Abstract
Background
IncobotulinumtoxinA (Bocouture®) is free from complexing proteins and effective for treating glabellar frown lines.
Purpose
To determine the efficacy, onset, and duration of action of incobotulinumtoxinA for the treatment of glabellar frown lines.
Patients and methods
In this single-arm, prospective, proof-of-concept study, 23 patients were treated with 25 U incobotulinumtoxinA, equally split between five injection sites in the glabella. Severity of glabellar frown lines was rated by an independent rater from standardized photographs using the validated Merz 5-point scale at several visits over 5 months following treatment. To assess patient satisfaction, patients completed a questionnaire before and 2 weeks after treatment.
Results
The percentage of responders at maximum frown 2–4 days after treatment was 95.2% and 85.0% when responders were defined as patients with ≥1-point and ≥2-point improvement on the 5-point scale compared with baseline, respectively. At this time point, 84% of the maximum effect had occurred. The responder rate at maximum frown, according to both definitions, was 100% for at least the next two visits (days 8 ± 1 and 14 ± 2). At all visits, the change from baseline in the mean glabellar frown-line score at maximum frown was statistically significant, with on average an almost 1-point improvement from baseline 5 months after treatment.
Conclusion
IncobotulinumtoxinA is an effective and well-tolerated treatment for glabellar frown lines, with a rapid onset of action and a long duration of effect lasting for more than 5 months.
Keywords:
Introduction
Botulinum toxin type A (BoNT/A) products have been used for a number of years to treat glabellar frown lines.Citation1–Citation5 IncobotulinumtoxinA (Bocouture®/Xeomin®, NT 201; Merz Pharmaceuticals, Frankfurt, Germany) is a BoNT/A product that is free from complexing proteins. It has proven efficacy for the treatment of glabellar frown lines,Citation6,Citation7 and has been licensed for this indication widely throughout Europe as Bocouture®. Recently, incobotulinumtoxinA was also approved in the US and Canada, where it is marketed as Xeomin® and Xeomin Cosmetic™, respectively, based on the results of two multicenter US studies (Carruthers et alCitation8 and Hanke et alCitation9).
Although the efficacy of BoNT/A in this indication is widely published, the rapidity of onset is less well documented. An improvement in glabellar frown lines usually takes place within 2–3 days, with the maximum effect being observed on day 30 with incobotulinumtoxinA.Citation10 However, the data reported from clinical trials have typically been obtained at a minimum of 1 week postinjectionCitation4 and often as long as 2 weeksCitation2,Citation3,Citation11 or 4 weeks after treatment.Citation6,Citation7 Nevertheless, the onset of effect in such studies has often been reported as “relatively quick” or “rapid.”Citation1,Citation4 In general, there are limited reports of exactly when, during the first week postinjection, the onset of the treatment effect is noted. A recent study concluded that one BoNT/A product provided rapid onset of effect, as rated by physician and patient assessment, in 45 patients with moderate-to-severe glabellar frown lines. Indeed, 77% of patients and 87% of physicians reported onset of effect by day 2, although this subjective estimate of response was not correlated on the facial wrinkle scale.Citation12 A subset analysis of four phase III trials of another BoNT/A for the treatment of glabellar frown lines reported a median time to onset of 2–4 days, which was recorded by patients in a diary but did not include any investigator assessment.Citation13 Clearly, the onset of effect is important to patients and affects patient satisfaction, and duration of effect is another important but infrequently reported factor affecting patient satisfaction. Therefore, the aims of this study were to investigate the time to the onset of treatment effect and the duration of the effect of incobotulinumtoxinA, and to examine its efficacy and safety for the treatment of glabellar frown lines.
Materials and methods
This was a single-arm, prospective, single-center, proof-of-concept clinical study performed in Germany. The study was conducted in accordance with local and international regulations, and informed consent was obtained from each patient. Patients were screened for eligibility according to the 5-point Merz scaleCitation14 at visit 1 (day 0), when baseline photographs were taken. Eligible patients were men and women aged ≥ 18 years with glabellar frown lines rated by the investigator as mild, moderate, severe, or very severe (grades 1–4) on the Merz 5-point facial wrinkle scale at maximum frown. Exclusion criteria were treatment with resorbable fillers and botulinum toxins in the previous 6 months, treatment with nonresorbable fillers or surgery in the treatment area (regardless of the time point of treatment), allergies to the study medication, contraindications against botulinum toxin treatment (including pregnancy and breastfeeding), severe concomitant disease, and circumstances that would not allow regular participation in the study.
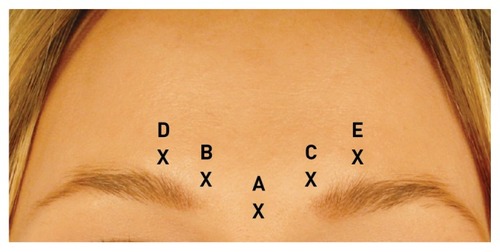
Eligible patients received one treatment at visit 1 (day 0, baseline) of five equal intramuscular injections of 5 U incobotulinumtoxinA, giving a total dose of 25 U (). The total dosage used in this study was comparable to that used in the large head-to-head study comparing incobotulinumtoxinA and onabotulinumtoxinA.Citation6 This dosage was chosen to ensure that the lateral part of the corrugator muscle was adequately treated, as treatment failure can often result from insufficient inhibition of contraction of the whole muscle. One injection was made into the procerus muscle, and two injections were made into the corrugator supercillii muscle (lateral and medial parts) on both the right and left sides (), as is approved for this indication. At the same treatment visit, patients also received incobotulinumtoxinA to improve the appearance of the platysmal bands as part of another study. Therefore, when patients were asked to assess the effect of their treatment, it should be noted that this was done after both areas had been treated. Nevertheless, for obtaining results for glabellar frown lines, patients were asked to evaluate solely the glabellar area.
Figure 1 Injection sites: A, procerus muscle; B, right medial corrugator muscle; C, left medial corrugator muscle; D, right lateral corrugator muscle; E, left lateral corrugator muscle.
The duration of the study was 5 months, during which time the patients attended the center for assessment at visit 2 (day 3 ± 1), visit 3 (day 8 ± 1), visit 4 (day 14 ± 2), visit 5 (weeks 12–13), visit 6 (weeks 16–17), and visit 7 (weeks 20–21). At all visits, standardized photographs of the treated region were taken at rest and at maximum frown. At visits 1–7, the severity of glabellar frown lines both at rest and at maximum frown was assessed by an independent rater according to the Merz 5-point scale using the standardized photographs. A patient questionnaire was completed at visits 1 (day 0) and 4 (day 14 ± 2). Patients were asked by what percentage they agreed with certain statements about their facial wrinkles on a visual analog scale, where 0% = I disagree and 100% = I agree. The patients also assessed the appearance of their glabellar frown lines at rest and at maximum frown compared with baseline as “markedly worse,” “worse,” “unchanged,” “improved,” or “markedly improved” at visits 2 (day 3 ± 1), 3 (day 8 ± 1) and 4 (day 14 ± 2).
The percentage of responders at visit 4 (day 14 ± 2) was assessed by an independent rater from standardized photographs. Two definitions of responders were used in this study. Firstly, a responder was defined as a patient with an improvement of at least 1 point on the 5-point Merz scale; this definition excluded patients with a baseline score of 0, as they could not satisfy this definition. Secondly, to bring these data in line with more stringent recent FDA-recommended definitions, a responder was also defined as a patient with an improvement of at least 2 points compared with baseline; this definition excluded any patient with a baseline score of 0 or 1 on the 5-point Merz scale, as they could not achieve a 2-point improvement and therefore could never qualify as a responder. Where data were missing, that patient was also excluded from the analysis at that time point, and the patient numbers were included in the results to indicate from how many patients the data points were collected.
To evaluate whether the mean score at each visit was statistically significantly different from the mean score at baseline, the Wilcoxon signed-rank test was used. To analyze whether the percentage agreement with certain statements differed at visit 4 (day 14 ± 2) compared with the percentage agreement at baseline, the two-sided Wilcoxon rank test was used.
Adverse events (AEs) were evaluated, and tolerance was rated by both the patient and the investigator according to the scale: “very good,” “good,” “indifferent,” “bad,” and “very bad.”
Results
In total, 23 patients met the eligibility criteria and were enrolled in the study. Patient demographics are summarized in . Of the 23 patients enrolled, only one patient had a score of 1 at maximum frown, ten patients had a score of 2, eight patients had a score of 3, and two patients had a score of 4. Two patients did not have photographs available at baseline at maximum frown, and one of these did not have a photograph available at baseline at rest either; these two patients were therefore excluded from the corresponding analyses by the independent rater.
Table 1 Patient demographics
Using the definition of a responder as a patient with at least a 1-point improvement compared with baseline, shows the responder rates assessed by the independent rater according to the Merz 5-point scale for glabellar frown lines at rest and at maximum frown. As early as visit 2, at 3 ± 1 days after treatment, 95.2% of patients were responders at maximum frown. The percentage of responders at maximum frown at visit 3 (8 ± 1 days posttreatment) was 100%, and remained at 100% until visit 6 (weeks 16–17 after treatment), when the responder rate decreased to 94.7%. Even at the final visit, 5 months after treatment, over 75% of patients were still responders at maximum frown. As expected, and in line with previous studies, the responder rates at rest were lower than those at maximum frown. The highest responder rate at rest was 56.3% at visit 5 (weeks 12–13 after treatment), indicating that treatment effect at rest improved more gradually over time.
Figure 2 Percentage of responders over time (A) when a responder was defined as a patient with at least a 1-point improvement from baseline at rest and at maximum frown; (B) when a responder was defined as a patient with at least a 2-point improvement from baseline at maximum frown.
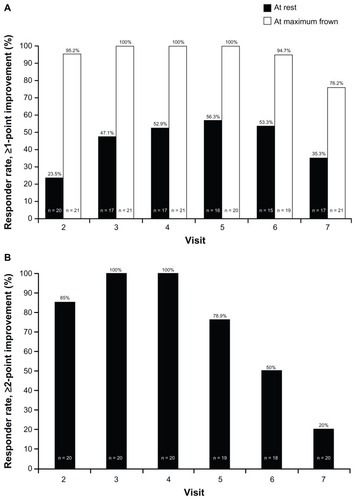
shows the results at maximum frown when a more stringent definition of responder was used (a patient with at least a 2-point improvement compared with baseline). One patient with a baseline score of 1 on the 5-point scale at maximum frown was excluded from this analysis. At visit 2 (3 ± 1 days posttreatment), 85.0% of patients were responders at maximum frown, and this increased to 100% at visit 3 (day 8 ± 1) and visit 4 (day 14 ± 2). At visit 5, 12–13 weeks after treatment, the responder rate was still high at 78.9%, and at visit 6 (weeks 16–17) the responder rate had decreased to 50% at maximum frown. When a responder was defined as a patient with at least a 2-point improvement compared with baseline, no patient was a responder at rest at any time point.
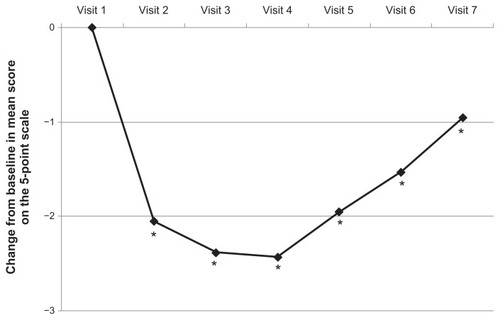
According to the 5-point scale, the mean score for glabellar frown lines at maximum frown at baseline (visit 1) was 2.53 (ie, between moderate and severe) when assessed by the independent rater. At visit 2 (day 3 ± 1 postinjection) the effect of incobotulinumtoxinA treatment was demonstrated by a statistically significant difference in the mean score from baseline at maximum frown of −2.05 (P < 0.001 between none and mild). At visit 3 (day 8 ± 1 postinjection) and visit 4 (day 14 ± 2 postinjection), the difference in the mean score from baseline at maximum frown assessed using the 5-point scale was −2.38 at both time points (P < 0.001 for both time points). The maximum effect was seen at visit 4 (day 14 ± 2). Therefore, 84% of this effect at maximum frown occurred within the first 2–4 days after injection, and 98% of this effect had occurred by the next visit at day 8 ± 1.
The mean change from baseline in the severity of glabellar lines at maximum frown according to the Merz 5-point scale at later time points (3 months, 4 months, and 5 months postinjection) was still significant (P < 0.001) at −1.95, −1.53, and −0.95, respectively, compared with baseline ().
Figure 3 Mean change from baseline glabellar frown lines score on the 5-point scale at maximum frown over time.
Patients were asked to rate their appearance relative to their actual age at baseline and at visit 4 (day 14 ± 2). Of those who responded, 18% (4/22) thought that they looked younger than their actual age before treatment, compared with 73% (8/11) after treatment.
At visits 2 (day 3 ± 1), 3 (day 8 ± 1), and 4 (day 14 ± 2), patients were asked to assess the improvement in the appearance of their glabellar frown lines at rest and at maximum frown compared with baseline. The possible responses were “markedly worse,” “worse,” “unchanged,” “improved,” or “markedly improved.” The percentage of patients giving a rating of improved or markedly improved is shown in . The majority of patients rated their glabellar frown lines as improved or markedly improved compared with baseline at all three time points. The percentage of patients increased at each time point for the results at rest. More patients rated their glabellar frown lines as improved or markedly improved at maximum frown than at rest at each time point except at visit 4 (day 14 ± 2), when this rating was reported by 95.5% of patients both at rest and maximum frown, again indicating that improvement at rest occurs at a later time point. At visit 4 (day 14 ± 2), 81.8% of patients rated their improvement from baseline at maximum frown as markedly improved.
Table 2 Percentage of patients who assessed themselves as having “improved” or “markedly improved” glabellar frown lines compared with baseline
Responses to the patient questionnaire at visit 1 (day 0) and visit 4 (day 14 ± 2) were compared. shows the mean percentages of patients who agreed with certain statements about the negative effects of their facial wrinkles at both time points. At visit 4 (day 14 ± 2), there was a trend for patients to agree less strongly with the statements than they did at visit 1 (day 0), suggesting that at this time point after treatment patients were happier with their appearance, though these results did not reach statistical significance.
Table 3 Patient questionnaire responses: mean percentage of patients who agreed with the statements at the time points shown
Five patients experienced AEs during the course of the study, although none was serious or deemed to be related to the study drug.
Discussion
This study was undertaken to examine the efficacy and safety of incobotulinumtoxinA for the treatment of glabellar frown lines, and the time to onset and duration of the effect, up to 5 months posttreatment.
By calculating the percentage of the maximal effect that occurred after only 2–4 days, we established that the onset of the effect of incobotulinumtoxinA treatment is rapid, with 84% of the effect occurring by this time point. In terms of the duration of effect, a significant reduction in the mean score of glabellar frown-line severity compared with baseline was seen at all time points, including the last time point assessed, 5 months postinjection. At this time point, patients averaged a −0.95-point improvement compared with baseline.
Factors such as time to onset of action and duration of treatment are important to patients. However, there is limited published information about the time to onset of treatment effect. Results of a study in 14 healthy volunteers who received injections into the extensor digitorum brevis muscle of the foot with incobotulinumtoxinA or onabotulinumtoxinA revealed a similar onset for both treatments, with over 50% of patients responding on day 1 postinjection.Citation15 A subset analysis of phase III trials of a botulinum toxin type A for the treatment of glabellar frown lines was conducted recently.Citation13 Integrated analysis of three trials, including patient self-assessment data, revealed that 19.7% of patients had an effect by 1 day, with the median time to onset being 3 days, based on patient assessment only, recorded in a patient diary. Two of these trials were fixed-dose studies; at 2, 3, and 7 days posttreatment, cumulative percentages of responders were 55%, 74%, and 90%, respectively, in one study, and 35.2%, 52.4%, and 80%, respectively, in the second study.Citation13 A study of another BoNT/A treatment for hyperfunctional facial lines, including glabellar frown lines, reported that all patients had an effect within the first 24–72 hours, but it was not clear how this assessment was made.Citation11 In a more recent study of glabellar frown lines, time to onset following onabotulinumtoxinA injection was determined by physicians and patients being asked to answer yes or no to whether they had noticed any effect on the appearance of the frown lines (lines between the eyebrows) since injection. The results of this assessment showed that 87% of patients had an effect 2 days after injection, 91% had an effect after 3 days, and 100% had a treatment effect after 4 days.Citation12 Patients also completed a 14-day diary; 48% reported an effect after 1 day and 77% reported a treatment effect 2 days after injection.Citation12
Early onset is a definite advantage in aesthetic treatment, and is related to patient satisfaction.Citation12 The results reported here demonstrate that incobotulinumtoxinA has a rapid onset of action. The only direct comparison of the onset of action of onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA has shown that the response to incobotulinumtoxinA was more rapid than that seen with the other two agents.Citation16
Since patients averaged an almost 1-point improvement in the appearance of glabellar frown lines at 5 months, this suggests that the improvement extends beyond this time point, demonstrating the durability of the incobotulinumtoxinA treatment effect. In addition, at the final visit at 5 months posttreatment, 76.2% of patients were still responders at maximum frown, using the definition of at least a 1-point improvement compared with baseline. Again, treatment duration is important, as it will affect the frequency with which a patient returns for further treatment. In the absence of any data that directly compare the treatment duration of the different BoNT/A products available, it is interesting to note the treatment duration reported for the products in separate trials. However, care should be taken when trying to make comparisons between trials, because doses, injection sites, and definitions of responder vary. Ascher et al reported that doses of 25, 50, and 75 U of abobotulinumtoxinA significantly improved the appearance of glabellar frown lines at maximum frown compared with placebo up to 3 months after treatment. However, responder rates (where a responder was defined as a patient with grade 0 or 1 glabellar frown lines on a 4-point scale) for any dose were not significantly different from placebo at 6 months postinjection,Citation1 suggesting that the treatment duration for abobotulinumtoxinA is somewhere between 3 and 6 months. This would seem to be supported by another study using the same definition of a responder: Monheit et al demonstrated that a statistically significant percentage of patients (26% and 27%) treated with 50 and 75 U of abobotulinumtoxinA, respectively, continued to show a response 120 days after treatment.Citation5 In a study of 20 U onabotulinumtoxinA for the treatment of glabellar frown lines, which defined a responder as a patient with grade 0 or 1 glabellar frown lines on a 4-point scale, 26.2% of patients (53/202) were still responders at maximum frown 120 days after injection, which was statistically significantly more than with placebo (0%; P < 0.001).Citation4
A recent review of published data found that treatment duration with one BoNT/A product in females ranged from 3 to 5 months, using a conservative definition of relapse rate as a return to baseline severity on two consecutive visits approximately 30 days apart.Citation17 Using this definition, none of the patients in this study would be considered to have relapsed, as only five out of 21 patients (24%) had returned to their baseline severity for the first time at visit 7 (5 months after treatment). Studies involving another BoNT/A product estimated retreatment intervals of a mean of 3.9 months.Citation17 In addition, a recent study has shown consistently high response rates following repeated treatments with incobotulinumtoxinA for glabellar frown lines over 2 years.Citation18 This field would benefit from a direct comparison of the treatment duration of the available products in order to clarify their relative longevity.
Time to onset and duration of treatment effect are key factors that contribute to patient satisfaction. There is likely to be a preference for a product with a rapid onset, especially in patients undergoing their first treatment,Citation12 and for longevity, as this influences treatment interval, with implications for cost and convenience for patients. The results shown here demonstrate that incobotulinumtoxinA treatment fulfills these criteria. In addition, incobotulinumtoxinA was reported to have a more rapid onset compared with onabotulinumtoxinA and abobotulinumtoxinA and may have a longer duration of action in a study where no AEs were reported for any product, giving incobotulinumtoxinA a favorable product profile.Citation16 This is supported by the fact that all the patients in this study reported that their glabellar frown lines at maximum frown were improved or markedly improved by visit 2, at 2–4 days after treatment. In addition, at visit 4 (day 14 ± 2), the average amount by which patients agreed with the statement “My facial wrinkles make me look older than I feel” had decreased by 13.6% compared with baseline, although this difference was not statistically significant (P = 0.148).
The high response rates achieved using the Merz 5-point scale and both definitions of responder (improvement of 1 or 2 points) confirm previously reported results of the efficacy of incobotulinumtoxinA for this indication.Citation6,Citation7 Response rates achieved using both definitions of responder had a similar profile, although, as expected, when the more stringent definition was used, the response rate dropped below 100% at an earlier time point than when the definition of at least a 1-point improvement was used.
Using a definition of a responder as a patient with at least a 1-point improvement, the percentage of responders at rest increased more gradually over time and peaked at visit 5, which has been observed in daily practice and may be due to remodeling of skin texture while the underlying muscle motion is inhibited.
When at least a 2-point improvement was required to qualify as a responder, no patient was a responder at rest at any time point. However, this was not unexpected, as the baseline scores of patients at rest were lower than those at maximum frown, and only three patients had glabellar frown lines rated as moderate (2 on the Merz 5-point scale) at rest, whereas the remainder had a score of 0 or 1 and therefore could not be responders.
The safety results show that incobotulinumtoxinA treatment was well tolerated, as reported previously,Citation6,Citation7,Citation19 and no serious AEs – or any deemed to be related to the study drug – occurred.
The small size of this study population may be considered a limitation; however, a dose-ranging study of BoNT/A in this indication enrolled 20 subjects per treatment arm, which was deemed to be a sufficient sample size to yield statistically significant results,Citation3 and this sample size is commonly used in such studies.Citation2,Citation20
Conclusion
In conclusion, the results presented here demonstrate that incobotulinumtoxinA is a well-tolerated and efficacious treatment for glabellar frown lines, with a rapid onset of action (within 2–4 days postinjection) and a long duration of effect lasting for more than 5 months. These are desirable characteristics that contribute to patient satisfaction, a key goal of aesthetic medicine.
Acknowledgments/disclosure
Welf Prager has acted as a consultant and lecturer for Allergan Inc, Merz Pharmaceuticals GmbH, and Galderma Pharma SA. Eva K Bee has acted as a consultant and lecturer for Merz Pharmaceuticals GmbH. Isabel Havermann reports no conflicts of interest in this work. Ina Zschocke reports no conflicts of interest in this work. Editorial assistance was provided by Ogilvy 4D, Oxford, UK and funded by Merz Pharmaceuticals GmbH, Frankfurt, Germany.
References
- AscherBZakineBKestemontPBaspeyrasMBougaraASantiniJA multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar linesJ Am Acad Dermatol200451222323315280841
- CarruthersACarruthersJProspective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytidsDermatol Surg200531101297130316188182
- CarruthersACarruthersJSaidSDose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in femalesDermatol Surg200531441442215871316
- CarruthersJALoweNJMenterMAA multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar linesJ Am Acad Dermatol200246684084912063480
- MonheitGCarruthersABrandtFRandRA randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal doseDermatol Surg2007331 SpecS51S5917241415
- SattlerGCallanderMGrablowitzDNoninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown linesDermatol Surg201036S42146215421134045
- ImhofMKühneUA phase III study of incobotulinumtoxinA in the treatment of glabellar frown linesJ Clin Aesthet Dermatol2011410283422010053
- CarruthersACarruthersJColemanWPIIIMulticentre, randomized, Phase III study of a single dose of incobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown linesDermatol Surg2013
- HankeWNarinsRSBrandtFA randomized, placebo-controlled, double blind Phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpointDermatol Surg2013
- Bocouture® [package insert] Summary of product characteristicsFrankfurtMerz Pharmaceuticals2010
- BlitzerABinderWJAvivJEKeenMSBrinMFThe management of hyperfunctional facial lines with botulinum toxin. A collaborative study of 210 injection sites in 162 patientsArch Otolaryngol Head Neck Surg199712343893929109785
- BeerKRBoydCPatelRKBowenBJamesSPBrinMFRapid onset of response and patient-reported outcomes after onabotulinumtoxinA treatment of moderate-to-severe glabellar linesJ Drugs Dermatol2011101394421197522
- SchlessingerJMonheitGKaneMAMendelsohnNTime to onset of response of abobotulinumtoxina in the treatment of glabellar lines: a subset analysis of phase 3 clinical trials of a new botulinum toxin type ADermatol Surg201137101434144221745254
- FlynnTCCarruthersACarruthersJValidated assessment scales for the upper faceDermatol Surg2012382 Spec30931922316187
- JostWHKohlABrinkmannSComesGEfficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (BOTOX) in healthy volunteersJ Neural Transm2005112790591315526142
- RapplTWiednerMKranzlbinderBHaasFIs there a difference in persistence, efficiency and efficacy of 3 BoNT/A-containing products? A double-blind, randomised studyPresented at the 4th International Master Course on Aging SkinHong Kong, China2010 Jul 10–12 Hong Kong, China
- FlynnTCBotulinum toxin: examining duration of effect in facial aesthetic applicationsAm J Clin Dermatol201011318319920369902
- RzanyBFlynnTCSchlöbeAHeinzMHarringtonLLong-term results for incobotulinumtoxinA in the treatment of glabellar frown linesDermatol Surg2013399510323190342
- PragerWWissmüllerEKollhorstBWilliamsSZschockeIComparison of two botulinum toxin type A preparations for treating crow’s feet: a split-face, double-blind, proof-of-concept studyDermatol Surg201036Suppl 42155216021134046
- CarruthersACarruthersJA single-center, dose-comparison, pilot study of botulinum neurotoxin type A in female patients with upper facial rhytids: safety and efficacyJ Am Acad Dermatol200960697297919467368