Abstract
Although growth hormone (GH) is primarily associated with linear growth in childhood, it continues to have important metabolic functions in adult life. Adult GH deficiency (AGHD) is a distinct clinical entity, and GH replacement in AGHD can improve body composition, strength, aerobic capacity, and mood, and may reduce vascular disease risk. While there are some hormone-related side effects, the balance of benefits and risks is generally favorable, and several countries have approved GH for clinical use in AGHD. GH secretion declines progressively and markedly with aging, and many age-related changes resemble those of partial AGHD. This suggests that replacing GH, or stimulating GH with GH-releasing hormone or a GH secretagogue could confer benefits in normal aging similar to those observed in AGHD – in particular, could reduce the loss of muscle mass, strength, and exercise capacity leading to frailty, thereby prolonging the ability to live independently. However, while most GH studies have shown body composition effects similar to those in AGHD, functional changes have been much less inconsistent, and older adults are more sensitive to GH side effects. Preliminary reports of improved cognition are encouraging, but the overall balance of benefits and risks of GH supplementation in normal aging remains uncertain.
Introduction
Frailty in the elderly is a syndrome of progressive loss of strength and aerobic capacity that can increase the risk of falls and their complications, and leads in part to this functional decline. The result is the need for costly home-based or institutional support in the rapidly growing part of the population older than 80 years (CitationMerriam et al 2002, Citation2003). Sarcopenia, or loss of muscle mass, leads to this progressive functional decline. Growth hormone (GH) also declines with age, and the findings in frail elders are similar in many ways to those signs and symptoms found in younger adults with GH deficiency (AGHD). Replacement of GH or stimulation of GH secretion with GH-releasing hormone (GHRH) or other GH secretagogues (GHS) would thus seem to be an appealing option to delay the onset of frailty in older adults and to prolong the capacity for independent living; but the balance of pros and cons is not necessarily the same as in AGHD. This review describes the components of the GH axis and their actions, compares and contrasts normal aging with AGHD; and summarizes GH replacement and the use of GHRH and GHS in these contexts.
Principal components of the growth hormone axis
GH is the most abundant pituitary hormone, accounting for 10% of pituitary dry weight (CitationMerriam et al 2002). It plays an important metabolic role in adult life as a partitioning hormone, regulating body composition and function (CitationMerriam and Cummings 2003). GH is a 191 amino acid protein whose secretion depends on stimulation by the hypothalamus and is regulated by tissue responses (CitationMerriam et al 2003). There are three hypothalamic factors or peptide systems that regulate GH synthesis and secretion (): somatostatin (SRIF), GHRH, and ghrelin (CitationAnawalt and Merriam 2001; CitationMelmed 2006). Somatostatin, a family of 14 and 28 amino acid peptides, is a potent noncompetitive inhibitor of the release of GH and other hormones. It modulates the pituitary GH response to GHRH. GHRH, a 44 amino acid peptide, is the principal stimulator of GH synthesis and secretion. Ghrelin, discovered in 1999 by Kojima and colleagues (CitationMerriam and Cummings 2003), is an endogenous ligand for a previously described GHS receptor. While the abbreviation GHS technically could be applied to any growth hormone secretagogue, it is generally used to refer to ghrelin and its mimetics rather than to GHRH. Ghrelin is secreted in large quantities by the stomach, and circulates systemically at levels high enough to stimulate central GHS receptors, with access facilitated by its unique lipophilic octanoyl side group, which is also required for binding to the GHS receptor (CitationMerriam 2002). Ghrelin also has appetite-stimulating activities distinct from its GH-stimulating effects (CitationAnawalt and Merriam 2001).
Figure 1 Major components of the GH neuroregulatory system. Question marks on the arrows leading from the stomach indicate uncertainty about the physiological role of gastric ghrelin in the regulation of GH; and on arrows from ghrelin in the hypothalamus indicate uncertainty as to whether ghrelin found in the hypothalamus is synthesized in neurons there, or is synthesized elsewhere and acts at hypothalamic or pituitary levels. IGF-1 is synthesized in many GH target tissues, but more than 85% of circulating IGF-1 is liver-derived. From CitationAnawalt and Merriam 2001.
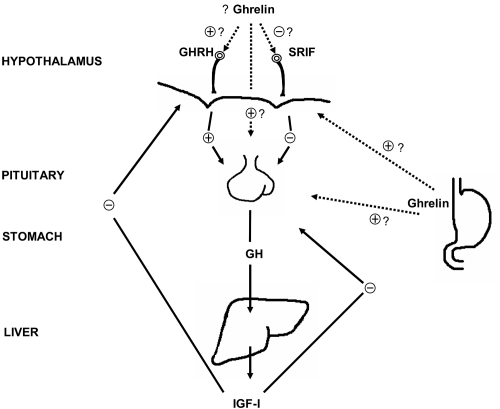
All of these peptides respond to a variety of stimuli and inhibitors, such as sleep, stress, exercise, food intake and body composition, and interact to generate the physiological pattern of pulsatile GH secretion (CitationAnawalt and Merriam 2001). There are approximately 10 pulses of GH secretion per day, each lasting about 90 minutes and separated by 120 minutes. Peak GH secretory activity occurs within an hour after the onset of deep sleep (CitationMelmed 2006). With increasing age, GH pulse amplitude is markedly reduced, and there is a loss of the nocturnal GH increase, but the number of GH pulses does not change greatly (CitationHo et al 1987). This secretion is modified by age and sex in addition to the stimuli mentioned above (CitationMolitch et al 2006). GH, in turn, stimulates the synthesis of insulin-like growth factor-I (IGF-I), which mediates many of GH’s effects and is a potent inhibitor of GH secretion (CitationMerriam 2002). GH has some direct effects as well via GH receptors present on the surface of many cell types (CitationCummings and Merriam 2003). Circulating IGF-I is synthesized mainly in the liver, but IGF-I is also locally generated in target tissues. The inhibition of IGF-I production can create a syndrome of relative GH resistance, causing increased GH secretion with decreased GH effects. Examples include fasting, malnutrition, and oral estrogen therapy (CitationMerriam 2002).
GH promotes lipolysis and inhibits lipogenesis, with a resultant redistribution of fat. It inhibits the conversion of cortisone to the active glucocorticoid cortisol, accelerates the conversion of l-thyroxine to the more biologically active triiodothyronine (CitationCummings and Merriam 2003), and exerts antinatriuretic effects by stimulating renal tubular sodium-potassium pumps and facilitating the renin-angiotensin-aldosterone system (CitationMerriam and Cummings 2003). GH influences bone physiology after linear bone growth has ceased, and is anabolic toward bone and muscle. It contributes to an increase in overall energy expenditure by stimulating protein synthesis and fat oxidation (CitationCummings and Merriam 2003). GH also enhances intestinal absorption of calcium and phosphate, vitamin D activity, renal tubular phosphate reabsorption, osteoblast proliferation, and synthesis of DNA and procollagen mRNA in bone (CitationMerriam and Cummings 2003).
Normal aging vs adult growth hormone deficiency
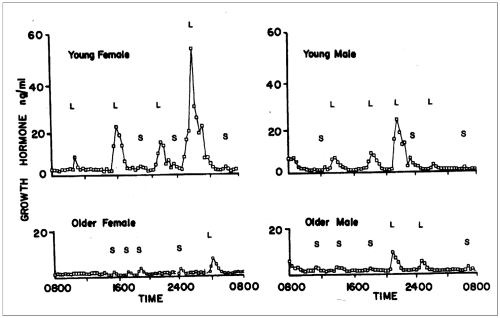
GH secretion rates decline exponentially from a peak of about 150 μg/Kg/day during puberty to about 25 μg/Kg/day by age 55 (CitationMelmed 2006). In this process there is a reduction in GH pulse amplitude, but little change in GH pulse frequency (CitationMerriam et al 2003). There is a particularly marked decline in sleep-related GH secretion, resulting in loss of the nocturnal pulsatile GH secretion seen in younger individuals and lack of a clear night-day GH rhythm () (CitationHo et al 1987; CitationMerriam et al 2000). It seems that the age-related decline in GH is not the cause of the decline in slow-wave sleep (SWS), however, since in most studies administering GH or GHRH does not enhance SWS in seniors (CitationVitiello et al 2001). The decline in GH production parallels the age-related decline in body mass index and is associated with alterations in body composition, hormonal status, and functional capacity that mimic the changes seen in AGHD or partial hypogonadism (CitationMerriam et al 1997). In addition to deteriorating memory and cognitive function, the changes in body composition that are most pronounced in normal aging include a reduction in bone density and in muscle mass and strength, an increase in body fat, and adverse changes in lipoprotein profiles (CitationAnawalt and Merriam 2001; CitationMerriam and Cummings 2003). While the aging pituitary remains responsive to GH, GHRH, and GHS, it is less responsive to stimuli such as exercise. This decline in GH production is initially clinically silent, but may contribute over time to sarcopenia and frailty.
Figure 2 Patterns of GH secretion in younger and older women and men. There is a marked age-related decline in GH secretion in both sexes and a loss of the nighttime enhancement of GH secretion seen during deep (slow-wave) sleep. This decrease is primarily due to a reduction in GH pulse amplitude, with little change in pulse frequency. L = large GH pulses, S = small GH pulses. From CitationHo et al 1987.
The decline in GH may also play a role in age-associated changes in cognition. While there are many systems for classifying different cognitive domains, often they are grouped as “crystallized” vs “fluid” intelligence. The former includes vocabulary and long-term memory; the latter includes short-term memory and active problem-solving and declines more markedly with aging. A number of studies have shown that in older adults there is a significant correlation between performance on tests of fluid intelligence and circulating levels of IGF-I (CitationAleman et al 1999), suggesting that GH may play a role in maintenance of fluid intelligence.
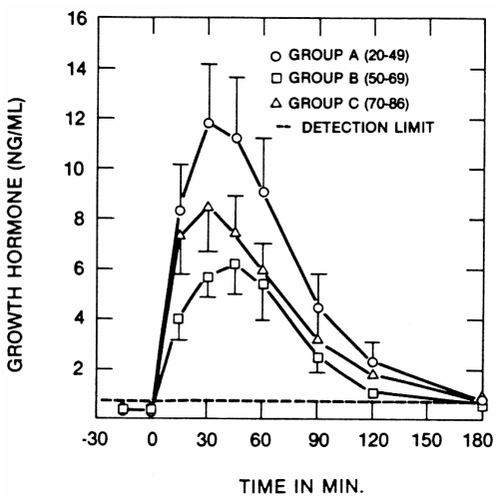
Several possible mechanisms for the age-related decline in GH secretion have been postulated: loss of (or decline in) pituitary responsiveness to GHS, increased sensitivity to the negative feedback by IGF-I, decline in hypothalamic stimulation, and increase in somatostatin inhibition of GH (CitationAnawalt and Merriam 2001; CitationMerriam and Cummings 2003). Published studies have pointed against the first two of these mechanisms as major factors (Pavlov et al 1996) (). The precise mix of the latter two factors, and of any others, is still not completely understood. Given that the aging pituitary can still respond to GHS, that there is no change in sensitivity to IGF-I, and that there may be some relative deficiency of GHRH and possibly ghrelin, it seems reasonable to infer that the cause of the overall decline of GH secretion with age is multifactorial and arises above the level of the pituitary (CitationMerriam and Cummings 2003).
Figure 3 Effects of a single intravenous bolus of GHRH on GH secretion in healthy subjects of different ages. While the highest responses are seen in young adults, there is no significant decrease with aging, and pituitary GH responses are well preserved even in the oldest subjects. From CitationPavlov et al 1986.
Aging is not a disease. Rather, it is a physiological state of relative GH deficiency. This is demonstrated by higher GH secretion and physiological responses seen in older adults when compared with AGHD patients of similar age (CitationMerriam et al 2002). It is important to distinguish true AGHD from normal aging, since the consequences of the two states differ.
Since all biochemical tests for GHD are imperfect, and their accuracy is strongly affected by the pre-test probability of the condition, the most important indicator of the likelihood of GHD is the clinical context (CitationMerriam and Cummings 2003). Among adults with AGHD, 85% acquire the deficiency as an adult, mostly from pituitary tumors or their treatment with radiation or surgery (CitationMerriam and Cummings 2003). Impairment of the hypothalamus may be present due to similar processes; although in the presence of pituitary damage, which renders them unresponsive to GHRH or GHS, this is more difficult to gauge. Traumatic brain injury is also becoming more frequently recognized as a cause of GHD in adults (CitationMerriam and Wyatt 2006; CitationMolitch et al 2006), and may produce deficiencies in other pituitary hormones as well. Studies have shown that adults with hypopituitarism have increased mortality compared with nonhypopituitary populations adjusted for age and sex. The main causes of the excess mortality were cardiovascular and cerebrovascular disease (CitationMolitch et al 2006). Patients who acquire GHD in adult life also have an increase in cardiovascular and cerebrovascular mortality and have clinically significant abnormalities in hormone profiles, body composition, and physical and mental functions (CitationMerriam and Cummings 2003).
GHD adults are physically and emotionally less healthy than their age-matched peers (). Their skin is cool, dry, and thin. They suffer psychological and social difficulties and cognitive impairment. Fat mass is increased by 7%–10%, with much of the excess located in the visceral compartment of the abdomen. Lean body mass is decreased by 7%–8% and skeletal muscle volume is diminished by up to 15% (CitationCummings and Merriam 2003; CitationMerriam and Cummings 2003). Cardiac muscle is also lost, with impaired ventricular function and cardiac capacity as a result. Hypertension is more common, thrombogenic blood components are increased, and an atherogenic lipid profile exists. All of this contributes to the cardiovascular (and cerebrovascular) disease seen in AGHD (CitationMerriam et al 2000; CitationCummings and Merriam 2003; CitationMerriam and Cummings 2003; CitationMerriam and Wyatt 2006).
Table 1 Clinical features of the adult GHD syndrome
Growth hormone replacement and its side effects
While a single case study in 1962 described improved vigor, ambition and well-being in a 35 year old hypopituitary adult who received GH, large-scale trials of GH replacement in AGHD could not be conducted with scarce extracted pituitary GH. With the availability of synthetic GH in unlimited qualities, clinical trials in AGHD were begun soon after recombinant GH was approved for pediatric use in 1985, and results of these studies began to appear in the late 1980’s. In 1996 the FDA approved the use of GH in GHD adults (CitationMerriam and Cummings 2003; CitationMolitch et al 2006). GH replacement in AGHD has been successful in reversing many structural and functional abnormalities in the condition () (CitationMerriam 2002; CitationMolitch et al 2006). The benefits and risks of GH replacement in AGHD have been documented in more than 1000 publications (CitationCummings and Merriam 2003; CitationMerriam et al 2003). While dosing was initially derived from pediatric practice, doses appropriate for growing children produced severe side effects in adults and were rapidly reduced. Over time, weight-based dosing as used in pediatrics gave way to the current adult practice of beginning with a low fixed dose unlikely to produce side effects, with subsequent dose titration until either an age- and gender-appropriate level of IGF-I or side effects are encountered. This titration must be conducted particularly carefully in older adults, who are more susceptible to adverse effects.
Table 2 Effects of GH replacement in GHD adults
Since aging is a milder GH-deficient state than AGHD, GH replacement seems a potentially reasonable approach to prevention or even reversal of the frailty symptoms of aging. The first studies in non-GHD older adults took place soon after its effects in AGHD were published. In a widely cited study by CitationRudman et al (1990), healthy men over 60 years old responded to 6 months’ GH treatment with an 8.8% increase in lean body mass, a 14.4% decrease in adipose tissue mass, and a 1.6% increase in vertebral bone mineral density (BMD). Since most studies of AGHD have required 12–18 months of treatment to show an improvement in BMD, this improvement was especially remarkable. Although the Rudman study did not include any functional measures, given these results, it was postulated that GH treatment might also improve muscle strength and functional performance. Studies of physical functional effects, however, have been generally disappointing and inconsistent. CitationPapadakis et al (1996) tried to determine whether GH treatment would improve functional ability in older men. The authors concluded that GH supplementation improved body composition but not functional status. Since the subjects were generally very fit and functional scores were close to the maximum at the beginning of the trial, it is not clear whether this was a true negative result or a “ceiling effect” related to the testing measures used.
Despite this lack of demonstrated functional efficacy, a number of clinics began to offer GH treatment to otherwise healthy older men and women. Faced with this growing practice and dearth of information, the NIH National Institute on Aging issued a call for applications in 1991 to study trophic factors in aging. Several studies of GH, either alone or in combination with sex steroids, IGF-I, or exercise conditioning, and one study of GHRH were funded and have now been completed. While a comprehensive review of the findings of these studies is beyond the scope of this article, there is a general consensus among these reports that GH replacement in normal seniors can elevate levels of IGF-I to the young adult normal range. While attempts to reproduce the doses used by Rudman and colleagues encountered severe side effects, forcing their reduction to 50% or less of those he used, target IGF-I levels could usually be reached at lower doses with tolerable short-term side effects. There is also a general consensus that GH treatment increases lean body mass and reduces body fat, especially abdominal visceral fat (CitationBlackman et al 2002). The studies that included exercise conditioning confirmed its beneficial effects, but GH did not augment exercise effects and there was no clear improvement in strength or aerobic capacity with GH alone. Studies published to date also provided no definitive proof that GH treatment could improve sleep or mood impairment (CitationMerriam 2002).
All of these studies were conducted for 6–12 months at a single site, and so only short-term intermediate outcomes and side effects, not long-term risks, could be observed. Their results provide no guidance on the effects of GH on long-term clinical outcomes or risks such as falls or fractures, maintenance of functional status, or effects on cardiovascular morbidity and mortality – factors that would establish more definitively the rationale for GH treatment in normal aging (CitationCummings and Merriam 2003; CitationMerriam and Cummings 2003). And while few long-term risks have been observed, this reflects more a lack of information than a demonstration of safety.
Since elders are more sensitive to replacement with GH (and GH resistance may not be uniform in all tissues), they are also more susceptible to the side effects of therapy. The side effects are due to the hormonal effects of over-replacement, so careful dose titration is extremely important. Patients who are older, heavier, or female are more prone to develop complications (CitationMolitch et al 2006). Common side effects of GH replacement include fluid retention, with peripheral edema (40% of patients), arthralgias (20% of patients), and carpal tunnel syndrome (10% of patients) (CitationAnawalt and Merriam 2001; CitationCummings and Merriam 2003; CitationMerriam and Cummings 2003). Studies have also shown increased fasting glucose levels. Although these levels generally return toward normal with the improvement in body composition and reduced insulin resistance, some studies have found a persistent increase in fasting glucose and insulin with chronic GH treatment, even after body composition changes have stabilized. Other less frequently reported side effects include headache, tinnitus, and benign intracranial hypertension (CitationMerriam and Cummings 2003; CitationMerriam and Wyatt 2006). GH can accelerate both the clearance of thyroxine and promote its conversion to triiodothyronine, and so can have variable effects in hypothyroid patients on fixed replacement doses. Since GH and IGF-I are growth factors, there are concerns for promotion of cancer cell growth, but studies to date have not demonstrated this (CitationMerriam 2002).
Besides these increased vulnerabilities in older patients, which are common to the use of GH both in GHD and in normal aging, there are concerns specific to the use of GH in non-GHD elders. In treatment of GHD, the target for dosing is replacement to age-appropriate normal levels. In anti-aging therapy, age-appropriate normal levels are the starting point, not the target; rather, the target is the normal range for young adults, and the balance of beneficial effects vs adverse effects and risks may thus be quite different in these two contexts. The ongoing controversy over the pros and cons of postmenopausal estrogen therapy, despite a large literature, should raise cautions that only studies conducted with the specific dosing targets and in the specific population for which the use is being proposed can adequately assess those benefits and risks.
Growth hormone-releasing hormone and growth hormone secretagogues
Growth hormone secretagogues such as GHRH, ghrelin, and their mimetics stimulate the secretion of GH, if the pituitary is intact and responsive. Since most AGHD is due to hypopituitarism, and these patients – unlike normal elders – are thus unresponsive to GHRH or GHS, there are not many studies of GHS replacement effects, and the use of GHS in normal aging has not been approved by regulatory authorities in any jurisdiction (CitationMerriam et al 2002, Citation2003). In principle, treatment with GHS should offer a more physiologic approach to GH replacement, leading to a pulsatile rather than prolonged elevation in GH and preserving the capability for negative feedback inhibition of GH by rising levels of IGF-I (CitationMerriam et al 2000; CitationMerriam 2002). GHS effects are influenced by the same factors which modulate endogenous GHRH secretion, such as negative feedback by somatostatin. This normal negative feedback regulation offers some buffering against overdose (CitationMerriam et al 2002). The side effects of GHRH treatment are similar in character to GH treatment, but are milder and less frequent. And, since the GHS are smaller molecules than GH, they can be administered orally, transdermally, or nasally (CitationMerriam et al 2003; CitationMerriam and Cummings 2003).
Once daily GHRH injections can stimulate increases in GH and IGF-I at least to the lower part of the young adult normal range (CitationMerriam et al 2000). The University of Washington study of 6 months treatment with daily bedtime subcutaneous injections of GHRH(1–29)NH2, alone or in combination with supervised exercise conditioning, was begun in response to the NIH initiative (CitationMerriam et al 2002, Citation2003). IGF-I levels rose approximately 35%. As with GH, subjects showed an increase in lean body mass and decrease in body fat (particularly abdominal visceral fat). However, there was no improvement in strength or aerobic fitness associated with GHRH injections. Testing again confirmed the benefits of exercise but showed no effect upon IGF-I levels; thus it appears that GH/GHRH and exercise work through different mechanisms (CitationVitiello et al 1997). Subjects receiving GHRH also showed no change in scores on an integrated physical functional performance test mimicking activities of daily living, but there was a significant decline in physical function in the placebo group (CitationMerriam et al 1997, Citation2003; CitationCummings and Merriam 2003). This tantalizing finding, suggesting that GHRH can stabilize if not improve physical function, needs confirmation. There is only one other published study of chronic GHRH in normal aging, which reported positive effects on exercise testing after 3 months of treatment (CitationVeldhuis et al 2005).
Sleep and cognition were also studied in the GHRH trial, with surprising results. GHRH failed to improve and may even have impaired deep sleep, despite the rise in IGF-I and pulsatile GH. However, GHRH treatment was associated with improved scores in several domains of fluid (but not crystallized) intelligence – those measures previously found correlated with circulating IGF-I levels (CitationVitiello et al 2006). This intriguing preliminary finding is now being studied more systematically at the University of Washington in a new NIH-funded study (the Somatotrophics, Memory, and Aging Research Trial, or “SMART”).
Thus as with GH, there is a consensus on hormonal and body composition effects but inconsistent functional effects on function; and in addition there is a very encouraging but still unconfirmed positive effect on some domains of fluid intelligence.
Ghrelin, which is produced in the stomach and increases during periods of fasting or under conditions associated with negative energy balance (such as starvation or anorexia), acts at both hypothalamic and pituitary levels via mechanisms distinct from GHRH, and thus has different effects from GHRH or GH; subjects often gain weight and do not lose, or even gain body fat) (CitationMerriam et al 2000, Citation2002; CitationLiddle 2006). The effects of ghrelin on GH secretion depend in part on the presence of GHRH; and thus if GHRH secretion declines with aging, ghrelin’s effects may be blunted. While the effects of these two GHS differ clinically, they have synergistic effects on GH release, and therefore supplementation of both substances may be more effective than either alone in aging (CitationMerriam et al 2000, Citation2002). Additionally, there are other substances which can enhance GH response to GHS by suppressing somatostatin secretion, including arginine and beta-adrenergic antagonists, which could potentially enhance treatment effects (CitationMerriam et al 1997).
Several studies have shown short-term effects of GHS on GH secretion, but so far only three groups have conducted studies of their chronic effects in normal aging. Bowers and colleagues showed that chronic repeated injections or subcutaneous infusions of GH-releasing peptide-2 (GHRP-2) could stimulate and maintain increases in episodic GH secretion and IGF-I (CitationBowers et al 2004). Thorner and colleagues at the University of Virginia have conducting a study of two years’ oral treatment with the non-peptidyl GHS MK-677. As with previous studies, there was a sustained increase in IGF-I and episodic GH secretion, and an increase in lean body mass (CitationThorner et al 2006). Preliminary functional results over one year of treatment, recently reported at an abstract presentation, however, did not show significant improvements.
In cooperation with investigators at Duke University and several other sites, we conducted a trial of the Pfizer investigational oral GH capromorelin in pre-frail older men and women (CitationMerriam et al 2006). This protocol recruited over 300 subjects and was initially planned as a two-year intervention. The study was unfortunately stopped, however, after all subjects had been treated for 6 and many for 12 months, due to failure to see an increase in per cent lean body mass, which was a pre-set non-efficacy termination criterion. Absolute lean body mass did increase significantly, but due to the appetite-stimulating effect of this ghrelin mimetic – unforeseen in early 1999 when the study was designed and ghrelin was still unknown – subjects also gained weight (about 1.5 Kg) and this washed out the effect on per cent lean body mass. However, even this truncated study is currently the largest clinical trial of chronic GHS treatment in aging. It showed the expected increases in IGF-I levels and (as noted) total lean body mass. There were also encouraging effects on physical functional performance. Of seven functional tests, one improved significantly after 6 months of treatment, and another after 12 months. Two other measures showed non-significant trends toward improvement, and the three remaining measures showed no effect. Effects on clinical endpoints such as falls could not be assessed with this relatively brief duration of treatment. Side effects were generally mild, including increases in fasting blood sugar within the normal range. Interestingly, there was a self-reported deterioration of sleep quality, though formal sleep testing was not performed. Cognition was not studied in this trial.
Thus as with GH and GHRH, reports of the hormonal and body composition effects of ghrelin mimetic GHS in normal aging are relatively consistent, but there is no consensus on functional effects among these very few studies, and of course none could assess clinical final outcomes or long-term risks.
Conclusion
Sarcopenia and subsequent frailty lead to loss of independence. While aging is not a disease, it results in significant body composition and functional changes which affect the individual and the community at large. Aging represents a milder form of adult GHD, and GH replacement in GHD has met with success. Since the aging pituitary remains responsive to GH and GHS, it is reasonable to suggest that GH replacement or stimulation might be indicated in aging. However, elders are more sensitive to GH, and thus more susceptible to the side effects of replacement. Stimulating GH with GHS instead of GH replacement has the advantage of a more physiological approach to increase endogenous GH pulsatility with theoretically decreased risk for side effects (CitationArvat et al 2000).
Reports of stabilization or even improvement of physical function with GHRH or with oral GHS are extremely tantalizing, but they are hardly proof that Ponce de Leon’s “Fountain of Youth” has been found. The failure to replicate these findings consistently across studies reminds us of the origins of the word “tantalizing.” In mythology, Tantalus, chained to a rock, bent down to drink from the pool of water around him – and the water receded just out of reach. So far, definitive conclusions regarding functional effects of GHRH and GHS in normal aging have also been out of our reach; and until we know whether the age-related decline in GH secretion is pathological or adaptive, and until more studies are undertaken to study this and the long term effects of GH and GHS supplementation, conclusive statements about the benefits of treatment cannot be made and we can only recommend their use in well-controlled clinical studies. Long term studies on hard clinical endpoints, such as decreased fractures and falls, increased function and quality of life, and decreased morbidity and mortality from vascular disease need to be performed in order to establish the role, if any, for GH and GHS treatment in normal aging.
Note
Presented in part at the Second Annual Meeting of the Society for Applied Research in Aging (SARA), 11 November 2006.
Acknowledgements
We thank our colleagues at the National Institutes of Health, the University of Virginia, the University of Washington, and Duke University, especially Drs Marc Blackman, Cyril Bowers, David Buchner, David Cummings, Marie Gelato, S Mitchell Harman, Ken Ho, the late Lawrence Larsen, Saul Malozowski, Karen Moe, the late Eugenia Pavlov, Robert Schwartz, Michael Thorner, Mary Lee Vance, Johannes Veldhuis, Michael Vitiello, and Heidi White, for their inspiration and collaboration; and Pamela Asberry, Suzanne Barsness, Colleen Carney, and Monica Kletke for expert professional assistance; and Dr Sharon Falzgraf for support and critical review of the manuscript.
References
- AlemanAVerhaarHJDeHaanEHInsulin-like growth factor-I and cognitive function in healthy older menJ Clin Endocrinol Metab199984471510022403
- AnawaltBDMerriamGRNeuroendocrine aging in men: andropause and somatopauseEndocrinology and Metabolism Clinics of North America2001306476911571935
- ArvatEGiordanoRBroglioFGH secretagogues in agingJ Anti-Aging Medicine2000314958
- BlackmanMRSorkinJDMunzerTGrowth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trialJAMA200228822829212425705
- BowersCYGrandaRMohanSSustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and womenJ Clin Endocrinol Metab200489229030015126555
- CummingsDEMerriamGRGrowth hormone therapy in adultsAnnu Rev Med20035451312471175
- HoKYEvansWSBlizzardRMEffects of sex and age on the 24 hour profile of growth hormone secretion in man: Importance of endogenous estradiol concentrationsJ Clin Endocrinol Metab1987645183782436
- LiddleRAGhrelin [online]2006 Up to Date Accessed 8 Sep 2006. URL: http://www.uptodate.com
- MelmedSPhysiology of growth hormone [online]2006 Up to Date Accessed 8 Sep 2006. URL: http://www.uptodate.com
- MerriamGRBlackmanMHoffmanAEffects of chronic treatment with an oral growth hormone (GH) secretagogue on nocturnal GH and insulin-like growth factor-I (IGF-I) in older men and womenFrontiers in Neuroendocrinology20062736 published abstract
- MerriamGRSchwarzRSVitielloMVGrowth hormone-releasing hormone and growth hormone secretagogues in normal agingEndocrine2003221714610292
- MerriamGRBarsnessSBuchnerDGrowth hormone-releasing hormone treatment in normal agingJ Anti Aging Med20024113
- MerriamGRCummingsDEMeikleWGrowth hormone and growth hormone secretagogues in adultsEndocrine replacement therapy in clinical practice2003Totowa, NJHumana Press6394
- MerriamGRGrowth hormone as anti-aging therapy, and other emerging (and submerging) indicationsClinical Endocrinology Update2002Chevy Chase, MDThe Endocrine Society
- MerriamGRKletkeMBarsnessSGrowth hormone-releasing hormone in normal aging: An UpdateToday’s Therapeutic Trends20001833554
- MerriamGRBuchnerDMPrinzPNPotential applications of GH secretagogs in the evaluation and treatment of the age-related decline in growth hormone secretionEndocrine19977139449025
- MerriamGRWyattFGDiagnosis and treatment of growth hormone deficiency in adults: current perspectivesCurrent Opinion in Endocrinology and Diabetes2006133628
- MolitchMClemmonsDMalozowskiSEvaluation and treatment of adult growth hormone deficiency: An Endocrine Society Clinical Practice GuidelineJ Clin Endocrinol Metab20069116213416636129
- PapadakisMAGradyDBlackDGrowth hormone replacement in healthy older men improves body composition but not functional abilityAnn Intern Med1996124708168633830
- PavlovEPHarmanSMMerriamGRResponses of growth hormone and somatomedin-C to growth hormone-releasing hormone in healthy aging menJ Clin Endocrinol Metab1986625956003080468
- RudmanDFellerAGNagrajHSEffects of human growth hormone in men over 60 years oldNEJM1990323162355952
- ThornerMONassRPezzoliSSOrally active ghrelin mimetic (MK-677) prevents and partially reverses sarcopenia in healthy older men and women: a double-blind, placebo controlled, crossover study2006Endocrine Society Annual Meeting24 June 2006Boston abstract OR5–5
- VeldhuisJDPatriJMFrickKAdministration of recombinant human GHRH-1,44-amide for 3 months reduces abdominal visceral fat mass and increases physical performance measures in postmenopausal womenEur J Endocrinol20051536697716260425
- VitielloMVMoeKEMerriamGRChronic growth hormone releasing hormone treatment improves cognition of healthy older adultsNeurobiology of Aging2006273182316399214
- VitielloMVSchwartzRSBuchnerKETreating age-related changes in somatotrophic hormones, sleep, and cognitionDialogs in Clinical Neuroscience2001322936
- VitielloMVWilkinsonCWMerriamGRSuccessful six-month endurance training does not alter insulin-like growth factor-I in healthy older men and womenJ Gerontol Med Sci199752A14954