Abstract
Chronic constipation is a common disorder in the general population, with higher prevalence in the elderly, and is associated with worse quality of life and with greater health care utilization. Lubiprostone is an intestinal type-2 chloride channel activator that increases intestinal fluid secretion, small intestinal transit, and stool passage. Lubiprostone is currently approved by the US Food and Drug Administration for the treatment of chronic idiopathic constipation and of irritable bowel syndrome with predominant constipation. This review outlines current approaches and limitations in the treatment of chronic constipation in the elderly and discusses the results, limitations, and applicability of randomized, controlled trials of lubiprostone that have been conducted in the general and elderly population, with additional focus on the use of lubiprostone in constipation in Parkinson’s disease and in opioid-induced constipation, two clinical entities that can be comorbid in elderly patients.
Introduction
The progressive increase in life expectancy in developed countries has led to a corresponding rise in the proportion of elderly population. In the United States, the population aged 65 and older was 40.2 million in 2010 and is projected to be 88.5 million in 2050.Citation1 This exponential increase poses a number of challenges, as diseases and conditions that are more prevalent in elderly patients will need a much greater allocation of resources and expertise in health care systems in future years.
Chronic constipation (CC) is a common disorder, with an estimated prevalence of 12%–19% in the general population (depending on the area and the criteria used for the diagnosis).Citation2 A multinational survey published in 2008 that included 13,879 adults from seven different countries (United States, United Kingdom, Germany, France, Italy, Brazil, and South Korea) found a prevalence of constipation symptoms of 12.3% in the adult population (range 5%–18%), with odds ratios for constipation in women and elderly of 2.43 (95% CI: 2.18–2.71) and 1.5 (95% CI: 1.25–1.73), respectively.Citation3 This same study reported a prevalence of use of laxatives among those with constipation between 16%–40%. Increasing age, symptom frequency, and lower income and education were individual factors associated with laxative use.
A survey performed in Olmsted (Minnesota, USA), specifically designed for elderly responders (≥65 years old, mean age 76), found a constipation prevalence of 40.1% (95% CI: 38.9–44.4). Functional constipation, the most frequent category encountered in this study, had a prevalence of 24.4% (95% CI: 22.0–26.9).Citation4 In another study, performed using face-to-face interviews with frail elderly individuals who were not institutionalized, constipation was spontaneously mentioned by 45% of participants and was considered by 11% to be a major burden to their quality of life.Citation5 CC affects the majority of long-term patients in hospitals and residents in nursing homes,Citation6 and often, with exorbitant prevalence (reported up to 50%–79%) in the long-term institutionalized elderly.Citation7
Although CC can be seen as a trivial medical problem, its impact on quality of life can be substantial and may result in considerable additional utilization of health care resources, including specialist visits, gastrointestinal diagnostic procedures, and medical treatment modalities.Citation6,Citation8 Using both disease-specific and generic quality of life measurement instruments, studies suggest constipation is associated with impaired health-related quality of life,Citation9,Citation10 with CC patients exhibiting lower scores for physical functioning, mental health, general health perception, and bodily pain when compared with individuals without constipation.Citation11 Medical relief of constipation in a group of 52 CC patients aged 65–89 years resulted in improvement of patient’s mood, sexual activity, and quality of life.Citation12
In the United States, CC accounts for more than 2.5 million visits to physicians and for laxative sales of several hundred million dollars a year.Citation13 In England and Wales, constipation generated some 450,000 general practice consultations per year in 1991 and 1992, at an estimated cost of £4.5 million per year.Citation8 Data from the UK National Survey of morbidity data in general practice showed an age-dependent increase in the consultation rates for constipation, from 75/100,000 person-year for the 45–64 age group, to 400/100,000 person-year for the 77–84 age group.Citation14
Lubiprostone (Amitiza®, Sucampo Pharmaceuticals) was approved in the United States in 2006 for the treatment of chronic idiopathic constipation (CIC) in men and women, and in 2008 for women with irritable bowel syndrome with predominance of constipation (IBS-C). Since then, several studies focusing on lubiprostone efficacy and use have been published. Aims of this review were: (1) to review the current available treatments of CC in the elderly population; (2) to summarize the pharmacological properties of lubiprostone and data on its effects on constipation, based on studies in the general population; (3) to evaluate the data supporting the use of lubiprostone in the elderly; and (4) to discuss the studies performed using lubiprostone in specific situations occurring more frequently in older people, including Parkinson’s disease (PD) and opioid-induced constipation (OIC).
For the purpose of the present review, publications in abstract form and full papers on lubiprostone clinical studies were searched from 1995 to October 2012 on the PubMed electronic database and from proceedings of gastroenterology international meetings, using combinations of the following keywords: constipation, chronic, idiopathic, elderly, lubiprostone, Amitiza, opioid-induced, and Parkinson’s.
Constipation in the elderly population
Pathophysiology, onset and clinical features
The underlying mechanisms for CC in older adults include primary slow colonic transit, pelvic floor dysfunction, or a combination of the two. The role of reduced fiber and fluid intake and decreased physical activity is questionable, whereas underlying health problems more prevalent with advanced age (eg, diabetes mellitus, PD, dementia) and the use of medications (ie, calcium supplements, opioids, certain antidepressants) potentially affecting gastrointestinal motility and evacuation of stools are often involved (). The above factors can intervene, with advancing age, to worsen common underlying disorders already present from a younger age, such as CIC or IBS-C. CC in the elderly, if severe, entails a substantial rate of complications, with subsequent increase of limitation to independent activities of daily life and hospitalization rate. A wide range of complications can be associated, from anorectal pathology (eg, fissures, hemorrhoids, rectal prolapse, etc) to sigmoid volvulus, fecal impaction, fecal incontinence, and urinary dysfunction.Citation15
Table 1 Common causes of constipation in elderly patients
Available therapies
Nonpharmacological treatments for constipated patients include lifestyle and dietary measures, such as exercise, modifications to the daily routine, adequate fiber, and water intake. These measures are often difficult to implement in the elderly population, as multiple obstacles ranging from limited mobility to deglutition and mastication problems frequently exist in this group.
In patients with CC and associated pelvic floor dysfunction, pelvic floor retraining using biofeedback techniques has shown to be effective and have durable results.Citation16,Citation17 The prevalence of pelvic floor dysfunction in CC has been estimated to be 50% or more, in studies in tertiary care centersCitation18 and is more frequent in patients with history of anorectal surgery or other pelvic floor trauma. A single study of 30 elderly adults with CC showed good response to a combined approach of physiological and psychological therapy.Citation19 When treating elderly populations, one should bear in mind that biofeedback efficacy may be limited by patients’ declining physical or mental abilities.
Pharmacological treatment comprises several types of laxatives, including bulk-forming agents (such as psyllium, calcium polycarbophil, and methylcellulose), stool softeners (such as docusate sodium and docusate calcium), osmotics or salines (such as magnesium hydroxide, lactulose, polyethylene glycol, sorbitol, and glycerin rectal suppositories), and stimulants (such as senna, cascara sagrada, castor oil, and bisacodyl).
Self-medication is common in CC. Laxatives are widely available and remain agents of first choice. It has been reported that up to 16% of individuals aged 65 and above use over-the-counter laxatives, women more often than men.Citation20–Citation24 The use of laxatives has been associated with a greater number of physician or emergency room visits and more frequent hospitalization, home health services utilization, and prescription drugs use.Citation21 Among laxatives, the osmotic preparation polyethylene glycol 3350 has been shown to be safe and effective in elderly populations.Citation25–Citation26 However, clinical trials targeted to the elderly are lacking.
Limited patient compliance, polypharmacy, and patient–provider miscommunication can all contribute to poor understanding of the laxative dose and mode of administration, resulting in suboptimal results.
In the elderly with CC, prescription agents have been, in general, much less used than over-the-counter laxatives, owing to the limited drug choice, to cost issues, and to potential safety concerns.
Linaclotide is an agonist of guanylate cyclase 2C that was recently approved by the US Food and Drug Administration (FDA) for treatment of CIC and IBS-C. A 4-week multicenter clinical trial with 310 CIC patients included 30 elderly subjects, who showed similar results in safety and improvement of weekly spontaneous bowel movements (SBMs), stool consistency, straining, abdominal discomfort, and quality of life to the entire study population.Citation27
Prucalopride is a 5-HT4 receptor agonist that was approved in some European countries in 2009 and in Canada in 2011, but not in the US, for the treatment of CIC. A 4-week, placebo-controlled, Phase III trial with 300 elderly patients (mean age of 76.4 ± 0.4 years) showed significant improvement in the number of SBMs/week and quality of life in the treatment group and that the treatment was safe and well tolerated.Citation28 A 28-day Phase II study of 89 elderly CIC institutionalized patients also showed good treatment tolerability and absence of significant adverse events.Citation30
Several reviews discuss clinical trials performed with elderly patients and the current recommendations for treating this population. Regrettably, the guidelines provided are only in part evidence-based, owing to the general lack of large, randomized, controlled trials.Citation8,Citation10,Citation30 Overall, they call for the need to take into account the patient’s specific features (eg, ambulatory versus institutionalized, use of concomitant medications), to individualize the clinical approach. The final goals remain the achievement of acceptable bowel function (associated with better quality of life) and prevention of adverse effects of medications, in this vulnerable population.
Lubiprostone: pharmacology and physiological effects
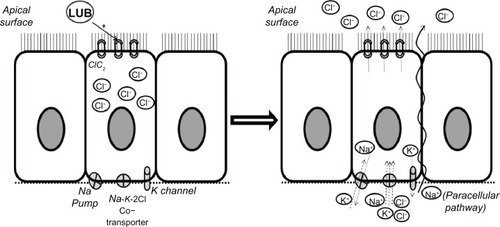
Lubiprostone is classified as a prostone, a bicyclic fatty acid compound derived from a metabolite of prostaglandin E1. Lubiprostone acts locally in the small intestinal lumen, inducing secretion of fluid and electrolytes through the activation of the type-2 chloride channels in the intestinal apical cell membrane (),Citation6,Citation31 and thereby accelerates the small bowel and colon transit times. In the upper gut, lubiprostone appears to delay gastric emptying and to increase stomach volumes in the fasting state.Citation32
Figure 1 Summary of the mechanism of action of lubiprostone.
Abbreviations: LUB, lubiprostone; ClC2, chloride channels-2.
Lubiprostone: clinical data in adults and elderly patients
A summary of results from lubiprostone open-label and placebo-controlled studies of adult and elderly populations with CIC and IBS-C is presented in –.
Table 2 Summary of clinical trials of lubiprostone in adult populations with chronic constipation
Table 3 Summary of clinical trials of lubiprostone in adult populations with IBS and predominant constipation
Table 4 Summary of clinical trials of lubiprostone in elderly adults
Patients with chronic idiopathic constipation
A Phase II, double-blind, dose-ranging study randomized 129 subjects with symptoms of CIC, between 18–75 years old, to four groups receiving placebo or lubiprostone 24, 48, or 72 mcg daily, for 3 weeks.Citation33 Mean SBMs frequencies were significantly higher for all lubiprostone groups, with better responses in the groups treated with higher doses. SBMs per week increased after the first 7 days of treatment with 48 and 72 mcg/day, and all three lubiprostone groups presented an increase from fewer than 2 to 5–6 SBMs/week after 2 weeks. Patients also reported an improvement of stool consistency, straining, and abdominal bloating symptoms. However, the percentage of patients using rescue medication during the trial was not significantly different across the four treatment arms and during the 3 weeks. The higher dose also was associated with greater severity of adverse effects, including nausea, headache, and diarrhea and did not appear to provide a clear risk-benefit advantage when compared with lubiprostone 48 mcg/day.
Subsequently, two Phase III trials randomized 479 adults with CIC to placebo or lubiprostone 48 mcg/day for 4 weeks.Citation2,Citation34 In the lubiprostone group, a significant increase of SBMs/week was seen starting after the first week of treatment, along with improvement in stool consistency, straining, and constipation severity that was maintained throughout the 4 weeks. The requirement of rescue laxatives was lower in the lubiprostone group, but only in one of the trials was this difference statistically significant.Citation2 More than 40% of patients in the active treatment groups reported at least one adverse effect during the studies, which led to the withdrawal of the drug in approximately 7% of patients overall. Nausea was the side effect reported most commonly (24%–32%).
A meta-analysis on lubiprostone data from 610 patients with CIC,Citation35 using self-reported relief of symptoms by patients as the main outcome measure, concluded that treatment during 3 or 4 weeks with lubiprostone 48 mcg/day was more effective than placebo (55% vs 33.1% of responders). The calculated number needed to treat was 5 (95% CI: 3–7). Adverse events, mostly diarrhea and nausea, were also overall more frequent in the lubiprostone-treated patients (60% vs 34% taking placebo).
A long-term, prospective, multicenter, open-label trial included 248 CIC patients to be treated with lubiprostone 48 mcg/day versus placebo, during 48 weeks.Citation36 Lubiprostone was more effective at reducing constipation severity, abdominal bloating, and discomfort. To minimize the impact of side effects occurrence on treatment withdrawals, the study design allowed dose reduction of lubiprostone when side effects occurred, at the discretion of the investigators, and redosing was performed in 17% of patients. Most lubiprostone-related adverse effects were mild (50%) or moderate (44%), and the most frequent were nausea (19.8%) and diarrhea (9.7%). Other adverse effects included abdominal distension, headache, abdominal pain, and vomiting. Overall, 13.3% of patients withdrew from the study due to adverse effects.
Patients with irritable bowel syndrome with predominant constipation
A combined analysis of two Phase III randomized trials involving 1171 patients who met Rome II criteria for IBS-C was performed comparing lubiprostone 16 mcg/day with placebo, for 12 weeks.Citation37 The patients were predominantly female (91.6%), 18–85 years old. The total number of overall responders (response was defined as moderate to significant IBS symptom relief, according to an electronic diary) was significantly higher in the lubiprostone group (17.9% versus 10.1%). Symptoms of abdominal discomfort or pain, bloating, constipation severity, straining, or stool consistency also improved significantly. The most frequent adverse effects were related to the gastrointestinal tract (ie, nausea, diarrhea, and abdominal distension) and had similar incidence in both groups.
An extension of this study was performed in 520 patients, mostly female (92.9%), with an age range of 21–82 years, for a total of 52 weeks.Citation38 Patients were randomized to three groups, according to the previous treatment, and received sequential treatment with placebo-lubiprostone, lubiprostone-lubiprostone, or lubiprostone-placebo-lubiprostone. The response rates to lubiprostone tended to improve over time, from 16% after 1 month, 32%–35% at 6–9 months, to 37%–44% after 10–13 months of treatment. Individually, symptoms of abdominal discomfort and pain, abdominal bloating, and stool consistency were significantly improved throughout the study. Rescue medication was required by 31.5% patients overall. Nausea and diarrhea were the most common treatment-related adverse effects, and 10.4% of patients discontinued treatment or experienced a dose reduction due to one of these adverse events.
Elderly patients
Generally, extrapolation of the results of clinical trials performed in the overall adult population to elderly patients must be done with caution. Data from three open-label clinical trials were combined to obtain a pool of elderly patients with CIC. These analyses were published as abstracts, with the available data assessing a limited range of outcomes.
A group of 57 patients aged ≥ 65 years was randomized to placebo or lubiprostone 48 mcg/day and followed for 4 weeks. In the lubiprostone group, a significant improvement in SBMs frequency (4.6–5.4 additional SBMs/week compared with 1.29–2.27 in the placebo group) and stool consistency was seen at week 4. Fewer patients in the lubiprostone arm experienced related adverse effects than those receiving placebo (46.2% vs 61.3%).Citation39
Another group of 163 elderly constipated patients was compared with a group of 715 nonelderly lubiprostone trial participants. Tolerability and efficacy of lubiprostone 48 mcg/day was assessed for 6 to 12 months. There was a significant improvement in constipation severity, abdominal bloating, and abdominal discomfort, at all postbaseline time points from week 1 to week 48, for both groups. Constipation severity appeared consistently improved throughout the study. The incidence rate for nausea, the most common adverse effect, was markedly less frequent in elderly subjects when compared to their nonelderly counterparts (17.8% vs 29.4%).Citation40 In a separate subanalysis, nausea occurred in only 18.8% of elderly patients, when the overall rate of nausea was 31.1%.Citation41
Although these results are encouraging, additional studies, with more patients, are required before confirming the efficacy and safety of the treatment in elderly patients.
Approved use of lubiprostone in CIC and IBS-C
Based upon the results of these pivotal studies, the FDA approved lubiprostone use for the treatment of adult men and women with CIC (at the dose of 24 mcg, twice daily) and for the treatment of adult women with IBS-C (at the dose of 8 mcg, twice daily).
The incidence of adverse effects appears to be dose-related, improving after the discontinuation of the treatment. These effects include diarrhea, nausea, abdominal pain or discomfort, and bloating and, less frequently, extraintestinal complaints, including stomatitis, headache, dyspnea, vertigo, or palpitations.Citation6,Citation31,Citation33 No alterations in the electrocardiogram were reported in one study performed on 177 patients with CIC and 68 healthy volunteers.Citation42
Lubiprostone: clinical data in specific situations
Parkinson’s disease
PD is the second most common neurodegenerative disease in the United States, after Alzheimer’s disease, with a point prevalence of at least one million affected individuals and about 50,000–60,000 new cases diagnosed annually. Incidence and prevalence of PD increase with age, with average disease onset at 60 years of age. Greater disease severity and higher doses of dopaminergic medications seem closely related to patients’ age and to more severe autonomic problems in PD. Autonomic dysfunction affects activities of daily life and health-related quality of life in PD.Citation43 Constipation is among the most common symptoms related to autonomic dysfunction in PD. Severe constipation in PD may eventually lead to megacolon, intestinal pseudo-obstruction, volvulus, and bowel perforation.Citation43 Its mechanisms include delayed gastric emptying, small intestine, and colonic transit time, difficulties with the volitional defecation process, and medication side effects. These digestive abnormalities are in part secondary to the early involvement in PD of the dorsal motor nucleus, which controls the majority of parasympathetic innervation to the gut; the loss of dopaminergic neurons and deposition of α-synuclein in the myenteric ganglia and in the abdominopelvic autonomic plexus also likely play a role, resulting in dysregulation of the enteric nervous system.Citation43–Citation45 Derangement of the volitional defecation process manifests clinically as impaired stool evacuation and is commonly the result of lack of coordination between the muscles in the pelvic floor and between these and the muscle groups involved in change of intra-abdominal pressure.Citation44
Although levodopa is generally not very effective in the treatment of gastrointestinal symptoms in PD patients,Citation43 its intrajejunal continuous infusion in patients with advanced PD showed improvement in constipation, in a study of 22 patients.Citation46 There are a few trials evaluating the use of bulking agents, such as dietary fibers, psyllium, and polycarbophil, performed with a small number of PD patients.Citation47–Citation49 Fiber supplementation seems to improve stool consistency and frequency, but not colonic transit, and also enhances the delivery of dopaminergic agents, which may allow better control of symptoms.Citation47–Citation50 The efficacy and safety of polyethylene glycol was evaluated in an 8-week, double-blind, placebo-controlled study of 57 PD patients, with significant improvement in SBMs frequency and stool consistency.Citation51 Pyridostigmine bromide, a reversible cholinesterase inhibitor, might have a possible beneficial effect and has been proposed as a safe option in case reports.Citation52 Cisapride, a 5-HT4 receptor agonist prokinetic, now withdrawn from the US market, was associated with faster colonic transit in a small, open-label PD study.Citation53 The selective 5-HT4 receptor agonist and partial 5-HT3 antagonist mosapride was also found to accelerate colonic transit time and improve evacuation, in another open-label, 3-month study of PD patients. Lastly, botulinum toxin injections have been proposed as treatment in cases of focal dystonia of the puborectalis muscle in PD patients; however, controlled data are lacking to support this.Citation54
In a double-blind, placebo-controlled study of 54 patients with CC and PD, mean age 67.0 ± 10.1 years,Citation44 treatment with lubiprostone 48 mcg/day over 4 weeks was well tolerated, increased stools per day, and improved subjective rating of constipation by visual analog scale score and questionnaires. The most commonly reported side effect was the occurrence of loose stools, in 48% of the lubiprostone-treated patients versus 3.7% in the placebo group. These were of mild severity and did not lead to treatment discontinuation. Of note, no patient receiving lubiprostone reported nausea. The duration of the study was limited; however, it suggests that use of lubiprostone in PD might be beneficial, without posing additional safety concerns.
Opioid-induced constipation
Opioid narcotics are commonly prescribed in the United States for both cancer and noncancer pain. It is estimated that 90% of patients presenting to pain centers and receiving treatment in such facilities are managed with opioids.Citation55 In elderly patients, chronic pain is highly prevalent, with 45%–85% of elderly patients reporting pain in moderate to severe degree,Citation56 and with the de facto common use of opioid narcotics, such as morphine, fentanyl, or oxycodone, despite the overall lack of clinical data to support the effectiveness and safety of these drugs in the elderly population.Citation57 Alongside their analgesic properties, opioids carry the burden of inducing cognitive impairment, cough depression, addiction, and sensory and motor dysfunction throughout the bowel, and are associated with a wide range of gastrointestinal manifestations.Citation58 Constipation is the most common gastrointestinal adverse effect of opioid narcotics, with an incidence about 40%.Citation59,Citation60
Alvimopan and methylnaltrexone, two peripherally acting antagonists of the μ-opioid receptors with restricted ability to cross the blood-brain barrier, have demonstrated efficacy and safety in the treatment of OIC, without reversing the central effects of analgesia.Citation61,Citation62 Alvimopan has been approved by the FDA as a short-term treatment to accelerate the time of upper and lower gastrointestinal recovery after bowel resection.Citation63 Methylnaltrexone has been approved to treat OIC in patients with advanced illness receiving palliative care, when the response to laxatives has been insufficient.Citation64 Additional pharmacodynamic and clinical data suggest methylnaltrexone has potential effects on gastric emptying, on relief of nausea and vomiting, and on reduction of episodes of airway aspiration.Citation65
Lubiprostone has been a potential candidate in OIC treatment. Studies performed on animalCitation66 and human intestinal tissuesCitation67 suggested lubiprostone has the potential to counteract the inhibition by morphine of secretomotor neurons in the enteric nervous system of the small intestine. Four double-blind, Phase III clinical trials, one of them an extension of two previous ones, were performed in Europe, Canada, and the United States. They compared lubiprostone 48 mcg/day to placebo, in adults with OIC secondary to treatment with a full-agonist opioid for chronic, noncancer-related pain. Two of these have been published as abstracts.Citation68,Citation69
One 12-week trial (NCT0595946) included 443 subjects. The lubiprostone-treated group showed significant improvement in SBMs/week, constipation severity, stool consistency, abdominal discomfort, and straining. This range of effects was not observed in patients on methadone.Citation68 A second 12-week trial (NCT1298219) enrolled 439 patients, excluding patients treated with methadone.Citation69 The lubiprostone-treated group reported significantly higher response in terms of SBMs compared with patients receiving placebo (26.9% vs 18.6%). Straining, stool consistency, abdominal discomfort, and constipation severity were also significantly improved. In a separate trial (NCT0597428), however, conducted in 420 adults over 12 weeks, lubiprostone did not show significant difference in the improvement of SBMs/week compared with placebo, although a significant improvement was found in the secondary end points of stool consistency, abdominal discomfort, constipation severity, and straining. As seen in the previous studies, the subgroup of patients on methadone treatment showed lower response rates.Citation70
A Phase III extension of NCT0595946 and NCT0597428 was later performed in the United States. It included 439 adults that were followed for 36 weeks. According to data filed by the drug manufacturer, a maintained improvement of SBM compared with baseline was observed, while remaining on lubiprostone.Citation71
The most common adverse effects in these trials were diarrhea, nausea, and abdominal pain or discomfort, which were reported by less than 10% of patients, but without affecting analgesia.
OIC is not an FDA-approved indication for treatment with lubiprostone. While lubiprostone might represent an additional option in OIC, it is unclear how its efficacy would compare with that of selective, peripheral opioid reversal agents available and in clinical development, and additional studies might be needed.
Conclusion
CC is highly prevalent in the general population and especially among elderly patients. It can worsen quality of life and significantly impair activities of daily living. There is an unmet need for safe and effective CC treatments in the elderly. Lubiprostone is approved for the treatment of CC and IBS-C in the general population. Lubiprostone has also shown efficacy in smaller studies of constipation in the context of PD and seems promising as a treatment modality for OIC, two situations found more frequently in elderly populations. Lubiprostone use is limited by the incidence of gastrointestinal side effects, most notably nausea, which can only in part be modulated by dose reduction.
Few data are available on lubiprostone use for CC in elderly patients. These data seem to support a comparable range of efficacy to that described in other adult populations and do not show a high incidence of nausea, although the statistical power of this elderly patient sample is likely limited. The available evidence supports, with the limitations discussed above, the efficacy and safety of lubiprostone for CC treatment in the elderly.
Disclosure
The authors report no conflicts of interest in this work.
References
- VincentGKVelkoffVAThe Next Four Decades. The Older Population in the United States: 2010 to 2050WashingtonUS Census Bureau2010 Available from: http://www.census.gov/prod/2010pubs/p25-1138.pdf. Accessed December 18, 2010
- JohansonJFMortonDGeenenJUenoRMulticenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipationAm J Gastroenterol2008103117017717916109
- WaldAScarpignatoCMueller-LissnerSA multinational survey of prevalence and patterns of laxative use among adults with self-defined constipationAliment Pharmacol Ther200828791793018644012
- TalleyNJFlemingKCEvansJMConstipation in an elderly community: a study of prevalence and potential risk factorsAm J Gastroenterol199691119258561137
- WolfsenCRBarkerJCMittenessLSConstipation in the daily lives of frail elderly peopleArch Fam Med1993288538588111515
- FukudoSHongoMKanekoHUenoREfficacy and safety of oral lubiprostone in constipated patients with or without irritable bowel syndrome: a randomized, placebo-controlled and dose-finding studyNeurogastroenterol Motil2011236544e20521303430
- BosshardWDreherRSchneggJFBülaCJThe treatment of chronic constipation in elderly people: an updateDrugs Aging2004211491193015554750
- MihaylovSStarkCMcCollEStepped treatment of older adults on laxatives. The STOOL trialHealth Technol Assess20081213iiiivix13918462572
- O’KeefeEATalleyNJZinsmeisterARJacobsenSJBowel disorders impair functional status and quality of life in the elderly: a population-based studyJ Gerontol A Biol Sci Med Sci1995504M184M1897614239
- RaoSSGoJTUpdate on the management of constipation in the elderly: new treatment optionsClin Interv Aging2010516317120711435
- GliaALindbergGQuality of life in patients with different types of functional constipationScand J Gastroenterol19973211108310899399387
- CharachGGreensteinARabinovichPGroskopfIWeintraubMAlleviating constipation in the elderly improves lower urinary tract symptomsGerontology2001472727611287730
- LemboACamilleriMChronic constipationN Engl J Med2003349141360136814523145
- McCormickAFlemingDCharltonJMorbidity Statistics from General Practice: Fourth National Study: 1991–1992LondonHMSO1995
- McCreaGLMiaskowskiCStottsNAMaceraLVarmaMGA review of the literature on gender and age differences in the prevalence and characteristics of constipation in North AmericaJ Pain Symptom Manage200937473774518789639
- HeymenSScarlettYJonesKRingelYDrossmanDWhiteheadWERandomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipationDis Colon Rectum200750442844117294322
- RaoSSSeatonKMillerMRandomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecationClin Gastroenterol Hepatol20075333133817368232
- CamilleriMLeeJSViramontesBBharuchaAETangalosEGInsights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older peopleJ Am Geriatr Soc20004891142115010983917
- SimónMABuenoAMBehavioural treatment of the dyssynergic defecation in chronically constipated elderly patients: a randomized controlled trialAppl Psychophysiol Biofeedback200934427327719618262
- HanlonJTFillenbaumGGRubyCMGraySBohannonAEpidemiology of over-the-counter drug use in community dwelling elderly: United States perspectiveDrugs Aging200118212313111346126
- StoehrGPGanguliMSeabergECEchementDABelleSOver-the-counter medication use in an older rural community: the MoVIES ProjectJ Am Geriatr Soc19974521581659033513
- GohLYVitryAISempleSJEstermanALuszczMASelf-medication with over-the-counter drugs and complementary medications in South Australia’s elderly populationBMC Complement Altern Med200994219906314
- AnrakuMInoueHSatoESurveillance study in collaboration with a university-daycare center for elderly people and nursery school for children on the use of over-the-counter drugs and health food in FukuyamaYakugaku Zasshi2010130810931103 Japanese20686214
- AlbertNMRathmanLRossDPredictors of over-the-counter drug and herbal therapies use in elderly patients with heart failureJ Card Fail200915760060619700137
- DiPalmaJAClevelandMBMcGowanJHerreraJLA comparison of polyethylene glycol laxative and placebo for relief of constipation from constipating medicationsSouth Med J2007100111085109017984738
- DiPalmaJAClevelandMVMcGowanJHerreraJLA randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipationAm J Gastroenterol200710271436144117403074
- LemboAJKurtzCBMacdougallJEEfficacy of linaclotide for patients with chronic constipationGastroenterology2010138388689520045700
- Mueller-LissnerSRykxAKerstensRVandeplasscheLRandomized double-blind placebo-controlled trial to evaluate efficacy and safety of prucalopride (Resolor®) in elderly patients with chronic constipationGastroenterology20081344 Suppl 1A-157 Abstract 1052
- CamilleriMBeyensGKerstensRRobinsonPVandeplasscheLSafety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled studyNeurogastroenterol Motil200921121256e111719751247
- FlemingVWadeWEA review of laxative therapies for treatment of chronic constipation in older adultsAm J Geriatr Pharmacother20108651455021356503
- ChamberlainSMRaoSSSafety evaluation of lubiprostone in the treatment of constipation and irritable bowel syndromeExpert Opin Drug Saf201211584185022834474
- CamilleriMBharuchaAEUenoREffect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteersAm J Physiol Gastrointest Liver Physiol20062905G942G94716603730
- JohansonJFUenoRLubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safetyAliment Pharmacol Ther200725111351136117509103
- BarishCFDrossmanDJohansonJFUenoREfficacy and safety of lubiprostone in patients with chronic constipationDig Dis Sci20105541090109720012484
- SuaresNCFordACEfficacy of lubiprostone in the treatment of chronic idiopathic constipation: systematic review and meta-analysisGut201160Suppl 1A164A165 Abstract
- LemboAJJohansonJFParkmanHPRaoSSMinerPBJrUenoRLong-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipationDig Dis Sci20115692639264521769655
- DrossmanDACheyWDJohansonJFClinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome – results of two randomized, placebo-controlled studiesAliment Pharmacol Ther200929332934119006537
- CheyWDDrossmanDAJohansonJFScottCPanasRMUenoRSafety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipationAliment Pharmacol Ther201235558759922251419
- UenoRJoswickTRWahleAZhuYHollandPCEfficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjectsGastroenterology2006130Suppl 2A189 Abstract S1262
- UenoRPanasRWahleAZhuYHollandPCLong-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjectsGastroenterology2006130Suppl 2A-188 Abstract S1260
- UenoRWahleARiveraEPooled analysis of the most frequent adverse events associated with the use of lubiprostoneAm J Gastroenterol2006101Suppl 2S489 Abstract 1264
- SprengerCCopaAMorganrothJEffect of lubiprostone, a unique agent for the treatment of chronic idiopathic constipation, on clinical electrocardiogram resultsGastroenterology2007132A325 Abstract
- MostileGJankovicJTreatment of dysautonomia associated with Parkinson’s diseaseParkinsonism Relat Disord200915Suppl 3S224S23220082997
- OndoWGKenneyCSullivanKPlacebo-controlled trial of lubiprostone for constipation associated with Parkinson diseaseNeurology201278211650165422573627
- KroghKChristensenPNeurogenic colorectal and pelvic floor dysfunctionBest Pract Res Clin Gastroenterol200923453154319647688
- HonigHAntoniniAMartinez-MartinPIntrajejunal levodopa infusion in Parkinson’s disease: a pilot multicenter study of effects on nonmotor symptoms and quality of lifeMov Disord200924101468147419425079
- AstarloaRMenaMASánchezVde la VegaLde YébenesJGClinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson diseaseClin Neuropharmacol19921553753801330307
- AshrafWPfeifferRFParkFLofJQuigleyEMConstipation in Parkinson’s disease: objective assessment and response to psylliumMov Disord19971269469519399219
- SakakibaraRYamaguchiTUchiyamaTCalcium polycarbophil improves constipation in primary autonomic failure and multiple system atrophy subjectsMov Disord200722111672167317149723
- BassottiGMaggioDBattagliaEManometric investigation of anorectal function in early and late stage Parkinson’s diseaseJ Neurol Neurosurg Psychiatry200068676877010811703
- ZangagliaRMartignoniEGloriosoMMacrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled studyMov Disord20072291239124417566120
- SadjadpourKPyridostigmine bromide and constipation in Parkinson’s diseaseJAMA1983249911486823069
- JostWHSchimrigkKLong-term results with cisapride in Parkinson’s diseaseMov Disord19971234234259159740
- CadedduFBentivoglioARBrandaraFMarnigaGBrisindaGMariaGOutlet type constipation in Parkinson’s disease: results of botulinum toxin treatmentAliment Pharmacol Ther20052210997100316268975
- TrescotAMGlaserSEHansenHBenyaminRPatelSManchikantiLEffectiveness of opioids in the treatment of chronic non-cancer painPain Physician200811Suppl 2S181S20018443639
- GianniWCeciMBustacchiniSOpioids for the treatment of chronic non-cancer pain in older peopleDrugs Aging200926Suppl 1S63S73
- van OjikALJansenPABrouwersJRvan RoonENTreatment of chronic pain in older people: evidence-based choice of strong-acting opioidsDrugs Aging201229861562522765848
- De SchepperHUCremoniniFParkMICamilleriMOpioids and the gut: pharmacology and current clinical experienceNeurogastroenterol Motil200416438339415305992
- KalsoEEdwardsJEMooreRAMcQuayHJOpioids in chronic non-cancer pain: systematic review of efficacy and safetyPain2004112337238015561393
- PappagalloMIncidence, prevalence, and management of opioid bowel dysfunctionAm J Surg2001182Suppl 5AS11S18
- WebsterLJansenJPPeppinJAlvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer painPain2008137242844018164818
- MichnaEBlonskyERSchulmanSSubcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled studyJ Pain201112555456221429809
- ItawiEASavoieLMHannaAJApostolidesGYAlvimopan addition to a standard perioperative recovery pathwayJSLS201115449249822643504
- SawhSBSelvarajIPDangaACottonALMossJPatelPBUse of methylnaltrexone for the treatment of opioid-induced constipation in critical care patientsMayo Clin Proc201287325525922386181
- GattiASabatoAFManagement of opioid-induced constipation in cancer patients: focus on methylnaltrexoneClin Drug Investig2012325293301
- FeiGRaehalKLiuSLubiprostone reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouseJ Pharmacol Exp Ther2010334133334020406855
- SunXWangXWangGDLubiprostone reverses the inhibitory action of morphine on mucosal secretion in human small intestineDig Dis Sci201156233033821181441
- CryerBLKatzSVallejoRA phase 3, randomised, double-blind, placebo controlled clinical trial of lubiprostone for the treatment of opioid-induced bowel dysfunction in patients with chronic non-cancer painGastroenterology2010138Suppl 1S-129 Abstract 906
- JamalMMareyaSWoldegeorgisFJoswickTUenoRLubiprostone significantly improves treatment response in non-methadone opioid-induced bowel dysfunction patients with chronic, non-cancer pain: Results from a phase 3, randomized, double-blind, placebo-controlled clinical trialGastroenterology20121425 Suppl 1S144S145 Abstract 848a
- Sucampo Pharmaceuticals. Takeda and Sucampo report top-line results of two phase 3 trials of lubiprostone in opioid-induced bowel dysfunction [press release]. Bethesda, MD: Sucampo Pharmaceuticals Inc; 2009 [Jul 21]. Available from: http://investor.sucampo.com/phoenix.zhtml?c=201197&p=irol-newsArticle&ID=1309679&highlight=. 2009. Accessed December 18, 2012
- Sucampo Pharmaceuticals. Positive top-line results from phase 3 long-term, open-label safety and efficacy trial of lubiprostone in opioid-induced bowel dysfunction patients [press release]. Bethesda, MD: Sucampo Pharmaceuticals; 2012 [Apr 5]. Available from: http://investor.sucampo.com/phoenix.zhtml?c=201197&p=irol-newsArticle&ID=1680449&highlight=. 2012. Accessed December 18, 2012