- YoshinoHSakuraiTHasegawaKYokonoKCauses of decreased activity of daily life in elderly patients who need daily living careGeriatr Gerontol Int201111329730321272178
- FujiwaraYChavesPHYoshidaHIntellectual activity and likelihood of subsequently improving or maintaining instrumental activities of daily living functioning in community-dwelling older Japanese: a longitudinal studyInt J Geriatr Psychiatry200924654755519132642
- ZuidemaSUBuursemaALGerritsenMGAssessing neuropsychiatric symptoms in nursing home patients with dementia: reliability and reliable change index of the neuropsychiatric inventory and the cohen-mansfield agitation inventoryInt J Geriatr Psychiatry201126212713420690131