Abstract
Statins have demonstrated substantial benefits in supporting cardiovascular health. Older individuals are more likely to experience the well-known muscle-related side effects of statins compared with younger individuals. Elderly females may be especially vulnerable to statin-related muscle disorder. This review will collate and discuss statin-related muscular effects, examine their molecular and genetic basis, and how these apply specifically to elderly women. Developing strategies to reduce the incidence of statin-induced myopathy in older adult women could contribute to a significant reduction in the overall incidence of statin-induced muscle disorder in this vulnerable group of patients. Reducing statin-related muscle disorder would likely improve overall patient compliance, thereby leading to an increase in improved short- and long-term outcomes associated with appropriate use of statins.
Introduction
Statins, or 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, represent a class of cholesterol-lowering drugs targeting low-density lipoproteins (LDLs). Nearly half of all men aged 65 years and above, and more than half of all women in the same age-group, meet diagnostic criteria for hyperlipidemia.Citation1Clinical trials involving prospective studies of individuals with hypercholesterolemia have demonstrated that long-term statin use is associated with a significant reduction in the risk of coronary artery disease (CAD) and other vascular disorders, including stroke.Citation2 Based upon these findings, the primary and secondary prevention of CAD and stroke has led to statin drugs being widely prescribed to both male and female adults, often beginning in early middle age.Citation3–Citation6
Since the introduction of lovastatin (Mevacor®, Merck and Co, Inc, Whitehouse Station, NJ, USA) to the US market in 1987, statins have become some of the most widely prescribed drugs both in the US and around the world.Citation7 According to the IMS Institute for Healthcare Informatics review on the use of medicines in the US, in 2011 a total of 19.8 million Americans used cholesterol-lowering medicines regularly, spending approximately $20.1 billion on these medications. Cholesterol-lowering medications as a group trail only oncologic and respiratory drugs in terms of annual patient pharmaceutical spending in the US. More than three-quarters of antihypercholesterolemia spending was for statins, with atorvastatin capturing more than half of all monies spent on statins.Citation8 The proportion of statin use and spending associated specifically with women has not been well defined in current literature.
Statins are generally safe and effective, and represent a very useful intervention for the prevention of CAD and stroke. However, as is the case for any medication, adverse effects have been associated with the use of statins. One of the most common of these adverse effects is statin-induced myopathy.Citation9–Citation11 Statin-related muscle disorders constitute a clinical spectrum ranging from a generally painless increase in serum creatinine kinase (CK) levels that do not exceed ten times the upper limit of normal (ULN), to a mild muscular discomfort termed myalgia, to potentially life-threatening rhabdomyolysis whereby myocytes degenerate, raising CK levels to beyond ten times the ULN, leading to renal dysfunction or failure in some individuals.Citation9
Advancing age has been associated with increased risk of statin-induced muscle disorder across the entire spectrum, as well as with a significantly greater incidence of the more severe forms of this disorder reported among the oldest groups of statin users.Citation9,Citation12,Citation13 To date, there have been relatively few reports on sex-specific relationships between advancing age and the incidence of myotoxic reactions among statin users. However, two published reports indicate that females demonstrate increased risk of developing muscle-related adverse events associated with statin use compared with men.Citation3,Citation9 In the relative vacuum of female-specific data on relationships between advancing age, statin use, and muscle dysfunction, this review will examine molecular and genetic factors associated with statin-induced myopathy, and how these factors specifically affect older adult women.
Specific side effects on muscle systems
Nomenclature
There have been several different terminologies for the description of muscle-related adverse events associated with statin use. In order to better understand the nature of adverse events, the American College of Cardiology, American Heart Association, and National Heart, Lung, and Blood Institute (ACC/AHA/NHLBI) developed standards for classifying and reporting events across the spectrum of this disorder.Citation9 The standardized descriptors range from myopathy, which is a nonspecific terminology representing any disease of the muscle, to myalgia, myositis, and at the most severe end of the spectrum, rhabdomyolysis, which can lead to acute renal failure and death.Citation9 The National Lipid Association (NLA) and Food and Drug Administration (FDA), however, have different definitions for certain terms.Citation10,Citation14 We reproduce a table () originally printed in a review by Joy and Hegele to illustrate the standardized definitions proposed by the ACC/AHA/NHLBI, compared to definitions employed by the NLA and FDA.Citation14 Inconsistency in the ways in which these conditions are defined and described limits the ability to compare findings from one study to those of another because the adverse-event definitions used may differ markedly.Citation7
Table 1 Manifestations of myopathy according to ACC/AHA/NHLBI clinical advisory on the use of statins, NLA and FDA . Reprinted with permission Joy TR, Hegele RA. Narrative review: statin-related myopathy.Citation14 © Annals of Internal Medicine 2009
Incidence
There is a great deal of variability in the reported incidence of statin-induced myopathy. The clinical advisory on the use and safety of statins, published in 2002 by Pasternak et al, cited an approximate 5% of study participants in clinical trials being affected by some form of statin-induced myopathy.Citation9 Depending on myopathy definitions employed in clinical trials, the incidence can range from 0%, such as was found in the Treating for New Targets (TNT) trial (n = 10,001), to 0.27%, which was identified in the Scandinavian Simvastatin Survival Study (4S) (n = 4444), and even up to 5% in other smaller trials.Citation12,Citation15,Citation16 However, in observational studies, the incidence of myopathy among statin users was as high as 5%–10%.Citation17,Citation18
Rallidis et alCitation6 enumerated several reasons that could explain this consistent underestimation of incidence rates in randomized clinical trials. These were primarily different definitions used to define myopathy, and the application of exclusion criteria that prevents patients with preexisting muscle symptoms or those at high risk for developing symptoms from being recruited into the trial.Citation6 Additionally, the higher clinician-based incidence reports may be a function of extended clinician follow-up.
In the Collaborative Association Diabetes Study (CARDS), which evaluated the safety and tolerability of atorvastatin 10 mg compared with placebo in 2838 diabetes patients aged 40–75 years with no history of coronary artery disease, the most common muscle symptoms reported were leg cramps and myalgia. However, the study reported fairly similar overall incidence of these disorders in both the treatment and placebo groups.Citation19 Other studies have also shown that the incidence of statin-induce myopathy varies when it is administered as monotherapy instead of as combination therapy. The incidence is estimated to be 0.1%–0.5% with statin monotherapy and 0.5%–2.5% in combination therapies with other cholesterol-reducing drugs.Citation20,Citation21 Severe myopathy has been reported in 0.8% of patients on lovas-tatin and simvastatin, while fatal rhabdomyolysis rarely occurs, with an incidence ranging between 0% and 0.1%.Citation9 A meta-analysis done recently using data available from 35 clinical trials did not find a significant difference in the incidence of rhabdomyolysis between statin treatment and placebo groups.Citation22
Among elderly women, the specific subgroup of interest for this review, incidence rates for statin-related muscle disorders are rarely reported in the medical literature. However, in a trial with cerivastatin over a decade ago, a subgroup analysis on elderly women aged 65 years and above, whereby 90 subjects were assigned to 0.8 mg cerivastatin and 27 subjects assigned to 0.4 mg cerivastatin, revealed that myopathy incidence was 5.6% and 7.4%, respectively. These incidence rates were higher than that seen in the overall study population, whereby incidence was consistently less than 2% in all study groups.Citation23,Citation24 In the medical review for FDA approval of the drug, elderly women ≥ 62 years and weighing ≤ 65 kg had increased incidence of CK elevations more than ten times ULN, even though it was not considered significant enough at that time to disapprove the drug.Citation24,Citation25 Cerivastatin was eventually withdrawn from the market in the year 2001 after a ten- to 100-fold increased risk of mortality was observed among individuals using cerivastatin compared with those on other statin drugs. A lesson to be learned from this is that clinical trials typically do not have sufficient sample sizes, and they may not have adequate length of study to capture rarely occurring conditions, such as rhabdomyolysis. This is a concern for all statin trials that attempt to assess this potentially fatal component of statin-induced myopathy.
A recent cross-sectional study performed on elderly women in Chile examined the association between statin use and loss of muscle mass and function. This small study compared 71 subjects on low-dose statins (rosuvastatin, lovastatin, simvastatin, or atorvastatin) with 57 subjects who were not on statins and had not taken a statin within the 2 months preceding the study. The investigators measured functional capacity by assessing quadriceps and hand-grip strength, and the time taken for subjects to perform the Timed Up and Go (TUG) test, as well as lean body mass and anthropometric measurements. They found no significant difference in the frequency of myalgia or in plasma CK levels between the two groups. They demonstrated that elderly women on low-dose statins did not appear to experience greater loss of muscle mass or function. On the contrary, in this study, statin users appeared to have better quadriceps strength and TUG time than noncurrent statin users; however, this association may have been confounded by participant socioeconomic status in that statin users tended to be from a higher socioeconomic background and may have benefited from unmeasured confounders, such as improved diet and greater physical activity. This study was limited by its small sample size, cross-sectional nature, and a study methodology that relied on self-reported behaviors (duration on statin treatment, muscle complaints, and level of physical activity). The results suggest that future prospective longitudinal studies employing objective methods for measuring muscle mass and function are needed to elucidate the relationship between statin use and temporal changes in muscle mass, strength, and functional ability.Citation26
Current findings regarding sex-associated risks associated with statin use are more equivocal. A recent meta-analysis by Kostis et al did not detect any sex-specific differences in statin-related adverse effects. However, this study reported that women seemed to be underrepresented in statin clinical trials.Citation27
Review of pharmacology and mode of action of statins related to effects on muscle
Competitive inhibitor of HMG CoA
Statins are potent competitive inhibitors of HMG CoA, binding to HMG CoA reductase with three times more efficacy than the natural substrate. This inhibition leads to a disruption in the cholesterol biosynthesis pathway mediated by HMG CoA, thereby decreasing LDL cholesterol levels in the body. There is as yet no single, precise biochemical mechanism that has been implicated as the main cause of statin-induced myopathy. Several possible mechanisms have been discussed in the literature; however, the most common relates to statin’s primary mechanism of action on the cholesterol biosynthetic pathway.Citation28,Citation29
Reduced production of geranyl pyrophosphate and farnesyl pyrophosphate
In vitro studies have demonstrated that statin-induced myopathy is most likely not due to the reduction in cholesterol synthesis itself. Instead, it is more likely due to the inhibition of the synthetic pathway, resulting in a reduction in the synthesis of crucial intermediary molecules such as geranyl pyrophosphate (GPP) and farnesyl pyrophosphate (FPP). GPP and FPP are responsible for the generation of various proteins essential in a variety of cellular signaling, transportation, and transformation processes that enhance cell-membrane integrity and support intracellular metabolic pathways.Citation30,Citation31
Apart from being intermediaries in the synthesis of cholesterol, GPP and FPP are also important in the prenylation, or posttranslational modification, of various cellular complexes, including proteins called lamins. Lamins are important for the structural and functional integrity of nuclei in cells by forming a nuclear lamina on the inner wall of the nucleus after interacting with nuclear membrane proteins. GPP and FPP are also precursors of central compounds, including dolichols and ubiquinone, which is also known as coenzyme Q10 (CoQ10). Dolichols help in glycosylation of intracellular polypeptides, a critical step in improving their function and thereby facilitating the formation of healthy structural proteins. CoQ10, on the other hand, is a hexameric compound found in the mitochondria of cells that plays a major role in the ultimate exchange of energy equivalents at the respiratory chain level.Citation32
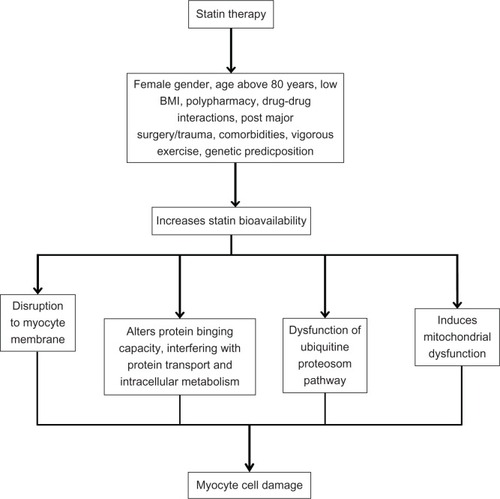
Statin-induced myopathy is hypothesized to occur via the following cascade: statin-induced disruption in the formation of GPP and FPP leads to dysprenylation of proteins, which drives production of dysfunctional lamins as well as dysfunctional Rab proteins and selenoproteins, which in turn interferes with transport of structural peptides within the cell, causing impaired intracellular signaling, sarcolemmal expression, structural alterations, and nuclear fragility. Concomitantly, the corresponding lack of dolichol and CoQ10 decreases expression of crucial membrane receptors responsible for cellular function and metabolism, leading to impaired energy production. Laboratory studies have shown that these changes tend to be more pronounced in skeletal muscle than in smooth muscle, which is why the cascade associated with the disruption of GPP and FPP has been thought to be an important driver of statin-induced myopathy.Citation29
CoQ10 depletion
There have been consistent reports through animal as well as human studies on the correlation between statin administration and corresponding depletion of CoQ10. In humans particularly, there have been reports of a 20%–40% decrease in CoQ10 levels associated with various statin treatments.Citation33 However, no direct association between decreased myocyte CoQ10 levels and myopathy has ever been demonstrated in any human or animal study.Citation32,Citation34 In fact, tissue levels of the CoQ10 enzyme have rarely been measured in any human study.Citation33 In addition, oral CoQ10 supplementation has not been shown to have any association with the risk of statin-related myopathy.Citation14 Furthermore, although this hypothesis implicating a disruption in the cholesterol synthesis pathway seems generally plausible, it does not explain why patients with hereditary hypocholesterolemia who are on statins do not report the occurrence of myopathy in the same way.Citation35
Myocyte-programmed cell death
More recent studies have suggested that statins do affect cellular FFP levels, which can lead to a dose-dependent programmed cell-death phenomenon. This occurs when a decrease in FFP causes a decrease in prenylated forms of various Rab proteins. In addition to altering cellular mechanics, this process leads to an increase in intracellular calcium, which in turn activates caspase enzymes responsible for cell death.Citation36 This mechanism of action may explain one aspect of the cholesterol-independent or pleiotropic effects of statins: therapeutic benefits may be related to statin-induced apoptosis in vascular smooth-muscle cells, which may lead to reduced atherosclerotic proliferation. However, in skeletal muscle cells, statin-induced apoptosis may lead to excessive cell mortality in susceptible individuals (perhaps, especially among older females) in whom myopathy ensues.Citation37
illustrates key steps by which statin-induced muscle-cell damage is hypothesized to occur.
Predisposing factors: sex, genetics, comorbidities, and drug interactions
Aging causes changes in body composition and function, including reduction in hepatic and renal clearance that alters the pharmacodynamics and pharmacokinetics of drugs.Citation38 The older one gets, the more likely one is to experience consequences of drug intensification leading to the manifestation of adverse effects, such as statin-induced myopathy.Citation39,Citation40
To complicate further the aging-related decline in bodily functions that alters drug effects, older adults are also more often inflicted with multiple comorbidities, some of which are likely to be treated pharmacologically, thereby exposing these individuals to the use of more drugs (polypharmacy). Both multiple comorbidities and polypharmacy worsen the already-compromised drug metabolic process in older aging individuals, leading to a heightened sensitivity to even low doses of drugs in general.Citation38 As a class, statins are generally safe and well tolerated. However, the risk for developing statin-induced myopathy in the elderly is substantially higher compared to their younger, healthier counterparts.Citation41
Sex
In general, adverse drug reactions are more common among females.Citation9,Citation42–Citation45 It has been reported that females are 1.5–1.7 times more at risk for clinically relevant adverse drug reactions compared to males.Citation44,Citation45 In addition, a recent hospital-based study in Germany reported that elderly females had significantly higher rates of adverse drug-related hospital admissions compared to men.Citation43 Evidence supporting sex-based differences in statin metabolism implicates, in part, well-known differences in body-fat content between men and women. This body fat-related differential drug metabolism is seen in other agents as well.Citation42,Citation46 This hypothesis has been well discussed in a general review on sex differences in pharmacological response that described the female preponderance towards adverse drug reactions.Citation42 Although men weigh more than women in general, drug doses in elderly adults are rarely titrated based on weight. In some cases, this may expose frail, elderly women to a dose that is higher than their bodies are able to metabolize and eliminate efficiently. Females also tend to have a higher percentage of body fat, which affects volume of distribution of some drugs and can significantly increase the half-life of a variety of medications, including the more lipophilic statins.Citation38,Citation42,Citation46
Most drugs are metabolized in the body through the hepatic cytochrome enzyme system. Lipophilic statins such as lovastatin, simvastatin, fluvastatin, atorvastatin, and pivastatin undergo first-pass metabolism in the liver, through reactions catalyzed by cytochrome P450 3A4 (CYP3A4). Women, however, have been found to have higher concentrations of CYP3A4.Citation42,Citation47,Citation48 This should mean that females theoretically are more capable of clearing statins out of their bodies. Therefore, at first glance, it seems counterintuitive that females have a heightened susceptibility to adverse effects. However, as discussed above, other factors such as body weight and body fat may offset any differences in CYP3A4 concentrations. Also, polypharmacy is common among elderly women, and concomitant use of other drugs that are also metabolized by CYP3A4 may cause a drug-drug interaction related to the competitive need for CYP3A4. This competition may lead to lower than optimal clearance rates for one or more of the drugs, thereby increasing the possibility of adverse effects associated with higher than optimal drug levels.Citation49,Citation50
Significantly higher mean physiological levels of CoQ10 (1.11 vs 0.86 (μmol/L) have been reported in males compared to females.Citation51 Whether this significantly lower CoQ10 level in females predisposes them to myopathy compared with males has not been well documented in the literature. Also, other processes in the body that lead to CoQ10 depletion, such as diabetes and hypothyroidism, have been considered risk factors for statin-induced myopathy.Citation6
It is also possible that a sociobehavioral perspective on pain perception may affect the likelihood of an individual complaining about discomfort or pain associated with statin-related muscle disorder.Citation52 Sex-based differences in pain perception favor a tendency for increased reporting of pain among females. There is a tendency for females to be more sensitive to pain and to describe pain as being more severe and recurrent in nature compared with males.Citation53 A complex framework comprising biological, psychological, environmental, and sociological factors has been suggested to play a role in possible differences in pain reporting between males and females.Citation52,Citation53 Genetic influences on pain perception have also been significantly related to myalgia, but how genetically mediated pain perception is associated with greater statin-related myopathies in elderly females is not well known.Citation37
Genetics
Although statin-induced CK elevations are dose-dependent, the correlation between plasma levels of statins and the risk for statin-induced myopathy has not been consistently demonstrated across populations.Citation37 To explain this discrepancy, there is a growing body of evidence describing various genetic factors that could contribute to differential reactions to the same drug from one individual to another.Citation37
Some researchers have proposed that a synergistic interaction between genetic and pharmacologic nuances might be a possible mechanism of statin myopathy.Citation54 It has been found that statin-induced myopathy is associated with a single-nucleotide polymorphism with intron 11 of the SLCOIBI gene on chromosome 12.Citation55 In the liver, statins enter hepa-tocytes using organic anion transport polypeptide (OATP) 1B1, which is coded by the SLCOIBI gene.Citation36,Citation56 It has been reported that plasma statin concentration tends to be higher in people with the above polymorphism, thus predisposing them to adverse effects.Citation55,Citation57 However, recent studies have shown that this polymorphism might be significantly associated only with simvastatin-induced myopathy.Citation58 Another recent population-based study showed that Native Americans might be at higher risk of having this polymorphism.Citation59
Another multisite study identified three genes - COQ2, ATP2B1, and DMPK - that are responsible for pathways related to CoQ10 biosynthesis, calcium regulation in the body, and muscular dystonia, respectively, as markers for myalgia in patients having statin-associated myalgia.Citation60 Benign CK elevation is also a marker for statin-induced myopathy. A retrospective case-control study involving 137 subjects taking simvastatin as a concomitant medication reported that nonexercise-induced CK elevation was associated with homozygosity in a genetic variant of the CYP3A enzyme, CYP3A5*3, which led to a greater degree of muscle damage.Citation61 To the best of our knowledge, there is no literature reporting the distribution of these alleles based on sex.
Comorbidities
Advanced age is a known risk factor for the presence of comorbid conditions. Without factoring in exposure to polypharmacy, comorbidities alone play a significant role in slowing statin metabolism and clearance from the body Knowing that muscle-related effects of statins have been shown to increase in a dose-dependent manner, a health condition that could potentially lead to an accumulation of statins in plasma would be considered a risk factor for statin-induced myopathy.Citation36,Citation62
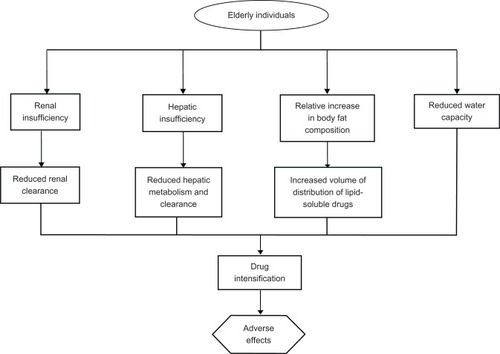
Hepatic and renal insufficiencies are examples of conditions that would naturally increase the levels of statins circulating in plasma.Citation11,Citation39,Citation63 Without titrating statin dose in patients suffering from such comorbid conditions, patients may face unnecessary overexposure to the drug when standard dosing regiments are applied. Dehydration is another risk factor for lower drug tolerance in the elderly. Though reduced water capacity may not be a diagnosis in itself, it is a condition that commonly accompanies other illnesses, especially in the elderly with limited self-care abilities.Citation39 Dehydration can interfere with normal drug clearance by reducing the body’s ability to eliminate drugs effectively, thus leading to higher than optimal drug levels.Citation39 is an adaptation of a figure originally produced by Szadkowska et al, illustrating a combination of factors that could stem from or be exacerbated by the presence of comorbid conditions, each of which increases the overall risk of developing nonspecific drug-related adverse effects in older individuals.Citation41
Drug-drug interaction
Negative drug-drug interactions involving statins occur when there is a drug-related disruption of the usual pharmacokinetic processes expected of statins. The dose-dependent nature of statin-induced myopathy leads to the understanding that any concomitant use of drugs that could increase plasma concentration of statins increases the risk for developing this adverse effect. In addition, agents that have the potential to alter statin pharmacodynamics, including statin response at the tissue level, also increase the likelihood for statin-induced myopathy to develop.Citation39
Most concerns with statin-related drug-drug interactions that have been described in the literature are related to altered pharmacokinetic properties, whether at the level of drug absorption, distribution, metabolism, or excretion. Drug interaction-related increases in the bioavailability of statins can be a result of a number of different factors, including: increase in the uptake or absorption of statins from the gut; decreased hepatic blood flow limiting the amount of statin that is carried to the liver for first-pass metabolism; inhibition of renal excretion, especially in the case of renal insufficiency or reduced renal blood flow, which is an expected normal phenomenon in the elderly; and disrupted statin metabolism, especially if the other agents interact with the pathway by which the statin is normally metabolized.Citation64
Drugs that utilize or interact with the CYP3A4 system tend to compete with predominantly lipophilic statins, such as simvastatin and atorvastatin, for CYP3A4. Drugs that are known to compete with lipophilic statins include amiodarone, azole antifungals, cyclosporine, calcium-channel blockers (eg, diltiazem), and antidepressants such as nefazodone, among others.Citation65 Gemfibrozil, another cholesterol-lowering drug primarily targeting triglycerides, is a competitive inhibitor of certain cytochrome P450 isoenzymes (CYP) and uridine diphosphate glucuronyltransferase. Both of these enzymes are necessary for hepatic metabolism of certain statins. By inhibiting oxidation and glucuronidation processes, statin clearance is reduced, and this has been shown to increase plasma statin levels substantially. This inadvertently contributes to the higher risk of developing rhabdomyolysis, which has been reported among patients on statin-gemfibrozil combination therapy.Citation66–Citation68 Also, the inhibition of OATP1B1 by drugs such as cyclosporine and protease inhibitors (eg, ritonavir) increases plasma statin concentrations by reducing hepatic uptake of statins.Citation69 A list of agents that have been described to influence statin bioavailability are presented in .
Table 2 Agents implicated in increasing statin bioavailability and the associated mechanism of action
Other predisposing factors
Of the seven statins presently used in practice, rosuvastatin and pravastatin are hydrophilic, while lovastatin, simvastatin, fluvastatin, atorvastatin, and pivastatin are more lipophilic. Hydrophilic statins are less capable of entering nonhepatic cells. This is one possible reason why statin-induced myopathy appears to be reported less frequently with the use of rosuvastatin and pravastatin. However, it is important to note that this possible relationship between statin lipophilicity/hydrophylicity and muscle disorder remains a matter of debate. It is also argued that statin lipophilicity should be positively associated with the removal of statins from intracellular compartments. There is a possibility of physiochemical changes to statin molecules when entering cells, which then reduces their ability to be transported out of the cells; this possibility has not been entirely ruled out. Further study will be required to elucidate how different statins with different lipophilic properties and safety profiles are associated with the risk of myotoxicity.Citation6,Citation11
Other factors associated with statin-induced muscle dysfunction that have been mentioned in the literature include a history of muscle pain during previous statin treatments, previous unexplained muscle cramps, family history of muscle aches, and previous CK elevations. Increased physical activity has been implicated as a trigger of statin-induced myopathy.Citation14,Citation40,Citation70 A 2011 study at the Boston Marathon found that statins appear to be associated with increased skeletal muscle injury, as evidenced by greater CK elevations in statin users (n = 37) compared to non-statin users (n = 40). However, the authors reported that instead of seeing a drug dose-dependent increase in risk, susceptibility to greater CK elevation appeared to be related to increasing age.Citation70 Conditions that increase predisposition for statin-induced myopathy are listed in .
Table 3 Risk factors for statin-induced myopathy
Long-term safety and tolerability issues, and patient-focused perspectives such as quality of life
The temporal relation between initiation of statin therapy and onset of myopathy remains unclear. Studies have reported symptom onset from anywhere between 1 and 12 months of initiating statin therapyCitation19 A small retrospective study involving 45 patients identified to have statin-induced myopathy between 1990 and 2003 reported a mean (standard deviation) duration of symptom onset since statin initiation as 6.3 (9.8) months. These patients also demonstrated a mean (standard deviation) duration for resolution of symptoms to be 2.3 (3.0) months after statins were discontinued.Citation71 These studies, however, included patients of all ages, and to our knowledge, a temporal profile unique to the elderly, much less to elderly females, is not yet available. However, preliminary information relating a probable time course for the presentation of statin-induced myopathy supports the need to monitor symptoms and CK levels carefully, particularly in the first year, so that timely reassurance and intervention can be instituted where necessary.
Link et al also documented a twofold increase in risk for developing statin-induced myopathy in females after the first year of therapy, from a baseline first-year risk of 1.6, further supporting the agenda that greater care be taken with female patients being prescribed with statins.Citation55 Again, it is difficult to ascertain, from this and other studies, a clear understanding of the interaction between age and sex in the development of statin-induced muscle disorder in older adults.
Among individuals suffering apparent statin-induced muscle disorder, muscular cramps and stiffness were the most commonly reported symptoms.Citation10,Citation72 Often, this was reported to be limited to the lower limbs.Citation72 These symptoms were also considered endurable in most cases, but were reported as being severely incapacitating in some cases.Citation10 In a survey done in 2003, Franc et al reported that approximately 90% of patients with muscle symptoms reported mild to moderate impact on daily activities, while 10% reported severe disability. More than a third of the patients in that study reported taking analgesics to ease their symptoms.Citation72 In patients diagnosed with claudication, statin-induced myalgia was reported not to be severe enough to limit walking.Citation73
Furthermore, most literature on statin-induced myopathy emphasizes that the disorder tends to be self-limiting and does not severely impact the daily functioning of afflicted statin users. Again, the current literature does not provide sufficient clarity to enable a complete understanding of age-and sex-related differences in the severity of patient-centered complaints regarding statin-induced muscle dysfunction. Patient-centered outcomes among statin users need to be elucidated more fully, and the limitations associated with patient complaints need to be studied in greater detail.
At present, there is a paucity of literature available to describe quality of life of patients with statin-induced myopathy, especially among elderly females. However, there is an ongoing study at Rockefeller University on quality of life among patients with statin-induced myopathy. To date, no interim findings have been reported; however, the results of this study should bring substantial knowledge regarding quality of life among statin users who suffer from statin-related muscle dysfunction. The results of this study should provide a solid foundation for future research in this important area, once they are made available.
Most studies that attempt to discuss quality of life among patients on statins have not focused exclusively on muscle-related complaints or changes, or on quality-of-life changes associated with the negative side effects of statins. Instead, these studies focused upon the improved quality and quantity of life enjoyed by statin users because of the successful prevention of adverse cardiovascular outcomes.Citation41,Citation74 Bearing in mind the substantial benefits associated with statin use, it is very important to recall that in general, drug-related adverse effects invariably result in reduced compliance. The occurrence of statin-induced myopathy compromises patient compliance in this way.Citation41
Statin prescription, whether to achieve a therapeutic benefit or as a preventive measure, requires that there is consistent, prolonged use of the drug. Statin-induced myopathy becomes a major barrier for achieving these targets in patients who develop symptoms of muscular dysfunction. Fortunately, in light of the well-documented benefits of statin therapy, a variety of statin medications and doses are available to patients who have not tolerated one particular drug well. For example, there are statins that may have a better safety profile or that may be metabolized by a different metabolic pathway. Beyond statins, where indicated, other cholesterol-lowering drugs can be tried. It is interesting to note that statin rechallenge in a small study revealed equivocal findings, and further research is warranted into understanding which particular drug at which particular dose may enable any specific patient to enjoy the maximum benefit of statins with the least possible risk of encountering statin-related muscle dysfunction.Citation69
Improving patient outcomes is central in medical care. Some aspects of care that physicians can keep in mind to increase safety and long-term tolerability of statins include the practice of prescribing statins at the lowest effective dose possible. Dose titration may be especially crucial in older female patients, given that elderly females have physiologic changes that predispose them to increased bioavailability of statins. Physicians should also strive to keep elderly females on as few medications as possible to avoid negative consequences of polypharmacy and minimize drug interactions with statins. When patients require drugs that may alter the metabolism of statins mediated by the CYP enzyme system, switching the patient to alternative statins, such as pravastatin and fluvastatin, which are hydrophilic and do not use the same metabolic pathway, should be considered in order to avoid drug-drug interactions and reduce the risk of possible statin-related adverse effects.Citation10,Citation74 The ACC/AHA/NHLBI clinical advisory on statins recommends that patients hospitalized for major surgery with high metabolic demands might benefit from short-term cessation of statins in order to prevent statin-induced myopathy in the perioperative period.Citation9 However, there has been some concern about a possible “rebound effect” when statins are discontinued, and several small trials have demonstrated a reduction in perioperative morbidity and mortality among vascular surgery patients who were continued on statin therapy compared to those who had discontinued treatment prior to surgery.Citation75–Citation77 Sustained-release formulations, those which do not require daily dosing of statins, have also been shown to be associated with fewer muscle-related adverse effects.Citation14 Considering the use of sustained-release formulations may be particularly important when addressing the issue of polypharmacy and impaired metabolism secondary to comorbidities in elderly females.
Discussion and conclusion
There is a paucity of sex- and age-specific information in the area of statin-induced myotoxicity. Hence, this review takes on an overall outlook of this topic, as reported by literature that is available.
Medication safety is a recognized indicator of quality of care. Measures to limit adverse drug reactions and medication-related adverse effects have been developed and implemented to varying degrees in most health-care settings. The incidence of statin-induced myopathy is relatively small, and this condition is usually self-limiting and relatively benign. However, because statins are so widely prescribed today, the experience with cerivastatin reminds us that the issue of medication safety in statins is vitally important.
Moving forward, it seems likely that increasing numbers of patients will be prescribed statins for more and more purposes. For example, there are studies being conducted to evaluate statin use in the management of conditions such as dementia, hypertension, and arthritis.Citation69,Citation77–Citation80 The pleiotropic effect of statins extends their ability to offer cardioprotection to myocardial cells. A recent in vitro study revealed that pravastatin offered significant cardioprotection to isolated human myocardium exposed to hypoxic injury. In the vascular surgery arena, the use of statins prophylactically in the preoperative period is not uncommon.Citation81 In addition, growing numbers of preclinical reports are revealing the potential for statins to enhance the effects of chemotherapeutic agents in cancer treatment, for varying types of cancers originating from different cell lines.Citation82
Other recent reports include those suggesting the role of statins in the prevention of certain cancers (eg, hematopoietic cancers), as well as in improving recovery potential among patients with head injury.Citation83,Citation84 As the indications for statin use expand, more individuals will be exposed to the risk of statin-induced myopathy.
Of particular concern with statins is that long-term use is required in order for the statins to be effective in the treatment of chronic disorders such as hypercholesterolemia. Long-term use of statins is also required for the primary or secondary prevention of cardiovascular disease. Chronic exposure to statins not only increases one’s likelihood of developing an adverse event just by virtue of continually challenging the body with the task of metabolizing the drug but also because of the long-term probability of developing other health conditions that may influence statin metabolism in a negative way. Currently, statin prescriptions are mostly concentrated among the middle-aged and the elderly. Even among statin users with good health, the aging process in itself inexorably increases the risk for statin-induced myopathy. The presence of greater comorbidity and exposure to polypharmacy in elderly individuals indicates that a careful focus on the safety of these patients is warranted when introducing any new medication, including a statin.
With this in mind, commencing statin therapy in elderly female patients, especially for primary or secondary prevention of cardiovascular disease, should follow a cautious approach. A thorough assessment of coronary mortality risk should be made, and only when clearly indicated should statins be prescribed, and then at an appropriately titrated dose. However, despite possible concerns about statin-induced muscle disorder, statin therapy should never be avoided in an appropriately screened patient when evidence shows the patient to be at high risk for coronary events.Citation74,Citation85 It is important to note that in the face of substantial level I evidence on the benefits of statins in primary and secondary prevention of coronary heart disease and stroke among the elderly, there still exists a significant therapeutic gap in this population, with a significant number of geriatric patients not reaching their therapeutic LDL goals.Citation86
One reason behind the therapeutic gap seen in elderly statin users may be the failure of the physician to titrate the patient to an optimal dose, mainly for fear of statin-induced myopathy. A retrospective cohort study analyzed databases that included 396,077 elderly residents in Ontario, Canada with coronary artery disease and diabetes, and found that only 19% of patients were on statin therapy. In this particular study, patients prescribed statins were younger and more likely to be male. The authors suggested that physician misconceptions about the risk-benefit tradeoff of statins were responsible, at least in part, for the low rate of statin prescriptions among these at-risk patients.Citation86
A recent nationwide review in the US revealed a reduction in spending for retail prescription of statins among the elderly.Citation8 It is interesting to note that advanced age has been shown to be an independent risk factor for underutilization of statins.Citation41 This is not unusual, as compliance is a recognized issue if a patient experiences any form of adverse effect, or if patients are unable to obtain the medications, whether due to financial reasons that prohibit them from continuing to purchase the medication in a pharmacy, or because of the lack of independence to travel to a facility to retrieve these medications. Improving evidence on the influence of aging, sex, and other patient-specific data on statin-induced myopathy would assist physicians in making risk-benefit tradeoffs when prescribing statins in practice. In the future, it may be possible for physicians to use the genetic profiles of their patients to guide their choice to prescribe statin medications and guide the choice of drug and dosage.Citation37,Citation55,Citation58
Despite the vast amount of information available in the field of statins and their adverse effects on muscles, the search for articles focusing only on elderly females was a futile effort. With the exception of the Chilean trial, none of the other cited studies focused specifically on the elderly female. Hence, this review strove to highlight essential findings that could be applied to elderly females based on existing knowledge on human physiology and the aging process. Further work is warranted to understand fully how statin-associated adverse effects actually affect elderly women.
Limitations
Recognizing potential risk factors for statin-induced muscle disorders is crucial. However, to properly develop strategies to address the increased risk of developing statin-induced myopathy in elderly women, the field requires further studies focusing on this population. The high variability in incidence of statin-induced myopathy reported in the literature could be attributed to the different definitions of myopathy employed in various trials, and the underrepresentation of women in some of them.Citation27,Citation87 Although statins have demonstrated a good safety profile in clinical trials, it is not unreasonable to hypothesize that muscle-related adverse effects might be significantly higher in the unmonitored, sicker, and older populations. Statin-induced muscle effects remain a concern, despite being generally mild and self-limiting. The paucity of information related to elderly females calls for more research focusing on this vulnerable subpopulation.
Developing strategies to reduce the incidence of statin-induced myopathy in older adult women could contribute to a significant reduction in the overall incidence of statin-induced muscle disorder in this vulnerable group of patients. Reducing statin-related muscle disorder would be likely to improve overall patient compliance, thereby leading to an increase in the improved short- and long-term outcomes associated with appropriate use of statins.
Disclosure
The authors report no conflicts of interest in this work.
References
- National Center for Health StatisticsHealth, United States, 2010: With Special Feature on Death and DyingHyattsville (MD)National Center for Health Statistics2011
- BaigentCKeechAKearneyPMEfficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet20053661267127816214597
- WalshJMEPignoneMDrug treatment for hyperlipidemia in womenJAMA20042912243225215138247
- Heart Protection Study Collaborative GroupMRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trialLancet200236072212114036
- LemaitreRNFurbergCDNewmanABTime trends in the use of cholesterol-lowering agents in older adults: the cardiovascular health studyArch Intern Med1998158176117689738605
- RallidisLSFountoulakiKAnastasiou-NanaMManaging the underestimated risk of statin-associated myopathyInt J Cardiol201215916917621813193
- BaysHStatin safety: an overview and assessment of the data - 2005Am J Cardiol2006976C26C
- IMS Institute for Health InformaticsThe Use of Medicines in the United States: Review of 2011Parsippany, NJIMS Institute for Health Informatics2012 Available from: http://www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/IHII_Medicines_in_U.S_Report_2011.pdfAccessed October 15,2012
- PasternakRCSmithSC JrBairey-MerzCNACC/AHA/NHLBI clinical advisory on the use and safety of statinsCirculation20021061024102812186811
- McKenneyJMDavidsonMHJacobsonTAGuytonJRNational Lipid Association Statin Safety Assessment Task ForceFinal conclusions and recommendations of the national lipid association statin safety assessment task forceAm J Cardiol20069789C94C
- SathasivamSStatin induced myotoxicityEur J Intern Med20122331732422560377
- AlexanderKPBlazingMARosensonRSManagement of hyperlipidemia in older adultsJ Cardiovasc Pharmacol Ther200914495819124599
- GaistDRodríguezLAHuertaCHallasJSindrupSHLipid-lowering drugs and risk of myopathy: a population-based follow-up studyEpidemiology20011256556911505177
- JoyTRHegeleRANarrative review: statin-related myopathyAnn Intern Med200915085886819528564
- LaRosaJCGrundySMWatersDDIntensive lipid lowering with atorvastatin in patients with stable coronary diseaseN Engl J Med200573521425143515755765
- ThompsonPDClarksonPKarasRHStatin-associated myopathyJAMA20032891681169012672737
- NicholsGAKoroCEDoes statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patientsClin Ther2007291761177017919557
- BruckertEHayemGDejagerSYauCBégaudBMild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients - the PRIMO studyCardiovasc Drugs Ther20051940341416453090
- NewmanCBSzarekMColhounHMThe safety and tolerability of atorvastatin 10 mg in the collaborative atorvastatin diabetes study (CARDS)Diab Vasc Dis Res2008517718318777490
- BallantyneCMCorsiniADavidsonMHRisk for myopathy with statin therapy in high-risk patientsArch Intern Med200316355356412622602
- EvansMReesAEffects of HMG-CoA reductase inhibitors on skeletal muscle: Are all statins the same?Drug Saf20022564966312137559
- MillsEJWuPChongGEfficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trialsQJM201110410912420934984
- InsullWJrIsaacsohnJKwiterovichPEfficacy and safety of cerivastatin 0.8 mg in patients with hypercholesterolaemia: the pivotal placebo-controlled clinical trial. Cerivastatin Study GroupJ Int Med Res200828476810898118
- JacobsonTAStatin safety: lessons from new drug applications for marketed statinsAm J Cardiol20069744C51C16377282
- Center for Drug Evaluation and ResearchBaycol medical review1999 Available from http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/20-740S008_Baycol_medr.pdfAccessed October 10, 2012
- BoetjeMBunoutDBarreraGde la MazaMPLeivaLHirschSEffects of statin use on functional capacity and muscle mass in elderly womenAgeing Res201123539
- KostisWJChengJQDobrzynskiJMCabreraJKostisJBMetaanalysis of statin effects in women versus menJ Am Coll Cardiol20125957258222300691
- KunclRWAgents and mechanisms of toxic myopathyCurr Opin Neurol20092250651519680127
- VaklavasCChatzizisisYSZiakasAZamboulisCGiannoglouGDMolecular basis of statin-associated myopathyAtherosclerosis2009202182818585718
- NishimotoTTozawaRAmanoYWadaTImuraYSugiyamaYComparing myotoxic effects of squalene synthase inhibitor, T-91485, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors in human myocytesBiochem Pharmacol2003662133213914609738
- MatznoSYamauchiTGohdaMInhibition of cholesterol biosynthesis by squalene epoxidase inhibitor avoids apoptotic cell death in L6 myoblastsJ Lipid Res199738163916489300786
- MarcoffLThompsonPDThe role of coenzyme Q10 in statin-associated myopathy: a systematic reviewJ Am Coll Cardiol2007492231223717560286
- BeltowskiJWôjcickaGJamroz-WisniewskaAAdverse effects of statins - mechanisms and consequencesCurr Drug Saf2009420922819534648
- ChatzizisisYSVaklavasCGiannoglouGDCoenzyme Q10 depletion: Etiopathogenic or predisposing factor in statin associated myopathy?Am J Cardiol2008101107118359340
- BakerSKMolecular clues into the pathogenesis of statin-mediated muscle toxicityMuscle Nerve20053157258015712281
- GuijarroCBlanco-ColioLMOrtegoM3-hydroxy-3-methylglutaryl coenzyme a reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in cultureCirc Res1998834905009734471
- GhatakAFaheemOThompsonPDThe genetics of statin-induced myopathyAtherosclerosis201021033734320042189
- MangoniAAJacksonSHAge-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applicationsBr J Clin Pharmacol20045761414678335
- ElDesokyESPharmacokinetic-pharmacodynamic crisis in the elderlyAm J Ther20071448849817890940
- ParkerBAThompsonPDEffect of statins on skeletal muscle: exercise, myopathy, and muscle outcomesExerc Sport Sci Rev20124018819423000957
- SzadkowskaIStanczykAAronowWSStatin therapy in the elderly: a reviewArch Gerontol Geriatr20105011411819217673
- AndersonGDGender differences in pharmacological responseInt Rev Neurobiol20088311018929073
- Hofer-DueckelmannCPrinzEBeindlWAdverse drug reactions (ADRs) associated with hospital admissions - elderly female patients are at highest riskInt J Clin Pharmacol Ther20114957758621961482
- FattingerKRoosMVergèresPEpidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicineBr J Clin Pharmacol20004915816710671911
- TranCKnowlesSRLiuBAShearNHGender differences in adverse drug reactionsJ Clin Pharmacol199838100310099824780
- CicconeGKHoldcroftADrugs and sex differences: a review of drugs relating to anaesthesiaBr J Anaesth19998225526510365004
- SicaDAGehrTWBRhabdomyolysis and statin therapy: relevance to the elderlyAm J Geriatr Cardiol200211485511773716
- WolboldRKleinKBurkOSex is a major determinant of CYP3A4 expression in human liverHepatology20033897898814512885
- GruerPJVegaJMMercuriMFDobrinskaMRTobertJAConcomitant use of cytochrome P450 3A4 inhibitors and simvastatinAm J Cardiol19998481181510513779
- YoshidaMMatsumotoTSuzukiTKitamuraSMayamaTEffect of concomitant treatment with a CYP3A4 inhibitor and a calcium channel blockerPharmacoepidemiol Drug Saf200817707517918187
- KaikkonenJNyyssönenKTuomainenTPRistonmaaUSalonenJTDeterminants of plasma coenzyme Q10 in humansFEB S Lett1999443163166
- RacineMTousignant-LaflammeYKlodaLADionDDupuisGChoinièreMA systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain201215361963522236999
- GoffauxPMichaudKGaudreauJChalayePRainvillePMarchandSSex differences in perceived pain are affected by an anxious brainPain20111522065207321665365
- VladutiuGDSimmonsZIsacksonPJGenetic risk factors associated with lipid-lowering drug-induced myopathiesMuscle Nerve20063415316216671104
- LinkEParishSArmitageJSLC01B1 variants and statin-induced myopathy - a genome-wide studyN Engl J Med200835978979918650507
- WilkeRARamseyLBJohnsonSGThe clinical pharmacogenomics implementation consortium: CPIC guideline for SLCOIBI and simvastatin-induced myopathyClin Pharmacol Ther20129211211722617227
- PasanenMKFredriksonHNeuvonenPJNiemiMDifferent effects of SLCOIBI polymorphism on the pharmacokinetics of atorvastatin and rosuvastatinClin Pharmacol Ther20078272673317473846
- BrunhamLRLansbergPJZhangLDifferential effect of the rs4149056 variant in SLCOIBI on myopathy associated with simvastatin and atorvastatinPharmacogenomics J20121223323721243006
- SantosPCSoaresRANascimentoRMSLCOIBI rs4149056 polymorphism associated with statin-induced myopathy is differently distributed according to ethnicity in the Brazilian general population: Amerindians as a high risk ethnic groupBMC Med Genet20111213621992719
- RuanoGWindemuthAWuAHMechanisms of statin-induced myalgia assessed by physiogenomic associationsAtherosclerosis201121845145621868014
- WilkeRAMooreJHBurmesterJKRelative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damagePharmacogenet Genomics20051541542115900215
- ThompsonPDClarksonPMRosensonRSNational Lipid Association Statin Safety Task Force Muscle Safety Expert PanelAn assessment of statin safety by muscle expertsAm J Cardiol20069769C76C
- BlomDJStatin therapy for the octogenarian? J Endocrinol Metab Diabetes South Afr2012173742
- BresslerRBahlJJPrinciples of drug therapy for the elderly patientMayo Clin Proc2003781564157714661688
- AroraRLieboMMaldonadoFStatin-induced myopathy: the two faces of JanusJ Cardiovasc Pharmacol Ther20061110511216891287
- SchneckDWBirminghamBKZalikowskiJAThe effect of gemfibrozil on the pharmakonietics of rosuvastatinClin Pharmacol Ther20047545546315116058
- CorsiniACeskaRDrug-drug interactions with statins: will pravastatin overcome the statins’ Achilles heel?Curr Med Res Opin2011271551156221682551
- JonesPDavidsonMReporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statinAm J Cardiol20069512012215619408
- NeuvonenPJNiemiMBackmanJTDrug interactions with lipid lowering drugs: mechanisms and clinical relevanceClin Pharmacol Ther20068056558117178259
- ParkerBAAugeriALCapizziJAEffect of statins on creatine kinase levels before and after a marathon runAm J Cardiol201210928228722036108
- HansenKEHildebrandJPFergusonEESteinJHOutcomes in 45 patients with statin-associated myopathyArch Intern Med20051652671267616344427
- FrancSDejagerSBruckertEChauvenetMGiralPTurpinGA comprehensive description of muscle symptoms associated with lipid-lowering drugsCardiovasc Drugs Ther20031745946515107601
- MascitelliLPezzettaFDoes statin therapy interfere with the ability of claudicant patients to exercise?Vasc Endovascular Surg20074147317942867
- MarooBPLavieCJMilaniRVEfficacy and safety of intensive statin therapy in the elderlyAm J Geriatr Cardiol2008179210018326948
- KulikARuelMStatins and coronary artery bypass graft surgery: preoperative and postoperatice efficacy and safetyExpert Opin Drug Saf2009855957119673591
- DesaiHAronowWSAhnCIncidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statinsArch Gerontol Geriatr20105114915119819571
- JickHZornbergGLJickSSSeshadriSDrachmanDAStatins and the risk of dementiaLancet20003561627163111089820
- 78FreemanDJNorrieJSattarNPravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention StudyCirculation200110335736211136689
- SpósitoACMansurAPCoelhoORNicolauJCRamiresJAAdditional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril)Am J Cardiol1999831497149910335771
- LeungBPSattarNCrillyAA novel anti-inflammatory role for simvastatin in inflammatory arthritisJ Immunol20031701524153012538717
- LemoineSAlloucheSCoulbaultLMechanisms involved in cardioprotective effects of pravastatin administered during reoxygenation in human myocardium in vitroAnesthesiology201211682483322343498
- OsmakMStatins and cancer: current and future prospectsCancer Lett201232411222542807
- LutskiMShalevVPorathAChodickGContinuation with statin therapy and the risk of primary cancer: a population-based studyPrev Chronic Dis20129E131
- SchneiderEBEfronDTMacKenzieEJRivaraFPNathensABJurkovichGJPremorbid statin use is associated with improved survival and functional outcomes in older head-injured individualsJ Trauma20117181581921986733
- MarooBPLavieCJMilaniRVSecondary prevention of coronary heart disease in elderly patients following myocardial infarction: are all HMG-CoA reductase inhibitors alike? Drugs Aging20082564966418665658
- KoDTMamdaniMAlterDALipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradoxJAMA20042911864187015100205
- SirventPMercierJLacampagneANew insights into mechanisms of statin-associated myotoxicityCurr Opin Pharmacol2008833333818243052