Abstract
Background
This study aimed to determine the safety of diclofenac sodium topical solution 1.5% (w/w) in 45.5% dimethyl sulfoxide (TDiclo) for the treatment of knee or hand osteoarthritis in persons aged 75 years or older.
Methods
A pooled analysis of safety data from seven multicenter, randomized, blinded, Phase III clinical trials (4–12 weeks’ duration) of TDiclo was conducted. The analysis focused on a subset of patients (n = 280) aged 75 years or older with a primary diagnosis of osteoarthritis of the knee (six trials) or hand (one trial). Patients received one of three topical treatments: TDiclo (n = 138); placebo (2.33% or 4.55% dimethyl sulfoxide, n = 39); or control (45.5% dimethyl sulfoxide, n = 103). Treatment groups were compared using Chi-square analysis, Fisher’s Exact test, or analysis of variance.
Results
The most common adverse events involved the skin or subcutaneous tissue, primarily at the application site. The incidence of dry skin was higher in the TDiclo (36.2%; P < 0.0001) and dimethyl sulfoxide control (18.4%; P = 0.0142) groups than in the placebo group (2.6%); the incidence of other skin or subcutaneous tissue adverse events was similar between the groups. Relatively few patients (<18%) experienced gastrointestinal adverse events, and group differences were not detected. In the TDiclo group, constipation (3.6%), diarrhea (3.6%), and nausea (3.6%) were the most common gastrointestinal adverse events. Cardiovascular and renal/ urinary adverse events were rare, and group differences were not detected. There was one case (0.7%) each of hypertension, spider veins, and vasodilation in the TDiclo group. Changes from baseline to the final visit in blood pressure and hepatic/renal enzyme levels were also similar between the groups.
Conclusion
TDiclo appears to be well tolerated for the treatment of osteoarthritis in persons aged 75 years or older.
Introduction
“The catastrophe of NSAID mortality is ultimately reduced to the individual collision of NSAID pharmacology conflated with the failure of host defences”
—Sanford H. RothCitation1
Musculoskeletal disorders are the leading cause of disability in the United States and present a major economic burden as a result of lost work wages and direct health care costs, which together account for an estimated $849 billion annually.Citation2 Of the various musculoskeletal disorders, arthritis is the most prevalent chronic condition. In the United States, 21.6% of the adult population aged 18 years or older is affected by arthritis.Citation2 Osteoarthritis is the most common form of arthritis and is characterized by cartilage degradation, osteophyte formation, synovial inflammation, subchondral sclerosis, and bone deformation of one or more joints, with the knees, hips, and hands more commonly affected than other joints, such as the ankles, wrists, or shoulders.Citation3,Citation4 Symptomatically, osteoarthritis is characterized by pain, stiffness, and an impairment or loss of function of the joint.Citation3,Citation4 Prevalence estimates vary depending on the criteria used to define the disease, but recent estimates indicate that 26.9 million American adults are affected by osteoarthritis in at least one joint.Citation5 The prevalence of osteoarthritis is greater among female and elderly individuals; among adults older than 70 years of age, 26.2% of women and 13.3% of men have symptomatic osteoarthritis of the hand.Citation6 The incidence of osteoarthritis is estimated to be 240 per 100,000 person-years for knee osteoarthritis and increases with age up to 80 years.Citation7
There is no cure for osteoarthritis; its treatment is focused on alleviating signs and symptoms of the disease and improving overall quality of life. Multimodal treatment strategies are frequently employed; these approaches generally incorporate weight management and exercise programs, physical and occupational therapy, and other types of nonpharmacologic and nonsurgical therapies.Citation8,Citation9 Total joint arthroplasty is employed as a definitive treatment in advanced disease; total hip and knee replacements are the most common procedures.Citation2 Although often effective, these procedures confer significant health risks and economic costs.Citation2 Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common pharmacologic treatment utilized for alleviating the symptoms of osteoarthritis. However, use of these agents is associated with an increased risk of serious adverse effects, particularly among the elderly and those with increased cardiovascular and gastrointestinal risk.Citation9–Citation13 For example, cohort and case-control epidemiologic studies have shown an increase in NSAID-related upper gastrointestinal adverse events (aspirin and nonaspirin), with a 2–6-fold increase in risk over non-NSAID users.Citation14
The most significant oral NSAID-related adverse events involve the gastrointestinal,Citation15,Citation16 cardiovascular,Citation17,Citation18 and renalCitation19,Citation20 systems. The Agency for Healthcare Research and Quality estimates that among adults aged 75 years or older receiving oral NSAIDs for osteoarthritis, 91 per 10,000 persons will experience a serious gastrointestinal bleed, 30 per 10,000 persons will experience a myocardial infarction from oral NSAIDs other than naproxen, and 20 per 10,000 persons will discontinue using oral NSAIDs due to renal dysfunction.Citation21
The gastrointestinal effects of oral NSAIDs have been recognized for more than 20 years, yet remain a problem despite the combined use of NSAIDs and gastroprotective agents.Citation15,Citation22–Citation24 Recent studies have shown that even shortterm use of oral NSAIDs can increase the risk of myocardial infarction and death among high-risk individuals. Celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, is associated with an increased risk of myocardial infarction or death within 14–30 days of treatment.Citation13 Rofecoxib, another selective COX-2 inhibitor, and ibuprofen, a nonselective COX inhibitor, are associated with increased risk of myocardial infarction or death within 7–14 days of treatment.Citation13 The nonselective NSAID diclofenac is associated with increased risk of myocardial infarction or death immediately after initiation of treatment.Citation13 In addition, NSAIDs increase blood pressure and can trigger blood pressure destabilization in hypertensive patients.Citation25,Citation26 These effects may be more pronounced in patients with compromised renal function, particularly the elderly, in whom renal function is compromised as a result of the normal aging process.Citation10,Citation19 The age-related compromise in renal function also confers greater risk of oral NSAID–induced renal toxicity in elderly patients.Citation10 NSAID-induced hepatotoxicity is rare, but because NSAIDs are widely prescribed, their use accounts for a large proportion of cases of acute liver failure.Citation27 The elderly are at greater risk for NSAID-induced hepatotoxicity secondary to the increased risk of hepatotoxicity related to advanced age rather than to NSAID use per se.Citation27
Topical NSAIDs have the potential to reduce the risk of systemic adverse events associated with oral NSAIDs by reducing the plasma level of active drug and metabolites.Citation28 A number of professional organizations have included topical NSAIDs as a therapeutic option for the treatment of osteoarthritis, including the American Geriatrics Society,Citation10 the European League Against Rheumatism,Citation29 the National Institute for Health and Clinical Excellence,Citation30 and the Osteoarthritis Research Society International.Citation31 The American College of Rheumatology (ACR) recently updated its osteoarthritis treatment guidelines, using published data available through 2010 and a formal grading process implemented by a broad range of experts drawn from numerous disciplines.Citation32 The new guidelines recommend topical NSAIDs as one option for first-line treatment of osteoarthritis of the knee or hand. For persons aged 75 years or older, the ACR guidelines recommend topical NSAIDs, rather than oral NSAIDs, as first-line treatment for osteoarthritis of the hand.
Currently, only two topical formulations of diclofenac have been approved by the United States Food and Drug Administration for the treatment of osteoarthritis. Diclofenac sodium topical solution 1.5% (w/w) in 45.5% dimethyl sulfoxide (abbreviated herein as TDiclo), approved in 2009, is one such formulation. Because of the increased risk of NSAID-induced adverse events among the elderly, as well as the ACR recommendation regarding the use of topical NSAIDs for the treatment of hand osteoarthritis in the elderly, this study aimed to determine the safety profile of TDiclo in persons aged 75 years or older through pooled analysis of safety data from seven multicenter, randomized, blinded, Phase III clinical trials of TDiclo.
Materials and methods
Patients
Eligible patients were men and nonpregnant women who received a diagnosis, verified radiologically and scored for severity, of primary osteoarthritis in at least one knee (six trials) or hand (one trial), with regular pain in the affected joint. If patients had two affected joints, the joint with the highest pain score or the dominant joint was assessed in the trial. The age of eligible patients varied between trials, but all patients were at least 18 years of age. Exclusion criteria that were common to all trials included were: secondary arthritis; known sensitivity to diclofenac, other NSAIDs, dimethyl sulfoxide, or any other component of the vehicle; concomitant skin disease or use of another topical product at the targeted application site; corticosteroid use; oral use of analgesics, glucosamine, or chondroitin; and clinically significant renal, hepatic, or peptic ulcer disease. A one-week washout period preceded baseline measurements. The use of aspirin was permitted for prophylactic cardioprotection.
Study design
This study involved a pooled analysis of safety data derived from seven multicenter, randomized, blinded, Phase III clinical trials of TDiclo (Pennsaid®; Mallinckrodt Inc, Hazelwood, MO)Citation33 conducted in Canada and the United States, and focused on a subset of patients 75 years of age or older with a primary diagnosis of osteoarthritis in the knee or hand. The trials were similar in design, although the comparison groups and the duration of the trials (4–12 weeks) differed. Each trial was approved by the appropriate institutional review board, and all patients provided written informed consent before study enrollment.
Three treatment groups were examined in the pooled analysis: TDiclo (diclofenac sodium topical solution 1.5% [w/w] in 45.5% dimethyl sulfoxide; n = 138); placebo (topical lotion consisting of 2.33% or 4.55% dimethyl sulfoxide; n = 39); and control (topical lotion consisting of 45.5% dimethyl sulfoxide; n = 103). The three lotions were identical in appearance. A small amount of dimethyl sulfoxide was included in the placebo lotion in order to control for the garlic odor produced by dimethyl sulfide when it is exhaled.Citation34 This blinding procedure was validated in earlier trials.Citation35 Patients were instructed to apply 40 drops (approximately 1.3 mL) of solution four times per day or 50 drops (approximately 1.55 mL) of solution three times per day to the affected knees or 5–40 drops of solution four times per day to the affected hands throughout the study period. Compliance was verified by weighing bottles at the start of each weekly visit and calculating the average dose applied per joint per day. Acetaminophen was permitted in these trials as a rescue medication.
Safety assessments
Vital signs were recorded at the baseline and final visits. Urine and blood samples were collected at the baseline and final visits for four of the seven trials. These were used for routine laboratory measurements, including measurement of hemoglobin and key hepatic (alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase) and renal (creatinine) enzymes. Patients were provided with a diary and instructed to record daily any adverse events that occurred. During each weekly clinic visit or telephone call, study personnel recorded adverse events using a standardized checklist containing common adverse events related to the use of NSAIDs. Study personnel verbally questioned patients using standardized open-ended questions and recorded any abnormalities in the checklist. Study personnel also assessed the affected joint at each weekly clinic visit and recorded any abnormalities using the same checklist.
Statistical analyses
All adverse events were categorized using the FDA Coding Symbols for Thesaurus of Adverse Reaction Terms.Citation36 Serious and severe adverse events were defined according to the definitions provided by Spilker.Citation37 Descriptive statistics were calculated, and continuous variables were represented as mean, standard deviation, minimum, and maximum values. Categorical variables were represented as frequencies and corresponding percentages. For selected continuous variables, change scores were calculated from values measured at the baseline assessment to those measured at the final visit. Treatment groups were compared (TDiclo versus placebo and control versus placebo) using the Chi-square or Fisher’s Exact test for categorical variables and analysis of variance with a main treatment effect for continuous variables. For all statistical tests, α = 0.05. All statistical analyses were conducted using SAS software (SAS Institute Inc, Cary, NC).
Results
Baseline demographics and clinical characteristics
Patients in the three treatment groups did not differ with respect to age, gender, or racial composition (). The baseline clinical characteristics of the patients were also similar among the three treatment groups (), except that the percentage of patients with a history of hypertension was higher in the TDiclo (60.9%; P = 0.013) and control (61.2%; P = 0.015) groups than in the placebo group (38.5%). However, mean blood pressure measurements were similar among the three treatment groups.
Table 1 Baseline demographics of patients
Table 2 Clinical characteristics of patients
Treatment-emergent adverse events
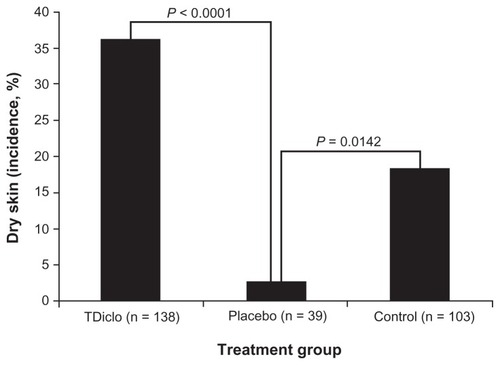
presents the incidence of skin or subcutaneous tissue and gastrointestinal adverse events affecting 1% or more of patients. The overall incidence of skin or subcutaneous adverse events was higher in the TDiclo (44.2%; P < 0.0001) and control (30.1%; P < 0.0042) groups than in the placebo group (7.7%). Among the specific skin or subcutaneous tissue adverse events, a significant difference between treatment groups was detected only for dry skin (), and the percentage of patients with dry skin was higher in the TDiclo (36.2%; P < 0.0001) and control (18.4%; P = 0.0142) groups than in the placebo group (2.6%). Other specific skin or subcutaneous tissue adverse events that were observed in the TDiclo group included erythema (5.8%) and contact dermatitis (5.1%); all other specific skin-related adverse events occurred in less than 3% of patients in the TDiclo group ().
Table 3 Incidence of treatment-emergent skin or subcutaneous tissue and gastrointestinal adverse events affecting ≥1% of patients
Figure 1 Incidence of dry skin among patients in the TDiclo, placebo, and control groups.
Abbreviation: TDiclo, diclofenac sodium topical solution.
The overall incidence of gastrointestinal adverse events was relatively low and did not differ between the TDiclo (17.4%), control (8.7%), and placebo (17.9%) groups (). Among the specific gastrointestinal effects observed in the TDiclo group, constipation (3.6%), diarrhea (3.6%), and nausea (3.6%) were the most frequently reported. All other gastrointestinal adverse events occurred in less than 3% of patients in the TDiclo group.
Cardiovascular adverse events occurred rarely, and the overall incidence of cardiovascular adverse events did not differ between the TDiclo (2.2%), placebo (2.6%), and control (0.0%) groups. Among the specific cardiovascular adverse events observed in the TDiclo group, there was one case (0.7%) of hypertension, one case (0.7%) of spider veins, and one case (0.7%) of vasodilation.
Renal or urinary adverse events were also rare and occurred with an incidence of 1.9% in the control group and 0.0% in both the TDiclo and placebo groups. One case (1.0%) of pollakiuria and one case (1.0%) of abnormal urine odor were reported in the control group.
Treatment-emergent serious adverse events
The overall incidence of serious adverse events was 0.7%, 0.0%, and 7.7% for the TDiclo, control, and placebo groups, respectively. The incidence was significantly higher for the placebo group than for the TDiclo (P = 0.034) or control (P = 0.020) groups. One patient (0.7%) in the TDiclo group developed a malignant neoplasm, and three patients (7.7%) in the placebo group reported a total of seven serious adverse events, including one instance (2.6%) each of cerebrovascular accident, dizziness, allergic transfusion reaction, feeling hot, and nausea, and two instances (5.1%) of asthenia. No patients experienced serious cardiovascular or renal/urinary adverse events.
Treatment-emergent severe adverse events
The overall incidence of severe adverse events was also higher for the placebo group (10.3%) than for the TDiclo (4.3%; not significant) or control (1.0%; P = 0.020) groups. The severe adverse events reported by patients in the TDiclo group included dry skin (0.7%), contact dermatitis (0.7%), erythema (0.7%), upper abdominal pain (0.7%), constipation (0.7%), and paresthesia (0.7%). No patients experienced severe cardiovascular or renal/urinary adverse events.
Treatment-emergent adverse events leading to study discontinuation
The overall percentage of patients who discontinued the study due to adverse events was higher in the placebo group (15.4%) than in the TDiclo (13.0%) or control (5.8%) groups, but these differences were not significant (). In the TDiclo group, dry skin (2.9%), erythema (2.9%), contact dermatitis (2.2%), and pruritus (2.2%) were the most common skin or subcutaneous tissue adverse events that resulted in study discontinuation. Gastrointestinal adverse events resulting in study discontinuation in the TDiclo group included upper abdominal pain (2.2%) and nausea (1.4%). Cardiovascular and renal/urinary adverse events were not associated with study discontinuation in the three treatment groups.
Table 4 Overall incidence of treatment-emergent skin or subcutaneous tissue and gastrointestinal adverse events that resulted in study discontinuation in ≥1% of patients
Application site–related treatment-emergent adverse events
The three treatment groups differed in overall incidence of application site–related adverse events, with a higher incidence in the TDiclo (39.1%; P < 0.0001) and control (23.3%; P = 0.014) groups than in the placebo group (5.1%). shows the specific types of application site–related adverse events for the three treatment groups. Similar to the results for all adverse events, a significant difference between treatment groups was detected only for dry skin, and the percentage of patients with dry skin at the application site was significantly higher in the TDiclo (31.9%; P < 0.0001) and control (15.5%; P = 0.041) groups than in the placebo group (2.6%). Other application site–related adverse events in the TDiclo group included erythema (5.8%) and contact dermatitis (5.1%); all other events occurred in less than 2% of patients in the TDiclo group.
Figure 2 Incidence of application site-related adverse events among patients in the TDiclo, placebo, and control groups.
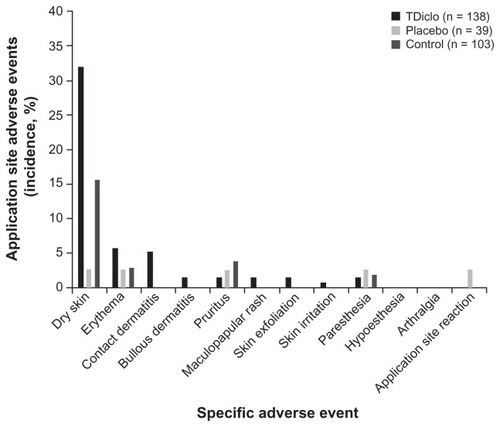
No patients experienced serious application site–related adverse events, but two patients (1.4%) in the TDiclo group experienced three severe application site–related adverse events (one case [0.7%] each of contact dermatitis, erythema, and paresthesia). The overall incidence of severe application site–related adverse events in the TDiclo (1.4%) and control (0.0%) groups was similar to that in the placebo group (0.0%).
The percentage of patients who discontinued study participation due to application site–related adverse events was higher in the TDiclo (5.1%) and control (1.9%) groups than in the placebo group (0.0%), but these differences were not significant. In the TDiclo group, the most common application site–related adverse events that resulted in study discontinuation were four cases (2.9%) of erythema and three cases (2.2%) of contact dermatitis.
Laboratory and blood pressure measurements
shows changes in blood pressure and key laboratory parameters from the baseline to the final visit for the three treatment groups. Of the 280 patients who were analyzed in this dataset, only 239 had both baseline and final readings (125 in the TDiclo group, 28 in the placebo group, and 86 in the control group). Changes in blood pressure from the baseline to the final visit did not differ between groups. Changes in levels of hepatic and renal enzymes and hemoglobin were similar between the TDiclo and placebo groups and between the control and placebo groups.
Table 5 Changes in blood pressure and key laboratory measurements from baseline to the final visit
Discussion
Osteoarthritis is a prevalent chronic disorder among the elderly.Citation5 NSAIDs are the most common drugs prescribed to the elderly, and they are often used to treat the symptoms associated with osteoarthritis.Citation38 The elderly deserve special consideration when prescribing NSAIDs due to the natural effects of aging on the gastrointestinal, cardiovascular, renal, and hepatic systems, the presence of comorbid conditions, and the potential for polydrug use.Citation9–Citation11 Topical NSAIDs are associated with less systemic bioavailability than oral NSAIDsCitation28 and thus may impart a particular benefit to the elderly.Citation10
TDiclo is a topical formulation of diclofenac that is approved for the treatment of osteoarthritis.Citation33 Several multicenter, randomized, blinded, Phase III clinical trials have demonstrated the efficacy of TDiclo in the treatment of osteoarthritis of the knee.Citation35,Citation39–Citation42 In addition, the efficacy of TDiclo has been demonstrated to be equivalent to that of oral diclofenac.Citation41
NSAID–induced gastrointestinal adverse events range from mild events such as heartburn and dyspepsia to more serious events such as gastric and duodenal ulcers, bleeding, and perforation.Citation15,Citation16,Citation22–Citation24 The risk of NSAID-induced gastrointestinal injury increases with age.Citation16 NSAIDs injure the gastrointestinal tract by blocking the production of prostaglandins in the gut, and this decreases mucosal blood flow, inhibits the secretion of mucus and bicarbonate, and decreases epithelial cell proliferation.Citation20,Citation43 In a pooled safety analysis comparing oral diclofenac with TDiclo, gastrointestinal adverse events were significantly more frequent with oral diclofenac than with TDiclo (39.0% versus 25.4%, P < 0.0001). The most common adverse events that occurred in the oral diclofenac group were dyspepsia (18.4%), diarrhea (13.4%), upper abdominal pain (12.1%), and abdominal distension (10.6%).Citation44 In the current study, 17.4% of patients in the TDiclo group experienced treatment-emergent gastrointestinal adverse events, with the most common being constipation (3.6%), diarrhea (3.6%), and nausea (3.6%). No serious gastrointestinal events were observed, and severe gastrointestinal events were rare in the TDiclo group. The incidence of gastrointestinal adverse events in the TDiclo group was similar to that in the placebo group. These results suggest that use of TDiclo by the elderly is not associated with a significant risk of gastrointestinal adverse events.
NSAID-induced cardiovascular adverse events are more common with selective COX-2 inhibitors than with nonselective NSAIDs,Citation17,Citation18 although one study using hospital registry data found that patients admitted to hospital with an initial myocardial infarction and treated subsequently with oral diclofenac had the greatest risk of subsequent myocardial infarction and death compared with patients treated with other NSAIDs.Citation13 The risk of NSAID-induced cardiovascular injury generally increases with age.Citation10,Citation45 NSAIDs adversely affect the cardiovascular system in part through their well established ability to increase blood pressure (about 5 mmHg)Citation25 and in part through their effects on renal function.Citation19 In the current study, cardiovascular adverse events were observed in 2.2% of patients in the TDiclo group, with one case (0.7%) each of hypertension, spider veins, and vasodilation, but the incidence of cardiovascular adverse events in the TDiclo group did not differ from that in the placebo group, and no serious or severe cardiovascular adverse events were reported among patients receiving TDiclo. No differences were detected between the TDiclo group and the placebo group in baseline blood pressure measurements or in mean change in blood pressure from the baseline to the final visit. These findings suggest a minimal risk of cardiovascular toxicity after TDiclo treatment in the elderly.
NSAID-induced renal adverse events are relatively uncommon, but can be serious. They include sodium retention and edema, acute tubular necrosis, acute renal failure, hyperkalemia, interstitial nephritis, and renal papillary necrosis.Citation19,Citation20 The risk of NSAID-induced renal toxicity increases with age due to a natural age-related decrease in the rate of glomerular filtration, which results in decreased urine excretion.Citation9–Citation11,Citation19 No renal adverse events were found among elderly patients in the TDiclo group in this analysis. Furthermore, the mean change in creatinine levels from the baseline to the final visit was similar in the TDiclo and placebo groups. These results suggest that the risk of TDiclo-induced renal dysfunction may be low among the elderly.
Hepatotoxicity related to NSAIDs is rare; however, because oral NSAIDs are so widely prescribed, particularly among the elderly,Citation38 they represent a major overall cause of drugrelated hepatotoxicity.Citation27,Citation46 Hepatotoxicity related to the use of oral diclofenac is especially high,Citation27 and acute liver injury is estimated to occur in 6.3 per 100,000 oral diclofenac users.Citation47 Diclofenac-induced hepatotoxicity is not well understood, but it is believed to result from direct toxicity related to diclofenac metabolites and indirect toxicity related to inflammation; both effects result in a hepatocellular pattern of liver injury.Citation27 The risk of NSAID-induced hepatotoxicity increases with age.Citation10,Citation27 An examination of key hepatic enzymes (alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase) at the baseline and final visits showed a similar mean change in hepatic enzyme levels for the TDiclo and placebo groups. These findings suggest minimal hepatotoxicity associated with TDiclo treatment in the elderly.
As noted previously, the ACR recently updated its osteoarthritis treatment guidelines to include the recommendation that topical NSAIDs be considered as one option for first-line treatment of osteoarthritis of the hand or knee.Citation32 The new guidelines do not specifically address treatment recommendations for osteoarthritis in persons at increased risk for NSAID-induced gastrointestinal, cardiovascular, renal, or hepatic adverse events. However, the updated guidelines do include the recommendation that topical rather than oral NSAIDs be used to treat knee osteoarthritis in persons aged 75 years or older. Presumably, this recommendation is based on the increased risk of gastrointestinal,Citation16 cardiovascular,Citation10,Citation45 renal,Citation9–Citation11,Citation19 and hepaticCitation10,Citation27 adverse events with oral NSAID use in the elderly.
Published short-term trials of TDiclo in the adult population report that the incidence of application site–related skin dryness ranges from 18.2% to 39.3%.Citation35,Citation39–Citation42 Paresthesia and rash are additional adverse events related to TDiclo.Citation39–Citation41 The rate of study withdrawal due to application site–related adverse events ranges from 3.2% to 6% in the published trials.Citation35,Citation39,Citation40,Citation42 A long-term (52-week) study of TDiclo in adults found that application site–related skin irritation is the most common type of adverse event after TDiclo treatment for primary osteoarthritis of the knee, with dry skin (25.3%) and contact dermatitis (22.5%) being the most common specific adverse events.Citation48 A systematic literature review on the use of topical NSAIDs in the elderly showed that application site–related adverse events are the most common type, with dry skin, erythema, dermatitis, rash, paresthesia, pruritus, and urticaria occurring frequently in the reviewed studies.Citation12 The same review found a high rate (0%–21%) of study discontinuation due to adverse events, although the rate of study discontinuation due specifically to application site–related adverse events was not reported. A study by Baraf et al compared the adverse event profile of diclofenac sodium gel 1% among persons aged 25–64 years and those aged 65 years or older, and for both groups, application site–related dermatitis was the most common treatment-emergent adverse event, affecting 4.0%–5.8% of patients.Citation49 The rate of study discontinuation due to application site–related dermatitis was low (2.0%–2.2%) and did not differ between the two age groups. The findings of the current study show that dry skin was the most common adverse event in the TDiclo group, affecting 36.2% of patients and occurring primarily near the application site. Erythema and contact dermatitis occurred in less than 6% of patients in the TDiclo group. Serious skin or subcutaneous adverse events were not reported by patients in the TDiclo group, and severe skin or subcutaneous adverse events were rare in the TDiclo group. Together, the current and previous findings suggest that the majority of adverse events after treatment with TDiclo are related to the application site, primarily involve the skin or subcutaneous tissue, and are minor, even among the elderly.
Of note is the fact that the incidence of skin or subcutaneous tissue adverse events in the control group, which received the TDiclo vehicle (45.5% dimethyl sulfoxide), was significantly higher than in the placebo group. This finding suggests that dimethyl sulfoxide was responsible for or contributed to the adverse events observed after TDiclo treatment. Indeed, the dermatologic effects of dimethyl sulfoxide, including skin rash and pruritus, are well established and have been exploited for the medical treatment of several dermatologic conditions.Citation34 The current finding confirms previous trial results suggesting that dimethyl sulfoxide likely contributes to some of the skin and subcutaneous tissue adverse events observed after treatment with TDiclo.Citation35,Citation39
This study has several limitations that should be considered when interpreting its results. First, the data were derived from a retrospective pooled analysis based on relatively short-term (4–12-week) trials. Long-term trials are needed to confirm the results of the current study, although a recent 52-week, open-label study of TDicloCitation47 reported results that are similar to those reported here. Also, this analysis included only one study involving hand osteoarthritis, which limits the ability for these findings to be generalized beyond knee osteoarthritis. Another limitation is that sample sizes were relatively low, particularly for the placebo group. This limitation is a consequence of the study design, which involved a pooled analysis of data from a subgroup of patients aged 75 years or older. Only a subset of the studies in this pooled analysis included laboratory measurements, hence reducing the sample sizes for those measurements. This may have limited the observation of the most serious upper gastrointestinal bleeds, which are most often asymptomatic in nature. Another limitation is that blood pressure measurements were not strictly controlled, and blood pressure variability was not examined. Finally, indirect measures of hepatic and renal toxicity were used for analysis.
Conclusion
TDiclo appears to be well tolerated in persons aged 75 years or older. The current findings suggest that TDiclo may be an appropriate treatment choice, particularly for patients who are resistant to or tolerant of application site–related skin or subcutaneous tissue adverse events. These findings support the new recommendation by the ACR that topical NSAIDs be used for the treatment of hand or knee osteoarthritis in the elderly.Citation32
Disclosure
SHR has served as a consultant/advisory board member and speaker for Covidien. He holds stock in Transdel Pharmaceuticals. PF is an employee of Mallinckrodt Inc, which sponsored the study and the preparation of this manuscript. Technical editorial and writing support for the preparation of this manuscript was provided by Karamarie Fecho, Synchrony Medical, LLC, West Chester, PA.
References
- RothSHComing to terms with nonsteroidal anti-inflammatory drug gastropathyDrugs201272787387922564130
- United States Bone and Joint DecadeThe Burden of Musculoskeletal Diseases in the United StatesRosemont, ILAmerican Academy of Orthopaedic Surgeons2008
- BusijaLBridgettLWilliamsSRMOsteoarthritisBest Pract Res Clin Rheumatol201024675776821665124
- BijlsmaJWBerenbaumFLafeberFPOsteoarthritis: an update with relevance for clinical practiceLancet201137797832115212621684382
- LawrenceRCFelsonDTHelmickCGfor the National Arthritis Data WorkgroupEstimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part IIArthritis Rheum2008581263518163497
- ZhangYNiuJKelly-HayesMChaissonCEAllabadiPFelsonDTPrevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham StudyAm J Epidemiol2002156111021102712446258
- OliveriaSAFelsonDTReedJICirilloPAWalkerAMIncidence of symptomatic hand, hip, and knee osteroarthritis among patients in a health maintenance organizationArthritis Rheum1995388113411417639811
- HuskissonECModern management of mild-to-moderate joint pain due to osteoarthritis: a holistic approachJ Int Med Res20103841175121220925992
- SeedSMDunicanKCLynchAMTreatment options for osteoarthritis: considerations for older adultsHosp Pract (Minneap)2011391627321441760
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older PersonsPharmacological management of persistent pain in older personsJ Am Geriatr Soc20095781331134619573219
- BarkinRLBeckermanMBlumSLClarkFMKohEKWuDSShould nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult?Drugs Aging2010271077578920883058
- MakrisUEKohlerMJFraenkelLAdverse effects of topical nonsteroidal antiinflammatory drugs in older adults with osteoarthritis: a systematic literature reviewJ Rheumatol20103761236124320360183
- Schjerning OlsenAMFosbølELLindhardsenJDuration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort studyCirculation2011123202226223521555710
- LaineLApproaches to nonsteroidal anti-inflammatory drug use in the high-risk patientGastroenterology2001120359460611179238
- LazzaroniMPorroGBManagement of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitorsDrugs2009691516919192936
- ScarpignatoCHuntRHNonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and preventionGastroenterol Clin North Am201039343346420951911
- FarkouhMEGreenbergBPAn evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugsAm J Cardiol200910391227123719406264
- TrelleSReichenbachSWandelSCardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysisBMJ2011342c708621224324
- HarirforooshSJamaliFRenal adverse effects of nonsteroidal anti-inflammatory drugsExpert Opin Drug Saf20098666968119832117
- JohnRHerzenbergAMRenal toxicity of therapeutic drugsJ Clin Pathol200962650551519474353
- Agency for Healthcare Research and Quality. US Department of Health and Human ServicesChoosing nonopioid analgesics for osteoarthritis: clinician summary guideJ Pain Palliat Care Pharmacother200923143345719947847
- SunDCRothSHMitchellCSEnglundDWUpper gastrointestinal disease in rheumatoid arthritisAm J Dig Dis19741954054104825946
- RothSHBennettRENonsteroidal anti-inflammatory drug gastropathy. Recognition and responseArch Intern Med198714712209321003318750
- RothSHNonsteroidal anti-inflammatory drugs: gastropathy, deaths, and medical practiceAnn Intern Med198810953533543044208
- JohnsonAGNguyenTVDayRODo nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysisAnn Intern Med199412142893008037411
- ZhaoSZBurkeTAWheltonAvon AllmenHHendersonSCBlood pressure destabilization and related healthcare utilization among hypertensive patients using nonspecific NSAIDs and COX-2-specific inhibitorsAm J Manag Care2002815 SupplS40141312416790
- AithalGPDayCPNonsteroidal anti-inflammatory drug-induced hepatotoxicityClin Liver Dis200711356357517723920
- KienzlerJLGoldMNollevauxFSystemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteersJ Clin Pharmacol2010501506119841157
- JordanKMArdenNKDohertyMEULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)Ann Rheum Dis200362121145145514644851
- National Institute for Health and Clinical ExcellenceOsteoarthritis: the care and management of osteoarthritis in adultsLondon, UKNational Institutes for Health and Clinical Excellence2008 NICE clinical guideline 59
- ZhangWMoskowitzRWNukiGOARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelinesOsteoarthritis Cartilage200816213716218279766
- HochbergMCAltmanRDAprilKTAmerican College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip and kneeArthritis Care Res2012644465474
- Pennsaid® [package insert]Hazelwood, MOMallinckrodt Brand Pharmaceuticals, Inc.2010
- SantosNCFigueira-CoelhoJMartins-SilvaJSaldanhaCMultidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspectsBiochem Pharmacol20036571035104112663039
- SimonLSGriersonLMNaseerZBookmanAAZev ShainhouseJEfficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritisPain2009143323824519380203
- US Food and Drug AdministrationCOSTART: Coding Symbols for Thesaurus of Adverse Reaction Terms4th edSpringfield, VANational Technical Information Service, US Department of Commerce1995
- SpilkerBCollecting adverse event and adverse reaction data in clinical trialsGuide to Clinical TrialsPhiladelphia, PALippincott Williams & Wilkins1991
- JonesRNonsteroidal anti-inflammatory drug prescribing: past, present, and futureAm J Med20011101A4S7S11165987
- BookmanAAMWilliamsKSAShainhouseJZEffect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trialCMAJ2004171433333815313991
- RothSHShainhouseJZEfficacy and safety of a topical diclofenac solution (Pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trialArch Intern Med2004164182017202315477437
- TugwellPSWellsGAShainhouseJZEquivalence study of a topical diclofenac solution (Pennsaid®) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trialJ Rheumatol200431102002201215468367
- BaerPAThomasLMShainhouseZTreatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886]BMC Musculoskelet Disord200564416086839
- BhattDLScheimanJAbrahamNSACCF/ACG/AHA2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID useAm J Gastroenterol20081031128902907
- RothSHFullerPDiclofenac topical solution compared with oral diclofenac: a pooled safety analysisJ Pain Res2011415916721811391
- LévesqueLEBrophyJMZhangBThe risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adultsAnn Intern Med2005142748148915809459
- TaberSSPaskoDAThe epidemiology of drug-induced disorders: the kidneyExpert Opin Drug Saf20087667969018983215
- de AbajoFJMonteroDMadurgaMGarcía RodriguezLAAcute and clinically relevant drug-induced liver injury: a population based case-control studyBr J Clin Pharmacol2004581718015206996
- ShainhouseJZGriersonLMNaseerZA long-term, open-label study to confirm the safety of topical diclofenac solution containing dimethyl sulfoxide in the treatment of the osteoarthritic kneeAm J Ther201017656657620216203
- BarafHSBGlothFMBarthelHRGoldMSAltmanRDSafety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trialsDrugs Aging2011281274021174485