Abstract
Purpose
To assess the safety and effectiveness of a novel, minimally invasive interspinous spacer in patients with moderate lumbar spinal stenosis (LSS).
Methods
A total of 53 patients (mean age, 70 ± 11 years; 45% female) with intermittent neurogenic claudication secondary to moderate LSS, confirmed on imaging studies, were treated with the Superion® Interspinous Spacer (VertiFlex, Inc, San Clemente, CA) and returned for follow-up visits at 6 weeks, 1 year, and 2 years. Study endpoints included axial and extremity pain severity with an 11-point numeric scale, Zurich Claudication Questionnaire (ZCQ), back function with the Oswestry Disability Index (ODI), health-related quality of life with the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores from the SF-12, and adverse events.
Results
Axial and extremity pain each decreased 54% (both P < 0.001) over the 2-year follow-up period. ZCQ symptom severity scores improved 43% (P < 0.001) and ZCQ physical function improved 44% (P < 0.001) from pre-treatment to 2 years post-treatment. A statistically significant 50% improvement (P < 0.001) also was noted in back function. PCS and MCS each improved 40% (both P < 0.001) from pre-treatment to 2 years. Clinical success rates at 2 years were 83%–89% for ZCQ subscores, 75% for ODI, 78% for PCS, and 80% for MCS. No device infection, implant breakage, migration, or pull-out was observed, although two (3.8%) patients underwent explant with subsequent laminectomy.
Conclusion
Moderate LSS can be effectively treated with a minimally invasive interspinous spacer. This device is appropriate for select patients who have failed nonoperative treatment measures for LSS and meet strict anatomical criteria.
Keywords:
Introduction
Lumbar spinal stenosis (LSS) is a progressive degenerative narrowing of the central spinal canal and/or lateral neuroforamen that commonly leads to neurogenic claudication by compression of the spinal nerves and associated vasculature.Citation1 LSS currently affects 1 million people in the United States and is the most common indication for spinal surgery in the elderly.Citation2 While some patients with LSS are asymptomatic, most present with leg pain, numbness, and tingling that is exacerbated with ambulation and extension movements of the spinal column. Ultimately, these symptoms result in lower quality of life and impaired functional capacity.Citation3
Despite the lack of convincing evidence to support their use, initial management of mild radicular symptoms focuses on nonsurgical options and includes activity modification, physical therapy, analgesic and anti-inflammatory medications, and epidural spinal injections.Citation4 However, none of these therapies has demonstrated long-term effectiveness since they do not alter the course of disease progression.Citation5,Citation6 As the disease worsens to yield moderate symptoms, patients must tolerate progressively persistent pain and functional impairment since no additional treatment options are available. Ultimately, as the disease advances to produce chronic and severe symptoms, open spinal surgery such as decompressive laminectomy with or without fusion is often required to alleviate symptoms.Citation7,Citation8
Minimally invasive lumbar procedures represent a viable alternative that addresses the therapeutic gap for patients with moderate radicular symptoms. In particular, interspinous process decompression utilizes a spacer that is implanted between contiguous spinous processes to limit back extension at the symptomatic level, thereby improving patient symptoms.Citation9 Potential advantages of this procedure versus other surgical procedures include lower neural injury risk, ability to intervene earlier in the disease process before symptoms become debilitating, and preservation of anatomical structures which allows the option of more invasive surgery in the future, should severe symptoms recur or further mechanical changes ensue. The X-STOP® Interspinous Process Decompression System (Medtronic, Inc, Minneapolis, MN) has been extensively studied in clinical trials,Citation10–Citation16 although reports with other spacers are less common.Citation17–Citation21 Despite overall favorable results with these devices in anatomically suitable patients, the clinical benefit of interspinous spacers is debatable given the paucity of available long-term data and the risk for device-related complications.Citation22,Citation23 This study was conducted to evaluate 2-year clinical outcomes in patients with moderate LSS who were treated with a novel, minimally invasive interspinous spacer.
Materials and methods
Patients
This single-arm prospective study enrolled 53 patients with moderate LSS between July 2007 and March 2008. All patients were treated with the Superion® Interspinous Spacer (VertiFlex, San Clemente, CA). Inclusion criteria for this study included: (1) diagnosis of moderate LSS, defined as 25%–50% reduction in lateral/central foramen diameter compared with adjacent levels and radiographic evidence (magnetic resonance imaging or computed tomography) of thecal sac and/or cauda equine compression, nerve root impingement by either osseous or nonosseous elements, and/or hypertrophic facets with canal encroachment; (2) persistent leg, buttock, or groin pain, with or without back pain, that was relieved by lumbar flexion; and (3) unsuccessful nonoperative treatment for at least 3 months. Exclusion criteria included (1) axial back pain only, (2) grade II–V spondylolisthesis, (3) unremitting back pain in any spinal position, (4) active systemic disease that may affect the welfare of the patient, (5) vertebral osteoporosis or history of vertebral fracture, and (6) pregnant or lactating female. The procedures used in this clinical study were in accordance with the recommendations of the Helsinki Declaration, and each patient provided written informed consent before surgery.
Intervention
The Superion device is a single-piece, self-expanding titanium implant that is delivered via percutaneous access and deployed between the spinous processes of the symptomatic vertebral levels (). The minimally invasive procedure began with the patient lying prone on a radiolucent table with the lumbar spine in a neutral or slightly flexed position. Using fluoroscopic guidance or direct visualization, the symptomatic level was identified and a 12–15 mm midline incision was made. The supraspinous ligament was longitudinally dissected at the symptomatic level and was then dilated to ensure adequate room to maneuver within the interspinous space. A cannula was inserted over the dilator, and proper alignment and depth were ensured before dilator removal. Next, an interspinous gauge was inserted through the cannula to determine proper implant size selection. Final midline positioning was confirmed under fluoroscopy.
Figure 1 (A) Postero-anterior and (B) lateral radiographic images showing a properly placed Superion® Interspinous Spacer (VertiFlex, Inc, San Clemente, CA).
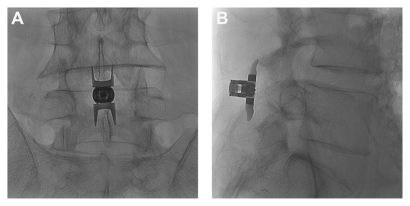
The appropriately sized spacer was delivered through the cannula using a device inserter that loaded the implant, inserted it into the interspinous space via the cannula, and then deployed the implant. Proper device placement was confirmed with fluoroscopy. Finally, the inserter and cannula were removed, and the incision was sutured in a standard fashion.
Outcomes
Patients were assessed pre-treatment and then returned for follow-up visits at 6 weeks and at 1 and 2 years post-treatment. Axial and extremity pain severity was measured with an 11-point (0–10) numeric pain scale at pre-treatment and postoperatively only. The Zurich Claudication Questionnaire (ZCQ) was utilized to assess patient-reported measures of symptom severity, physical function, and patient satisfaction. Citation14 Back-specific functional disability was measured with the Oswestry Disability Index (ODI) (version 2) on a 0%–100% scale.Citation24 Health-related quality of life was assessed with the SF-12®, version 2, (Medical Outcomes Trust, Hanover, NH) and Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were recorded.Citation25 Safety was assessed by incidence of reported adverse events through the 2-year follow-up period.
Data analysis
Data were analyzed using Predictive Analytics Software, version 18 (SPSS, Inc, Chicago, IL). Patients included in this report had 2-year data available for at least one of the following variables: axial pain, extremity pain, ZCQ, ODI, PCS, and MCS. Continuous data were reported as mean ± standard deviation, and categorical data were reported as frequencies and percentages. Longitudinal changes in patient outcomes were analyzed with repeated measures analysis of variance. Clinical success at each follow-up period was defined as a ≥30% improvement in pain scores,Citation26,Citation27 ≥0.5 point improvement in ZCQ symptom severity and physical function,Citation14 ZCQ patient satisfaction score ≤2.5,Citation14 ≥30% improvement in ODI,Citation26,Citation28 ≥5.7-point improvement in PCS,Citation29 and ≥6.3-point improvement in MCS.Citation29
Results
Patient characteristics included mean age of 70 ± 11 years, 45% female, 98% (52 of 53) with single level disease, moderate disability, and severe back pain with a mean duration of 30 ± 31 months. Implant size ranged from 8 to 16 mm with the 11–13 mm devices accounting for two-thirds of implants ().
Table 1 Patient characteristics
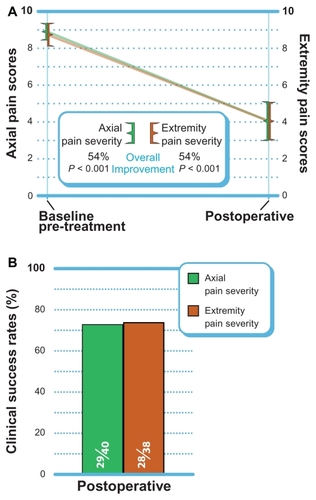
Axial and extremity pain severity
Axial pain decreased 54% (P < 0.001) from 8.9 ± 1.4 at pre-treatment to 4.1 ± 3.4 postoperatively (). At the postoperative follow-up, 73% (29 of 40) of patients achieved the clinical success threshold of a ≥30% improvement (). Similar improvements in extremity pain were realized with a mean improvement of 54% from 8.7 ± 1.9 at pre-treatment and 4.1 ± 3.2 postoperatively (P < 0.001). Extremity pain clinical success was 74% (28 of 38) postoperatively.
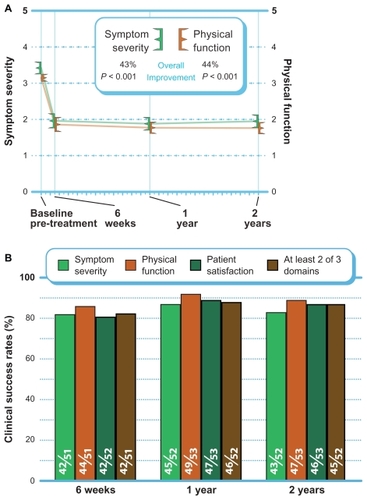
ZCQ
ZCQ symptom severity scores improved 43% (P < 0.001), and ZCQ physical function improved 44% (P < 0.001) from pre-treatment to 2 years post-treatment (). The mean ZCQ patient satisfaction score was 1.9 at all follow-up visits. When using the standard criteria of an improvement of 0.5 points or greater to define 2-year clinical success, 83% (43 of 52) of patients achieved ZCQ symptom severity clinical success, and 89% (47 of 53) of patients achieved ZCQ physical function clinical success. Patient satisfaction clinical success (score ≤2.5) at 2 years was 87% (46 of 53). The proportion of patients that achieved at least two of three clinical success criteria at 2 years was 87% (45 of 52) ().
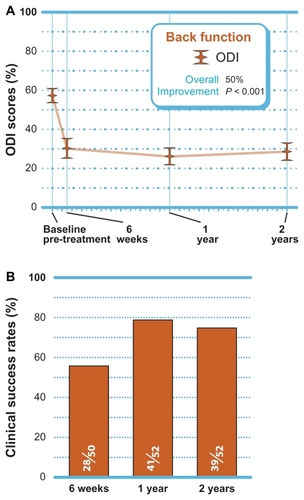
Back-specific functional impairment
A statistically significant 50% improvement (P < 0.001) in back function was noted through 2 years post-treatment (). When using the standard criteria of an improvement of 30% or greater to define clinical success, 75% (39 of 52) of patients achieved ODI clinical success at 2 years ().
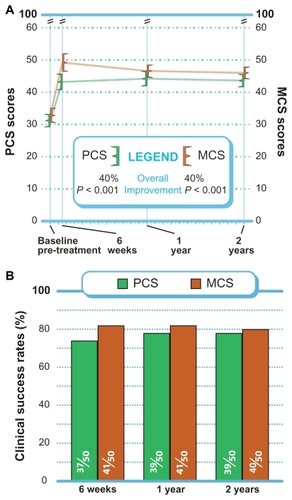
Quality of life assessment
PCS and MCS each improved 40% (P < 0.001) from pre- treatment to 2 years (). Through 2 years, MCS clinical success was achieved in 80% (40 of 50) of patients, while PCS clinical success was achieved in 78% (39 of 50) of patients ().
Adverse events
No device infection, implant breakage, migration, or pull-out was observed through the 2-year follow-up visit. Two (3.8%) patients underwent explant with subsequent laminectomy due to persistent radicular symptoms.
Discussion
The Superion Interspinous Spacer is a novel, minimally invasive device that offers excellent safety and effectiveness for the patient with intermittent neurogenic claudication secondary to moderate LSS based on the 2-year outcomes reported in the current study. All clinical markers of pain, function, and health-related quality of life improved by approximately 50% over the 2-year follow-up period.
The outcomes observed in this study compare favorably to those reported with the X-STOP spacer with improvements of 44% for ZCQ physical function, 45% for ZCQ symptom severity, 36% for extremity pain, and 38% for ODI in these studies.Citation12,Citation16 Furthermore, the 3.8% reintervention rate in the current series approximates the 1.0%–4.6% reintervention rate reported in the largest X-STOP trials.Citation12,Citation16
The safety and effectiveness of the Superion device also appears to be similar to that of laminectomy. Success rates of 74%–89% were observed, depending on the outcome, in the current series. Overall success rates with laminectomy range from 26% to 100%.Citation8,Citation30–Citation32 No device- complications were observed in the current series, although persistent radicular symptoms requiring device explant were reported in two (3.8%) patients. Common complications of laminectomy are dural tear (6%), infection (3%), and deep vein thrombosis (3%).Citation8
Despite the low incidence of complications in this series, several concerns have been raised regarding use of interspinous spacers including device subsidence, spinous process fracture, treated segment destabilization, and adjacent segment degeneration. Although concern for device subsidence has been acknowledged with interspinous spacers, no known study has reported this complication to date. Spinous process fracture has been noted in 0%–6% of patients treated with interspinous spacers who were followed with plain radiographs,Citation16,Citation33,Citation34 although one study reported a 29% fracture incidence with computed tomographic imaging.Citation35 Low lumbar bone mineral density has been suggested as a potential risk factor for device fracture, and severe osteoporosis is a common exclusion criterion for these devices.Citation35 Destabilization of the treated segment is purported as a potential risk of interspinous spacer treatment. Biomechanical studies have yielded mixed conclusions on the effect of an implanted interspinous spacer on segmental range of motion,Citation36,Citation37 and no definitive evidence from human trials currently exists. Despite concern for adjacent segment degeneration with long-term interspinous spacer implant, no biomechanical or human study has demonstrated that an interspinous spacer has deleterious effects on adjacent levels.Citation36,Citation38,Citation39 Overall, complications with interspinous spacers are uncommon and seem to be primarily attributable to improper patient selection.Citation34
The appropriateness of patients with low-grade slip for interspinous spacer treatment is debatable. The study of Verhoof and colleaguesCitation40 investigated interspinous spacer use in 12 patients (9 with grade I slip and 2 with grade II slip) and reported a 58% reintervention rate within 2 years. In the largest study in interspinous spacer use in patients with low-grade slip, patients treated with interspinous spacer (n = 42) had a similar reintervention rate (12%) as subjects treated nonoperatively (n = 33).Citation33 Implantation of an interspinous spacer prevents motion at the implanted levelCitation41 and results in no progression of spondylolisthesis over time.Citation33 Most studies of interspinous spacers allow enrolment of patients with grade I slip, and no compelling evidence exists to exclude these patients from treatment consideration.Citation23,Citation34
Patient selection is critical to ensure a high probability of treatment success with an interspinous spacer. Patients must have confirmatory imaging evidence of LSS, relief of symptoms during lumbar flexion, adequate vertebral bone mineral density, and must be nonresponsive to conservative care efforts in order to realize maximum benefit from this therapy. The favorable safety profile observed with this interspinous spacer for treatment of LSS is attributed not only to careful patient selection but also because resection of the posterior spinal elements was not required, and therefore, motion of the lumbar spine is preserved.
The primary limitation of this study was the lack of a concurrent control group, which may have introduced bias into the interpretation of study outcomes. However, the magnitude of benefit with the interspinous spacer was so dramatic that nonspecific study effects such as placebo could not reasonably account for all of the noted clinical improvement.
Interspinous spacer implant with the Superion device is a safe and effective treatment option for carefully selected patients with moderate LSS who are unresponsive to conservative care but are not yet eligible candidates for traditional spinal surgery such as laminectomy.
Acknowledgments
The authors thank Mr Randy Asher for assistance with graphical illustrations. Vertiflex, Inc, (San Clemente, CA) provided financial support for development of this manuscript.
Disclosure
The authors have no conflicts of interest in this work.
References
- ArbitEPannulloSLumbar stenosis: a clinical reviewClin Orthop Relat Res200138413714311249158
- AtlasSJKellerRBRobsonDDeyoRASingerDESurgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine studySpine (Phila Pa 1976)200025555656210749631
- LinSILinRMHuangLWDisability in patients with degenerative lumbar spinal stenosisArch Phys Med Rehabil20068791250125616935063
- AtlasSJDelittoASpinal stenosis: surgical versus nonsurgical treatmentClin Orthop Relat Res200644319820716462443
- MarkmanJDGaudKGLumbar spinal stenosis in older adults: current understanding and future directionsClin Geriatr Med2008242369388viii18387461
- SimotasACNonoperative treatment for lumbar spinal stenosisClin Orthop Relat Res200138415316111249160
- FritzJMDelittoAWelchWCErhardRELumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurementsArch Phys Med Rehabil19987967007089630153
- TurnerJAErsekMHerronLDeyoRSurgery for lumbar spinal stenosis. Attempted meta-analysis of the literatureSpine (Phila Pa 1976)1992171181531550
- BonoCMVaccaroARInterspinous process devices in the lumbar spineJ Spinal Disord Tech200720325526117473649
- HsuKYZuchermanJFHartjenCAQuality of life of lumbar stenosis-treated patients in whom the X STOP interspinous device was implantedJ Neurosurg Spine20065650050717176013
- KondrashovDGHannibalMHsuKYZuchermanJFInterspinous process decompression with the X-STOP device for lumbar spinal stenosis: a 4-year follow-up studyJ Spinal Disord Tech200619532332716826002
- KuchtaJSobottkeREyselPSimonsPTwo-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosisEur Spine J200918682382919387698
- LeeJHidaKSekiTIwasakiYMinoruAAn interspinous process distractor (X STOP) for lumbar spinal stenosis in elderly patients: preliminary experiences in 10 consecutive casesJ Spinal Disord Tech20041717277 discussion 7814734979
- SiddiquiMSmithFWWardlawDOne-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosisSpine (Phila Pa 1976)200732121345134817515824
- ZuchermanJFHsuKYHartjenCAA prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year resultsEur Spine J2004131223114685830
- ZuchermanJFHsuKYHartjenCAA multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up resultsSpine (Phila Pa 1976)200530121351135815959362
- BuricJPulidoriMLong-term reduction in pain and disability after surgery with the interspinous device for intervertebral assisted motion (DIAM) spinal stabilization system in patients with low back pain: 4-year follow-up from a longitudinal prospective case seriesEur Spine J20112081304131121279392
- BuricJPulidoriMSinanTMehrajSDIAM device for low back pain in degenerative disc disease : 24 months follow-upActa Neurochir Suppl201110817718221107955
- NardiPCabezasDReaGPettoriniBLAperius PercLID stand alone interspinous system for the treatment of degenerative lumbar stenosis: experience on 152 casesJ Spinal Disord Tech201023320320720065864
- SobottkeRSchluter-BrustKKaulhausenTInterspinous implants (X Stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome?Eur Spine J200918101494150319562386
- FabriziAPMainaRSchiabelloLInterspinous spacers in the treatment of degenerative lumbar spinal disease: our experience with DIAM and Aperius devicesEur Spine J201120Suppl 1S202621409561
- KabirSMGuptaSRCaseyATLumbar interspinous spacers: a systematic review of clinical and biomechanical evidenceSpine (Phila Pa 1976)20103525E1499150621102279
- KimDHAlbertTJInterspinous process spacersJ Am Acad Orthop Surg200715420020717426291
- FairbankJCPynsentPBThe Oswestry Disability IndexSpine (Phila Pa 1976)2000252229402952 discussion 295211074683
- JenkinsonCLayteRJenkinsonDA shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?J Public Health Med19971921791869243433
- OsteloRWDeyoRAStratfordPInterpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important changeSpine (Phila Pa 1976)2008331909418165753
- HaggOFritzellPNordwallAThe clinical importance of changes in outcome scores after treatment for chronic low back painEur Spine J2003121122012592542
- ZiglerJDelamarterRSpivakJMResults of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc diseaseSpine (Phila Pa 1976)2007321111551162 discussion 116317495770
- WareJEJrSF-36 health survey updateSpine (Phila Pa 1976)200025243130313911124729
- DunlopRBAdamsMAHuttonWCDisc space narrowing and the lumbar facet jointsJ Bone Joint Surg Br19846657067106501365
- JohnssonKERosenIUdenAThe natural course of lumbar spinal stenosisClin Orthop Relat Res199227982861534726
- KatzJNLipsonSJChangLCLevineSAFosselAHLiangMHSeven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosisSpine (Phila Pa 1976)199621192989122770
- AndersonPATribusCBKitchelSHTreatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesisJ Neurosurg Spine20064646347116776357
- BarbagalloGMOlindoGCorbinoLAlbaneseVAnalysis of complications in patients treated with the X-Stop Interspinous Process Decompression System: proposal for a novel anatomic scoring system for patient selection and review of the literatureNeurosurgery2009651111119 discussion 119–12019574832
- KimDHTantorskiMShawJOccult spinous process fractures associated with interspinous process spacersSpine (Phila Pa 1976)20113616E1080108521343860
- LindseyDPSwansonKEFuchsPHsuKYZuchermanJFYerbySAThe effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spineSpine (Phila Pa 1976)200328192192219714520030
- FuchsPDLindseyDPHsuKYZuchermanJFYerbySAThe use of an interspinous implant in conjunction with a graded facetectomy procedureSpine (Phila Pa 1976)2005301112661272 discussion 1273–126415928550
- WisemanCMLindseyDPFredrickADYerbySAThe effect of an interspinous process implant on facet loading during extensionSpine (Phila Pa 1976)200530890390715834334
- SwansonKELindseyDPHsuKYZuchermanJFYerbySAThe effects of an interspinous implant on intervertebral disc pressuresSpine (Phila Pa 1976)2003281263212544951
- VerhoofOJBronJLWapstraFHvan RoyenBJHigh failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesisEur Spine J200817218819217846801
- GoyalAGoelVKMehtaADickDChinthakuntaSRFerraraLCyclic loads do not compromise functionality of the interspinous spacer or cause damage to the spinal segment: an in vitro analysisJ Long Term Eff Med Implants200818428930220370641