Abstract
Post-herpetic neuralgia is a neuropathic pain syndrome resulting from an insult to the peripheral and central nervous systems caused by the varicella zoster virus. Spontaneous pain may result in the persistent sensation of burning, tingling, or aching and may be associated with thermally or mechanically provoked pain, resulting in hyperalgesia or allodynia. The majority of cases occur in patients over the age of 50 years. Gabapentin is a structural analog of gamma aminobutyric acid that binds to the α2-δ site of voltage-dependent calcium channels and modulates the influx of calcium, with a resulting reduction in excitatory neurotransmitter release. Gabapentin is effective in reducing neuropathic pain due to post-herpetic neuralgia when given at least three times per day, due to its short half-life, resulting in demonstrable fluctuations in plasma levels. Gabapentin has dose-limiting side effects that prevent some patients from achieving therapeutic plasma levels, such as somnolence (27.4%), dizziness (23.9%), and ataxia (7.1%). Gralise™ is a once-daily extended-release formulation of gabapentin that has been developed using AcuForm™ technology. AcuForm is a polymer-based drug delivery system that retains the tablet in the stomach and upper gastrointestinal tract for a sustained period of time. Once-daily dosing has been shown to provide comparable drug exposure with an identical daily dose of the immediate-release formulation when administered three times daily. Participants given Gralise 1800 mg daily had a statistically significant reduction in average daily pain intensity scores compared with placebo, reduced sleep interference due to pain, and a greater percent of participants reporting being much or very much improved on the patient global impression of change. An analysis comparing the efficacy and safety profiles in the aging population (≥65 years) with those younger than 65 years showed that Gralise is effective and well tolerated in both age groups.
Introduction
The International Association for the Study of Pain defines neuropathic pain as “pain caused by a lesion or disease of the somatosensory nervous system”. Post-herpetic neuralgia is a neuropathic pain syndrome that results from an insult to the peripheral and central nervous system caused by the varicella zoster virus. Neuropathic pain can have components of both spontaneous and provoked pain, positive symptoms such as paresthesia and dysesthesia, and sensory deficits that reflect nerve damage. Nerve lesions caused directly by the virus or the inflammatory response can trigger molecular changes in nociceptive neurons leading to channelopathies contributing to the experience of pain.Citation1–Citation3 Spontaneous pain may result in the persistent sensation of burning, tingling, or aching and may be associated with thermally or mechanically provoked pain, resulting in hyperalgesia or allodynia.Citation4 The pain poses a tremendous burden on the patient, as reflected by a significantly lower level of health-related quality of life compared with the general public. Individuals with neuropathic pain generally report a lower health-related quality of life even when compared with individuals with chronic conditions, such as cancer, heart failure, diabetes, and stroke.Citation5 The lower quality of life involves areas of vitality, activities of daily living, and social functioning.Citation6
Figure 1 Rate of herpes zoster and post herpetic neuralgia with increasing age.
Adapted from Harpaz 2008, with permission Depomed (Menlo Park, CA, USA).
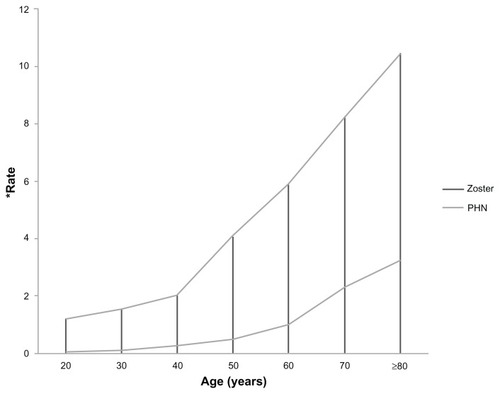
Table 1 Comparison of adverse events with gabapentin extendedrelease 1800 mg versus placebo
Post-herpetic neuralgia is one of the commonest and most studied neuropathic pain conditions. It is most common in the aging population because the majority of cases occur in patients over the age of 50 years, with the incidence doubling by the age of 80 years.Citation7 Of patients with herpes zoster who are aged ≥50 years, as many as 10%–20% will develop post-herpetic neuralgia.Citation6 Post-herpetic neuralgia results from a reactivation of the varicella zoster virus contracted years beforehand, which typically produces a well defined dermatomal rash. Post-herpetic neuralgia is typically defined as pain and/or dysesthesia at 12 weeks after resolution of the rash. Some patients experience the pain as a continuation of the pain experienced with the acute herpes zoster eruption, which has been described as a burning or stabbing sensation, itching, tingling, or even angina-like. The intensity of post-herpetic neuralgia pain is often in the moderate to severe range and has been reported as being ranked higher in intensity than labor pain or post-surgical pain.Citation6 Others have reported completely different sensations, but the most distressing and consistent component of post-herpetic neuralgia pain is mechanical allodynia, which is present in ≥70% of patients with the diagnosis.Citation8 In addition to the physical pain reported, patients with post-herpetic neuralgia also note an increase in sleep disturbances, anxiety, mood disturbances, loss of appetite, and a decrease in interest in social interactions.Citation9 A decrease in a patient’s ability to perform activities of daily living or an inhibition of social interactions due to zoster-related pain may significantly impact a patient’s ability to maintain an independent lifestyle.Citation10
FDA-approved therapies for neuropathic pain
There are a number of medications that are used in clinical practice to treat neuropathic pain states. In clinical trials assessing the efficacy of various medications for neuropathic pain, typically ≤50% of patients experience satisfactory pain relief, and side effects (including inability to tolerate treatment) are common. However, many of these medications are not approved by the US Food and Drug Administration (FDA) for this indication and their use is considered off-label. Of the medications that are approved by the FDA, each is approved for one or more specific neuropathic pain syndromes and not for treatment of neuropathic pain regardless of the etiology. As a result of this widespread on-label and off-label use of various medications, the International Association for the Study of Pain developed consensus guidelines for the pharmacologic treatment of neuropathic pain. In these guidelines, they recommended the use of particular medications and ranked them as first-line, second-line, and third-line medications. The medications recommended as first-line treatment have undergone multiple, randomized, controlled trials and have demonstrated consistent efficacy in treating neuropathic pain.Citation11 The first-line medications include tricyclic antidepressants, selective serotonin and norepinephrine reuptake inhibitors (duloxetine and venlafaxine), calcium channel α2-δ ligands (gabapentin and pregabalin), and topical lidocaine. Of these medications, only gabapentin, pregabalin, and the 5% lidocaine patch have been approved by the FDA specifically for the treatment of post-herpetic neuralgia.Citation12 However, in January of 2011, the FDA approved Gralise™ as a once-daily medication for the treatment of post-herpetic neuralgia.Citation13 Gralise is an extended-release form of gabapentin developed by Depomed Inc (Menlo Park, CA, USA), that not only has been shown to significantly decrease post-herpetic neuralgia pain scores, but may also be associated with fewer side effects than its immediate-release counterpart.
Overview of gabapentin
Gabapentin is an anticonvulsant that was initially developed and approved as an adjunctive therapy for the treatment of partial seizures in both the adult and pediatric populations.Citation14 Since that time, it has been approved by the FDA for the treatment of post-herpetic neuralgia, and has been a mainstay of therapy for many years. Gabapentin is a structural analog of gamma aminobutyric acid (GABA), but does not bind to the GABAA or GABAB receptor to achieve its effect. Instead, it binds to the α2-δ site of voltage-dependent calcium channels and modulates the influx of calcium with a resulting reduction in excitatory neurotransmitter release.Citation15 A decrease in excitatory transmitter release results in decreased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and glutamate receptor activation, and a decrease in pain transmission signaling.
Gabapentin is effective at reducing neuropathic pain due to post-herpetic neuralgia when given at least three times per day.Citation14 This is due to the pharmacokinetics of the drug, which show a peak plasma concentration observed at 2–3 hours after oral administration.Citation16 Less frequent dosing would not achieve therapeutic plasma levels that can be maintained over a 24-hour period. Gabapentin is absorbed in the upper gastrointestinal tract via a saturable L-amino acid transport system. This saturable transport system is partially responsible for the nonlinear absorption of gabapentin and the inverse correlation between bioavailability and dose. In addition, it has a short half-life (5–7 hours) requiring a dosing regimen of at least three times daily, resulting in demonstrable fluctuations in plasma levels throughout the day.Citation17 Although there is an increase in the mean steady-state plasma concentration with increasing dose, the increase is not proportional to the increase in the dose, and there is a gradual plateauing of the plasma concentration levels at higher doses.Citation16
Gabapentin has dose-limiting side effects that may prevent some patients from achieving therapeutic plasma levels. The most consistent and common of these side effects are somnolence (27.4%), dizziness (23.9%), and ataxia (7.1%).Citation18 While these side effects are considered to be minor, the experience of somnolence and dizziness accounts for most of the adverse event-related withdrawals in randomized controlled trials, and is especially worrisome in the elderly population prone to injuries from falls. Additionally, these side effects are a principal reason why clinicians discontinue or stop titration of this medication in clinical practice. One possible explanation for these side effects are the multiple peaks and troughs in plasma concentration levels with the multiple dose per day dosing schedule required for mean therapeutic plasma levels. However, most clinical trials with gabapentin and other voltage-dependent calcium channel modulators demonstrate improvement in sleep. Therefore, there is an advantage of a formulation of gabapentin that peaks during the night, with lower levels during the day.
Once-daily extended-release gabapentin formulation
Gralise is a once-daily extended-release formulation of gabapentin that has been developed using AcuForm™ technology. AcuForm is a polymer-based drug delivery system that retains the tablet in the stomach and upper gastrointestinal tract for a sustained period of time. When administered with a meal, the tablet expands, is retained in the stomach, and gradually releases the drug over time via a polymer matrix.Citation18
Gralise was approved by the FDA for the treatment of post-herpetic neuralgia after an 11-week, randomized, placebo-controlled trial in patients with post-herpetic neuralgia confirmed that once-daily gabapentin 1800 mg provided significantly greater pain relief than placebo.Citation17 Gabapentin extended-release 1800 mg once daily has been shown to provide comparable drug exposure with an identical daily dose of the immediate-release formulation when administered three times daily and allows for continuous delivery to the intestine over an approximately 10-hour period.Citation19 Gralise is available in 300 mg and 600 mg tablets, allowing titration to a clinically effective dose in a manner similar to that of gabapentin immediate-release. The total trialed dose of 1800 mg/day was preceded by a titration schedule to optimize tolerability.Citation17
Immediate-release versus extended-release gabapentin
Oral administration of gabapentin immediate-release results in peak gabapentin plasma levels that can be observed within 2–3 hours. The absorption of gabapentin is dose-dependent, with an inverse relationship between dose and bioavailability.Citation19 The absolute bioavailability of gabapentin decreases from 60% to 33% as the dosage increases from 900 to 3600 mg/day.Citation16 Gabapentin extended-release with AcuForm technology increases the bioavailability of gabapentin via two separate mechanisms, ie, it releases the medication at a slower rate, which prevents saturation of the active transport mechanism, and the AcuForm technology allows retention in the stomach for up to 8 hours, enabling delivery of gabapentin to the optimal site of absorption for approximately 10 hours.Citation19 Interestingly, while once-daily (1800 mg) and twice-daily dosing (600 mg in the morning, 1200 mg in the evening) of gabapentin extended-release revealed comparable drug exposure with three times daily administration of an identical daily dose of gabapentin immediate-release, only the twice-daily administration of gabapentin extended-release resulted in an apparently lower maximum and numerically higher minimum serum concentration at steady state, reflecting less serum concentration fluctuation.Citation19
Clinical efficacy of gabapentin extended-release
In an 11-week study performed by Sang et al, 452 patients were randomized in the US (n = 259), Russia (n = 161), and Argentina (n = 32) in order to assess the analgesic efficacy of gabapentin extended-release 1800 mg taken once daily at bedtime in patients with post-herpetic neuralgia. Eligible for inclusion were generally healthy adults with post-herpetic neuralgia of 6 months’ to 5 years’ duration following resolution of the herpes zoster rash and a baseline daily pain intensity score ≥ 4 on an 11-point Likert scale. Key exclusion criteria were concomitant use of analgesics and an insufficient analgesic response or inability to tolerate doses of gabapentin ≥ 1200 mg/day or pregabalin ≥ 300 mg/day. The primary endpoint was the mean change from baseline in average daily pain intensity score via baseline observation carried forward (BOCF) scoring. At the end of ten weeks of active treatment, the BOCF change in average daily pain intensity score was −2.1 and −1.6 for gabapentin extended-release and placebo, respectively (P = 0.013).Citation17 This study showed that participants who were given Gralise 1800 mg daily had a statistically significant reduction in average daily pain intensity scores compared with placebo (), reduced sleep interference due to pain, and a greater percentage of participants reporting being much or very much improved on the patient global impression of change ().Citation17
Figure 2 Mean change in average daily pain score (by week, and baseline observation carried forward analysis). Adapted from Sang et al.Citation17
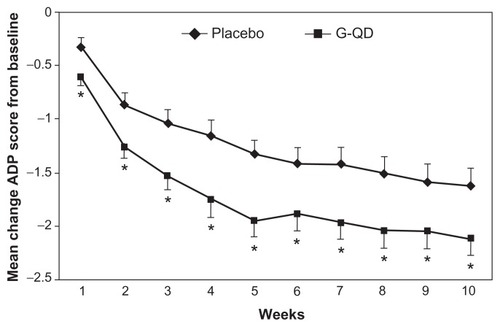
Figure 3 Patient global impression of change at endpoint: intent-to-treat population. Adapted from Sang et al.Citation17
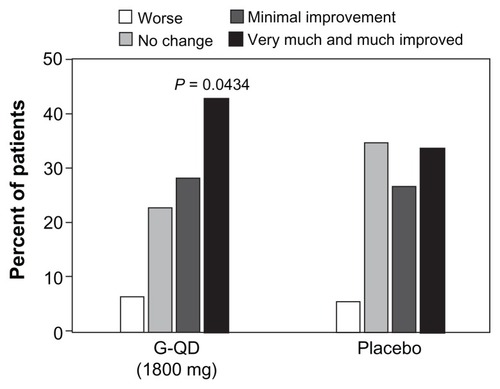
Figure 4 Percentage of safety population reporting dizziness and somnolence for all patients (overall) and the subgroup of patients aged ≥65 years.
Adapted from Sweeney et al.Citation25
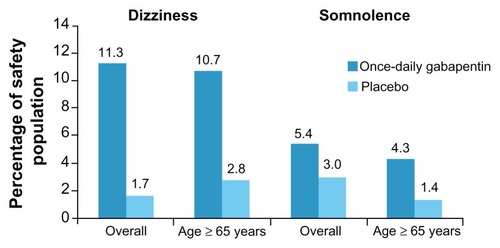
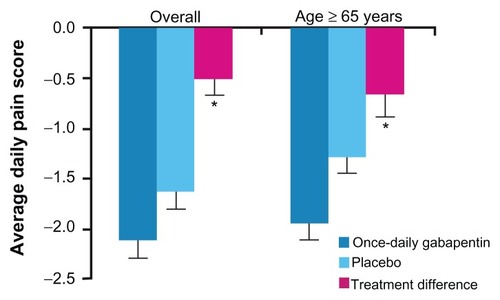
Figure 5 Least squares mean from baseline to endpoint (using baseline observation carried forward) in average daily pain scores and treatment differences for all patients (overall) and in patients aged ≥65 years.
Adapted from Sweeney et al.Citation25
The clinical efficacy seen with gabapentin extended-release parallels that shown in studies using three times daily dosing of gabapentin immediate-release in the 1800–3600 mg total daily dose range.Citation20,Citation21 A meta-analysis by Edelsberg et alCitation22 evaluated data from randomized controlled trials of drugs used to treat post-herpetic neuralgia that showed a reduction in daily pain scores, with a weighted mean difference of 21.93% when comparing gabapentin immediate-release with placebo. Edelsberg et al also showed similar efficacy when comparing pregabalin with placebo, ie, a weighted mean difference of 22.39%.
Safety and tolerability
Gabapentin immediate-release is commonly used for the treatment of post-herpetic neuralgia. It is efficacious but is associated with a high incidence of dose-limiting side effects, namely dizziness (28% versus 8% for placebo) and somnolence (21% versus 5% for placebo). In an analysis of the incidence of adverse events associated with gabapentin extended-release when compared with placebo, the incidence was consistently higher for gabapentin extended-release.Citation23 The most common treatment-emergent adverse events were the same as those previously identified in studies of immediate-release gabapentin, namely dizziness, somnolence, headache, and peripheral edema. While the rates of dizziness and somnolence were significantly lower than previously stated for gabapentin immediate-release, it should be noted that this was a partially enriched study and while α2-δ ligand naïve volunteers were included, participants who had a prior lack of response to gabapentin at dosages of ≥1200 mg/day, pregabalin at dosages of 300 mg/day, previous dose-limiting adverse effects with gabapentin, or hypersensitivity to gabapentin were excluded.Citation23 A subsequent subgroup analysis of the study by Sang et al was performed, and once-daily gabapentin was found to have similar efficacy and safety profiles both in patients who were naïve to α2-δ ligand therapy and in those with prior exposure.Citation24 While this does imply that there is little effect due to the partial enrichment study design, no direct head-to-head studies have been performed comparing the incidence of adverse events in the immediate-release formulation versus the extended-release formulation.
Gralise and the aging population
A subgroup analysis of the Sang study looked at the clinical efficacy and tolerability of gabapentin extended-release in participants aged ≥65 years. Among the intent-to-treat population, 280 patients were aged ≥65 years (once-daily gabapentin, n = 139; placebo, n = 141). Baseline average daily pain scores (± standard deviation) in patients aged ≥65 years were similar to the overall study group (once-daily gabapentin, 6.8 ± 1.5; placebo, 6.5 ± 1.3). Differences using BOCF analysis change in least squares mean daily pain scores favored once-daily gabapentin in patients from the overall intent-to-treat population (P = 0.013) and in the older subgroup of patients (P = 0.009), but this was not powered for subgroup analysis. The incidence of dizziness and somnolence was greater in the once-daily gabapentin groups compared with placebo both in the overall safety population and in the subgroup of patients aged ≥65 years.Citation25 This information, when taken into consideration with the information gained from the prior subgroup analysis implying that the partial enrichment design is unlikely to have had an effect on the study outcomes, provides some evidence that Gralise is likely to be efficacious and well tolerated in the aging population.
Commentary
Gabapentin has long been considered a first-line agent in the treatment of painful post-herpetic neuralgia. While it has been shown to be efficacious in treating this disorder, it requires at least three times daily dosing in order to achieve mean plasma levels throughout a 24-hour period that allows for its efficacy. In addition, it is not an uncommon finding that patients are not able to tolerate dosing in the therapeutic range due to common side effects, such as somnolence (27.4%), dizziness (23.9%), and ataxia (7.1%).
Gabapentin for once-daily bedtime treatment of postherpetic neuralgia (Gralise) has been shown in randomized clinical trials to be significantly more effective in reducing daily pain scores, improving quality of sleep, and having a greater overall patient global impression of change when compared with placebo.Citation17 It also showed a favorable side effect profile, but these data are based on a partially enriched enrollment study and not a head-to-head trial with gabapentin immediate-release. A subgroup analysis comparing the efficacy and tolerability of Gralise in individuals previously exposed to α2-δligands and individuals who were α2-δligand-naïve, showed little difference between the two groups. Taken together, the data support evidence for better tolerability of gabapentin once daily at bedtime compared with gabapentin immediate-release for the treatment of post-herpetic neuralgia pain. This improved tolerability should allow easier titration to a total daily dose of gabapentin in the clinically effective range. A subgroup analysis comparing the efficacy and side effect profile in the aging population (≥65 years) with those younger than 65 years showed that Gralise is effective and well tolerated in both groups. In addition to the sustained steady-state plasma levels seen with Gralise, one must acknowledge the convenience of a once-daily dosing regimen. It is commonplace for the aging population to be on multiple daily medications, and once-daily dosing is not only convenient but also affords a greater likelihood of patient compliance with the medication.
Gralise has been shown to be effective in reducing daily pain scores, and improving overall patient global impression of change and sleep when compared with placebo in both aging and younger populations. It has also been shown to be well tolerated and may have a lower incidence of side effects. The ease of once-daily bedtime dosing is likely to improve patient compliance over a three times daily dosing regimen, and it is likely to be of great benefit in the clinical treatment of pain associated with post-herpetic neuralgia. Because peak plasma levels occur during sleep, this is likely to have a positive impact on sleep and better daytime tolerability.
Disclosure
Dr Wallace has received payment for consulting for Depomed Inc, other authors report no conflicts of interest in this work.
References
- WoolfCJMannionRJNeuropathic pain: aetiology, symptoms, mechanisms, and managementLancet19993531959196410371588
- CostiganMScholzJWoolfCJNeuropathic pain: a maladaptive response of the nervous system to damageAnnu Rev Neurosci20093213219400724
- ArgoffCEKatzNBackonjaMTreatment of postherpetic neuralgia: a review of therapeutic optionsJ Pain Symptom Manage20042839641115471658
- LindsayTJRodgersBCSavathVHettingerKTreating diabetic peripheral neuropathic painAm Fam Physician20108215115820642268
- DothAHHanssonPTJensenMPTaylorRSThe burden of neuropathic pain: a systematic review and meta-analysis of health utilitiesPain201014933834420227832
- JohnsonRWZoster-associated pain: what is known, who is at risk and how can it be managed?Herpes200714Suppl 2303417939893
- BaderMSMcKinseyDSViral infections in the elderly. The challenges of managing herpes zoster, influenza, and RSVPostgrad Med2005118454816329530
- DworkinRHGnannJWOaklanderALRajaSNSchmaderKEWhitleyRJDiagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgiaJ Pain200891 Suppl 1S37S4418166464
- Vander StratenMCarrascoDLeePTyringSKReduction of postherpetic neuralgia in herpes zosterJ Cutan Med Surg2001540941611907852
- BouhassiraDChassanyOGaillatJPatient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practicePain201215334234922138256
- O’ConnorABDworkinRHTreatment of neuropathic pain: an overview of recent guidelinesAm J Med2009122Suppl 10S22S32
- American Pain FoundationTreatment options for post-herpetic neuralgia (long-term nerve pain) Available at: http://www.painfoundation.org/learn/pain-conditions/shingles/treatment-options-phn.htmlAccessed on May 4, 2012
- Depomed IncDepomed announces US Food and Drug Administration approval of Gralise™ (gabapentin) once-daily tablets for treatment of post-herpetic neuralgia Available at: http://investor.depomedinc.com/phoenix.zhtml?c=97276&p=irol-newsArticle_pf&ID=1521435Accessed May 4, 2012
- Neurontin [package insert]New York, NYPfizer Inc2009
- SinghDKennedyDHThe use of gabapentin for the treatment of postherpetic neuralgiaClin Ther20032585288912852705
- BockbraderHNWescheDMillerRChapelSJaniczekNBurgerPA comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentinClin Pharmacokinet20104966166920818832
- SangCNSathyanarayanaRCarterFJSweeneyMExtended release for the once-daily treatment of post herpetic neuralgiaPoster presented at the International Association for the Study of Pain, 13th World Congress on PainAugust 29–September 2, 2010Montreal, Canada
- RowbothamMHardenNStaceyBBernsteinPMagnus-MillerLGabapentin for the treatment of postherpetic neuralgia: a randomized controlled trialJAMA1998280183718429846778
- GordiTHouEKasichayanulaSBernerBPharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive extended-release and immediate-release tablets: a randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjectsClin Ther20083090991618555937
- RiceASMatonSPostherpetic Neuralgia Study GroupGabapentin in postherpetic neuralgia: a randomized, double blind, placebo controlled studyPain20019421522411690735
- RowbothamMHardenNStaceyBBernsteinPMagnus-MillerLGabapentin for the treatment of postherpetic neuralgiaJAMA1998280183718429846778
- EdelsbergJSLordCOsterGSystematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgiaAnn Pharmacother2011451483149022085778
- IrvingGJensenMCramerMEfficacy and tolerability of gastric-retentive gabapentin for the treatment of postherpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trialClin J Pain20092518519219333167
- SweeneyMSathyanarayanaREffect of prior exposure to immediate-release α2-δligands on the efficacy and adverse event profile of once-daily gabapentin for the treatment of postherpetic neuralgiaPoster presented at the 63rd Annual Meeting of the American Academy of NeurologyApril 9–16, 2011Honolulu, HI
- SweeneyMSathyanarayanaREfficacy and tolerability of once-daily gabapentin for the treatment of postherpetic neuralgia in patients at least 65 years oldPoster presented at the 34th Annual Meeting of the Society of General Internal MedicineMay 4–7, 2011Phoenix, AZ