Abstract
Purpose
Male breast cancer (MBC) is a rare disease that tends to occur in elderly men. Little is known about the causes of death in MBC because of the small sample size of most studies. This study aimed to investigate the causes of death in MBC patients.
Patients and Methods
MBC patient data were obtained from the Surveillance, Epidemiology, and End Results database (1975–2016). Time trends of MBC mortality in the US population were analyzed using Joinpoint software. We calculated the proportion of each cause of death in the overall cohort and in different patient subgroups. Competing risk models were used to calculate cumulative mortality at different follow-up times. The risk of cardiovascular death (CVD) in MBC patients was compared to that of the age-matched general population by calculating standardized mortality ratio (SMR).
Results
In total, 6426 patients were included in the analysis. MBC mortality rate increased between 2004 and 2019 (annual percentage change=1.16, 95% confidence interval [CI]: 0.50, 1.80). There were 1757 patients (27.3%) who died of non-breast cancer causes. CVD was the leading cause of death in patients who were elderly or had localized disease. MBC patients had a 6.58-fold higher risk of CVD than the general population (SMR=6.58, 95% CI: 6.14, 7.05).
Conclusion
Non-breast cancer death accounts for the majority of deaths in MBC patients who are elderly or have localized cancer. Compared to the general population, MBC patients have an increased risk of CVD. These results highlight the importance of monitoring cardiovascular comorbidities in MBC patients.
Introduction
Male breast cancer (MBC) is rare, accounting for approximately 1% of breast cancer cases and <1% of malignancies in men.Citation1,Citation2 It is estimated that 2650 new MBC cases will be diagnosed and 530 men will die of this disease in the US in 2021.Citation3 The incidence of MBC is increasing.Citation4–Citation6 Owing to its rarity, not many clinical studies have been carried out in MBC patientsCitation1 and treatment strategies have mostly been extrapolated from research on female breast cancer (FBC).Citation7 However, MBC is distinct from FBC in both biological and clinical features;Citation8,Citation9 therefore, optimal management strategies also differ.Citation10,Citation11 Most previous researches on MBC were small single-center case series that did not yield clear evidence,Citation7 which has limited progress in the improvement of MBC prognosis. Large-scale studies of MBC are needed to fill the gap in knowledge.
Non cancer death accounts for a large proportion of deaths in breast cancer survivors; thus, identifying and controlling risk factors for mortality can improve patients’ overall survival.Citation12 Previous studies have shown that non cancer death—especially from cardiovascular causes—accounts for the largest proportion of deaths in FBC patients.Citation13,Citation14 Evidence-based clinical guidelines of the American Society of Clinical Oncology (ASCO)Citation15 and statements from the American Heart AssociationCitation16 have highlighted non cancer diseases associated with FBC and provide recommendations for their monitoring and management. However, the causes of death in MBC patients have rarely been reported; a few studies that examined different causes of death reported a high rate of non-cancer death,Citation17,Citation18 although these studies had small sample sizes and may not reflect the actual epidemiology of MBC. MBC tends to occur at an older age than FBCCitation7,Citation19 and therefore, patients have a higher risk of cardiovascular disease and other comorbidities.
The first as well as the latest ASCO guidelines on MBC propose additional studies to provide evidence for post-treatment surveillance and management.Citation7 To identify high risk diseases in certain groups of MBC survivors is the basic of precise surveillance and management. To this end, we conducted a population-based analysis of data from the Surveillance, Epidemiology, and End Results (SEER) database in order to identify high-risk diseases causing mortality in MBC patients, including in different patient subgroups. We mainly focused on the risk of cardiovascular and other causes of death in MBC patients and their cardiovascular death (CVD) risk was compared to the general population. Our findings can help clinicians monitor and manage high-risk conditions as well as balance cancer treatment side effects and benefits in MBC patients in order to maximize their overall survival.
Materials and Methods
Data Source
Data for this population-based analysis were downloaded using SEER*Stat v8.3.7 software (National Cancer Institute, Bethesda, MD, USA) from the SEER-18 database, which contains data from 18 cancer registries in the US covering approximately 34.6% of the US population.Citation20 We obtained permission to retrieve and use the data after signing the data use agreement. Data on men in the general population who died from breast cancer between 1999 and 2019 were downloaded from the Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research (CDC WONDER)Citation21 for time trend analysis. Data for a standardized cohort reflecting the US general population were also obtained from CDC WONDER.Citation21 Ethics approval and informed consent were not required as the data were publicly available.
Study Population and Variables
This was a population-based epidemiologic study spanning a long period (42 years). The inclusion criteria were as follows: (1) diagnosed with MBC as the primary tumor; (2) diagnosed between 1975 and 2016; and (3) active follow-up and clear cause of death. The exclusion criteria were as follows: (1) death certification or autopsy only; and (2) multiple primary cancers. Patients aged 0–34 years were excluded from the competing risk analysis and standardized mortality ratio (SMR) calculation due to their small number. Patient data that were extracted from the database included age at diagnosis, marital status, race, year of diagnosis, grade, laterality, stage, surgery, radiation, and chemotherapy.
Study Design and Outcomes
We first analyzed time trends of breast cancer mortality in the US male general population between 1999 and 2019. The proportion of each cause of death in the overall cohort and subgroups (categorized by age at diagnosis, marital status, race, year of diagnosis, grade, laterality, stage, surgery, radiation, and chemotherapy) was calculated. Cumulative mortality rates by follow-up time were calculated and cumulative mortality curves were generated with the competing risk method. Additionally, we calculated SMRs for CVD in different age groups. SMR in this study represented the CVD risk of MBC patients compared to the US general population. The primary endpoint in this study was death from any cause including cardiovascular diseases. Causes of death were classified according to the SEER Cause of Death Recode—which is based on the World Health Organization International Statistical Classification of Diseases and Related Health Problems (8th, 9th, and 10th Revisions)Citation22—into 10 categories as previously describedCitation23 (Supplemental Table 1). Patients who were still alive at the last follow-up were considered as censored observations. The follow-up period was the time from the date of first diagnosis with MBC to the date of death or last follow-up. The final follow-up date was December 31, 2016.
Statistical Analysis
Time trends of MBC mortality were analyzed using Joinpoint v4.8.0.1 software (Statistical Research and Applications Branch, National Cancer Institute, USA) (https://surveillance.cancer.gov/joinpoint/). The proportion of each cause of death was calculated using Excel software (Microsoft, Redmond, WA, USA). Cumulative mortality rates were calculated with the competing risk model using R v4.0.3 software (https://www.r-project.org). SMR was calculated as the ratio of observed to expected deaths; the latter was calculated as person-years multiplied by the rate of CVD in the general population obtained from CDC WONDER, and person-years was the sum of MBC patients’ survival time—ie, the interval from MBC diagnosis to CVD or the last follow-up of the study. The 95% confidence interval (95% CI) and P value of SMRs were calculated as previously described.Citation24,Citation25 P<0.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Population
There were 6426 eligible patients diagnosed with MBC between 1975 and 2016 in the SEER database; 3068 (47.7%) died during the follow-up period. Patients’ baseline characteristics are shown in . The median age at diagnosis was 65 years. Most patients (87.0%) were diagnosed between the age of 45 and 84 years. A higher proportion of young patients survived while a higher proportion of elderly patients died. Most patients (82.7%) were diagnosed between 1995 and 2016. Of the patients who died, 67.4% were diagnosed between 1995 and 2016; on the other hand, 96.7% of survivors were diagnosed between 1995 and 2016. White patients made up the majority (80.4%) of the cohort. Most patients had localized (40.2%) or regional (40.8%) disease; about half (50.4%) had low-grade tumors; and most (89.8%) received surgery, whereas radiotherapy (26.0%) and chemotherapy (35.0%) were less frequently used.
Table 1 Characteristics of Male Breast Cancer Patients
Time Trends of MBC Mortality
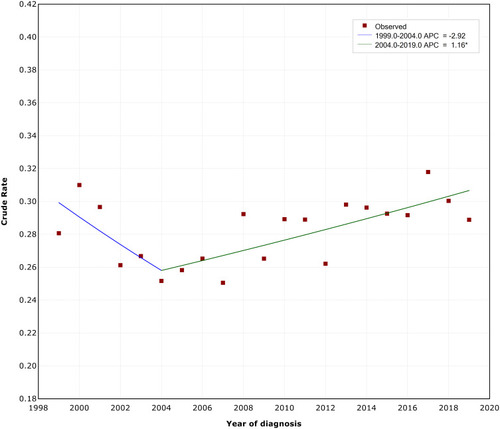
The final selected model showed one joinpoint, indicating that MBC mortality rate did not change significantly between 1999 and 2004 (annual percentage change [APC]=−2.92, 95% CI: −6.20, 0.50). However, on average, the crude rate of MBC mortality increased by 1.16% each year between 2004 and 2019 (APC=1.16, 95% CI: 0.50, 1.80) (). The crude rate of MBC mortality increased significantly from 0.26 per 100,000 in 2004 to 0.31 per 100,000 in 2019.
Causes of Death in MBC Patients
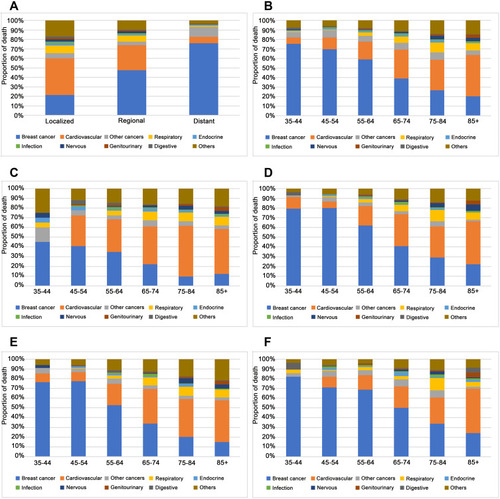
Among patients who died in the overall cohort, 1311 (42.7%) died of breast cancer and 1757 (57.3%) died of non-breast cancer causes including CVD (26.9%), other cancers (6.7%), respiratory disease (6.1%), and nervous system disease (1.8%) (Supplemental Table 2). The proportion of breast cancer deaths decreased whereas that of CVDs increased with age (). The proportion of CVDs was much higher than breast cancer deaths (38.5% vs 21.4%) among patients with localized disease (). For patients ≥75 years old, CVD was more common than breast cancer death and was the leading cause of death (). In the subgroup analyses, the proportion of CVDs increased with age in both localized and regional disease stage subgroups (), with CVD more frequently observed in patients with localized cancer than in age-matched patients with regional cancer. Similarly, the proportion of CVDs increased with age in both low- and high-grade subgroups, with a higher percentage of CVDs in low-grade cancer patients as compared to age-matched high-grade cancer patients (). Furthermore, in the localized or low-grade cancer subgroups, CVD was the leading cause of death in patients aged ≥65 years. Causes of death in other subgroups are shown in Supplemental Figure 1.
Cumulative Mortality Rates by Cause of Death
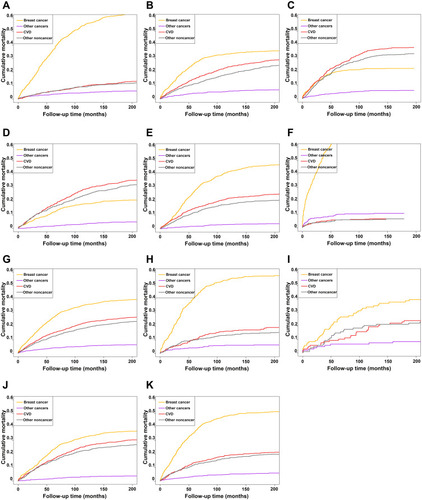
The competing risk model confirmed the risk of all competing events and showed cumulative mortality with follow-up time (). We found that risk of death from breast cancer was much higher than from other competing causes in patients aged 35–64 years (); CVD risk increased and was the second most common cause of death in patients aged 65–79 years (), followed by other non-cancer causes of death; meanwhile, CVD was the leading cause of death in patients aged 80+ years (). The same trend was observed in patients with different stages of cancer: CVD was the leading cause of death in patients with localized disease although other noncancer deaths were also important (). Patients with regional or distant cancer had a higher risk of breast cancer death (). Regarding racial differences in mortality rates, the competing risk model indicated that Black patients had a higher risk of breast cancer death than those who were White or of other races (). Patients with low-grade cancer had a higher risk of CVD compared to those with high-grade cancer (). Cumulative mortality rates in the overall cohort for different follow-up times are shown in Supplemental Table 3, and the rates in patients aged 80+ years or with localized cancer are shown in Supplemental Tables 4 and 5, respectively.
Figure 3 Cumulative mortality curves by follow-up time in different MBC patient subgroups. (A–C) Patients aged 35–64 years (A), 65–79 years (B), and 80+ years (C). (D–F) Patients with localized (D), regional (E), and distant (F) cancer. (G–I) White (G), black (H), and other ethnic group (I) patients. (J and K) Patients with low-grade (J) and high-grade (K) cancer.
MBC Patients’ CVD Risk Compared to the General Population
SMRs of CVD are listed in . In the overall cohort, MBC patients’ CVD risk was 6.58-fold higher than that of the general population (SMR=6.58, 95% CI: 6.14, 7.05). Young patients (35–44 years old) had the highest CVD risk compared to the age-matched general population (SMR=14.45, 95% CI: 5.79, 29.78). SMRs decreased with age: in MBC patients aged 85+ years, CVD risk was 1.71-fold higher than that of the general population (SMR=1.71, 95% CI: 1.45, 2.01).
Table 2 Age-Specific Cardiovascular Death-Related Standardized Mortality Ratios in Male Breast Cancer Patients
Discussion
In this population-based study, we found that MBC mortality in the US population has been increasing since 2004. Non-breast cancer death, especially CVD, accounted for a high proportion of deaths in MBC patients; for elderly patients or those with localized disease, CVD was the leading cause of death. Compared to the age-matched general population, MBC patients—especially those of a young age—had elevated CVD risk. This is the first study to analyze the causes of death and CVD risk in MBC at the population level.
Non-breast cancer death accounted for a high proportion of deaths in MBC patients, exceeding breast cancer deaths. In the overall cohort of MBC patients, the top 3 causes of death were breast cancer, CVD, and other cancers. As one cause of death precludes others, they were considered as competing events. We used a competing risk model to evaluate the risks of different causes of death and calculated cumulative mortality rates, which ensured the accuracy of our results.Citation26 Our findings are supported in part by the study of Donegan et al, which showed that heart diseases and other cancers were the most common non cancer causes of deathCitation17 and by the study of Gnerlich et al, in which the proportion of non-breast cancer deaths exceeded that of breast cancer deaths.Citation27 However, the former study had a small sample size and only analyzed the proportion of death, whereas the latter did not provide detailed causes of death. In the present investigation, we analyzed population-based epidemiologic data that included detailed causes of death, for which we calculated competing risks and cumulative mortality rates.
CVD was the leading cause of death in elderly patients or patients with localized disease. This is in line with earlier studies on FBC. An investigation based on the SEER-Medicare database found that CVD was the leading cause of death among elderly or early-stage FBC patients,Citation13 while another reported that cardiovascular disease risk was equal to or higher than breast cancer recurrence risk in 80% of postmenopausal patients with hormone receptor-positive FBC 10 years after diagnosis.Citation14 We found that MBC patients had a higher CVD risk than the general population based on calculated SMRs. Similarly, a large-scale SEER-based study showed that CVD risk was 1.62- to 90.07-fold higher in FBC patients than in the general population.Citation12 It is worth noting that SMR decreased with age in our study—that is, compared to the age-matched general population, young MBC patients had a much higher CVD risk whereas the disparity was less obvious in the elderly population. Similar trends have been reported in FBCCitation12 and other cancers,Citation28 possibly because CVD risk is high among the elderly in the general population. Our results suggest that CVD risk should also be monitored and managed in younger patients.
The high CVD rate in MBC patients may be explained by cardiotoxic anticancer treatments and high prevalence of cardiovascular comorbidities. In one study, 97.4% of MBC patients were estrogen receptor-positive and most received tamoxifen therapy.Citation29 Side effects of tamoxifen include thromboembolism and cardiovascular problems;Citation29,Citation30 moreover, elderly MBC patients have an increased risk of arterial thromboembolism.Citation31 Chemotherapy is a risk factor for CVD, as it was shown to be cardiotoxic and increase the risk of cardiac dysfunction in FBC patients.Citation16 Compared to female lymphoma patients, male patients who received chemotherapy had an elevated risk of cardiac events.Citation32 Radiotherapy causes inflammation, endothelial damage, and atherosclerosis, which increase the risk of cardiovascular events.Citation16 Because of their older age, MBC patients may be more likely to have cardiovascular comorbidities than FBC patients.Citation33
We examined causes of death in different subgroups of MBC patients. Our results were in line with those of a previous study demonstrating that Black men had a higher breast cancer mortality rate, which may be attributable to lower income and a lack of health insurance.Citation34 Patients with low-grade tumors had a higher CVD rate, possibly because they live longer and have more time to experience cardiovascular events. Our results indicate that more attention should be paid to the prevention of breast cancer death in Black men and of CVD in MBC patients who are elderly, have localized or low-grade cancer.
This study had some limitations. Firstly, treatment and nursing practices have changed over the last 4 decades, which could affect the causes of death in MBC patients; however, this could not be verified as detailed treatment and nursing information was not available in the SEER database. Nonetheless, we did analyze causes of death in different years of diagnosis. Secondly, the SEER database does not provide comorbidity information for patients, which precluded an analysis of the effect of comorbidities on different causes of death. On the other hand, the main purpose of the present epidemiologic study was to analyze the major causes of death in MBC patients and not to identify prognostic factors.
Conclusion
The results of this study demonstrate that more MBC patients die from causes other than breast cancer than from the disease itself, and that CVD is the leading cause of death in patients who are old or have localized disease. Our findings highlight the importance of monitoring not only breast cancer but also other risk factors for death—especially cardiovascular comorbidities—in these patients.
Abbreviations
APC, annual percentage change; ASCO, American Society of Clinical Oncology; CDC, Centers for Disease Control and Prevention; CI, confidence interval; CVD, cardiovascular death; FBC, female breast cancer; MBC, male breast cancer; SEER, Surveillance, Epidemiology, and End Results; SMR, standardized mortality ratio; WONDER, Wide-ranging Online Data for Epidemiologic Research.
Data Sharing Statement
The datasets analyzed in the current study are publicly available from the SEER database (http://seer.cancer.gov).
Statement of Ethics
Ethics approval was not required because the analyzed data are publicly available.
Acknowledgments
We thank all the staffs from the SEER and CDC WONDER for providing the research data.
Disclosure
The authors have no conflicts of interest to declare.
References
- Giordano SH, Longo DL. Breast cancer in men. N Engl J Med. 2018;378(24):2311–2320. doi:10.1056/NEJMra170793929897847
- Abdelwahab Yousef AJ. Male breast cancer: epidemiology and risk factors. Semin Oncol. 2017;44(4):267–272. doi:10.1053/j.seminoncol.2017.11.00229526255
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.2165433433946
- Konduri S, Singh M, Bobustuc G, Rovin R, Kassam A. Epidemiology of male breast cancer. Breast. 2020;54:8–14. doi:10.1016/j.breast.2020.08.01032866903
- Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115(2):429–430. doi:10.1007/s10549-008-0053-y18478326
- Reddington R, Galer M, Hagedorn A, et al. Incidence of male breast cancer in Scotland over a twenty-five-year period (1992–2017). Eur J Surg Oncol. 2020;46(8):1546–1550. doi:10.1016/j.ejso.2020.01.00931955992
- Hassett MJ, Somerfield MR, Baker ER, et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020;38:1849–1863.32058842
- Piscuoglio S, Ng CK, Murray MP, et al. The genomic landscape of male breast cancers. Clin Cancer Res. 2016;22(16):4045–4056. doi:10.1158/1078-0432.CCR-15-284026960396
- Masci G, Caruso M, Caruso F, et al. Clinicopathological and immunohistochemical characteristics in male breast cancer: a retrospective case series. Oncologist. 2015;20(6):586–592. doi:10.1634/theoncologist.2014-024325948676
- Gucalp A, Traina TA, Eisner JR, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. 2019;173(1):37–48. doi:10.1007/s10549-018-4921-930267249
- Fentiman IS. Male breast cancer is not congruent with the female disease. Crit Rev Oncol Hematol. 2016;101:119–124. doi:10.1016/j.critrevonc.2016.02.01726989051
- Zaorsky NG, Churilla TM, Egleston BL, et al. Causes of death among cancer patients. Ann Oncol. 2017;28(2):400–407. doi:10.1093/annonc/mdw60427831506
- Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a Retrospective Cohort Study. Breast Cancer Res. 2011;13(3):R64. doi:10.1186/bcr290121689398
- Bardia A, Arieas ET, Zhang Z, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012;131(3):907–914. doi:10.1007/s10549-011-1843-122042368
- Runowicz CD, Leach CR, Henry NL, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34(6):611–635. doi:10.1200/JCO.2015.64.380926644543
- Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American heart association. Circulation. 2018;137(8):e30–e66. doi:10.1161/CIR.000000000000055629437116
- Donegan WL, Redlich PN, Lang PJ, Gall MT. Carcinoma of the breast in males: a multiinstitutional survey. Cancer. 1998;83(3):498–509. doi:10.1002/(SICI)1097-0142(19980801)83:3<498::AID-CNCR19>3.0.CO;2-R9690543
- Kwong A, Chau WW, Mang OW, et al. Male breast cancer: a population-based comparison with female breast cancer in Hong Kong, Southern China: 1997–2006. Ann Surg Oncol. 2014;21(4):1246–1253. doi:10.1245/s10434-013-3377-824337541
- Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232–239. doi:10.1200/JCO.2009.23.816219996029
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER data & software. Available from: https://seer.cancer.gov/data-software/. Accessed 219, 2021.
- Centers for Disease Control and Prevention. About multiple cause of death, 1999–2019. Available from: https://wonder.cdc.gov/mcd-icd10.html. Accessed 219, 2021.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER cause of death recode 1969+ (03/01/2018) SEER data reporting tools. Available from: https://seer.cancer.gov/codrecode/1969_d03012018/index.html. Accessed 219, 2021.
- Ye Y, Otahal P, Marwick TH, Wills KE, Neil AL, Venn AJ. Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015: an Australian Population-Based Study. Cancer. 2019;125(3):442–452. doi:10.1002/cncr.3180630311655
- Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343(aug08 1):d2304. doi:10.1136/bmj.d230422803193
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II – the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406.
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi:10.1093/aje/kwp10719494242
- Gnerlich JL, Deshpande AD, Jeffe DB, Seelam S, Kimbuende E, Margenthaler JA. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol. 2011;18:1837–1844.21484520
- Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. doi:10.1093/eurheartj/ehz76631761945
- Eggemann H, Brucker C, Schrauder M, et al. Survival benefit of tamoxifen in male breast cancer: prospective cohort analysis. Br J Cancer. 2020;123(1):33–37. doi:10.1038/s41416-020-0857-z32367072
- Eggemann H, Bernreiter AL, Reinisch M, et al. Tamoxifen treatment for male breast cancer and risk of thromboembolism: prospective cohort analysis. Br J Cancer. 2019;120(3):301–305. doi:10.1038/s41416-018-0369-230655614
- Reiner AS, Navi BB, DeAngelis LM, Panageas KS. Increased risk of arterial thromboembolism in older men with breast cancer. Breast Cancer Res Treat. 2017;166(3):903–910. doi:10.1007/s10549-017-4433-z28836029
- Myrehaug S, Pintilie M, Yun L, et al. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood. 2010;116(13):2237–2240. doi:10.1182/blood-2010-01-26376420595518
- Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367(9510):595–604. doi:10.1016/S0140-6736(06)68226-316488803
- Crew KD, Neugut AI, Wang X, et al. Racial disparities in treatment and survival of male breast cancer. J Clin Oncol. 2007;25(9):1089–1098. doi:10.1200/JCO.2006.09.171017369572