Abstract
Asthma is a disease of all ages. This assumption has been challenged in the past, because of several cultural and scientific biases. A large body of evidence has accumulated in recent years to confirm that the prevalence of asthma in the most advanced ages is similar to that in younger ages. Asthma in the elderly may show similar functional and clinical characteristics to that occurring in young adults, although the frequent coexistence of comorbid conditions in older patients, together with age-associated changes in the human lung, may lead to more severe forms of the disease. Management of asthma in the elderly follows specific guidelines that apply to all ages, although most behaviors are pure extrapolation of what has been tested in young ages. In fact, age has always represented an exclusion criterion for eligibility to clinical trials. This review focuses specifically on the safety and efficacy of leukotriene modifiers, which represent a valid option in the treatment of allergic asthma, both as an alternative to first-line drugs and as add-on treatment to inhaled corticosteroids. Available studies specifically addressing the role of montelukast in the elderly are scarce; however, leukotriene modifiers have been demonstrated to be safe in this age group, even though cases of acute hepatitis and occurrence of Churg-Strauss syndrome have been described in elderly patients; whether this is associated with age is to be confirmed. Furthermore, leukotriene modifiers provide additional benefit when added to regular maintenance therapy, not differently from young asthmatics. In elderly patients, the simpler route of administration of leukotriene modifiers, compared with the inhaled agents, could represent a more effective strategy in improving the outcomes of asthma therapy, given that unintentional nonadherence with inhalation therapy represents a complex problem that may lead to significant impairment of asthma symptom control.
Introduction
Bronchial asthma is one of the most common chronic diseases worldwide, and by definition is not expected to recover with aging. However, the concept that asthma can affect older individuals has been largely denied in the past, leading to underdiagnosis and/or misdiagnosis and, consequently, undertreatment or inappropriate treatment. The heterogeneity of the clinical and functional presentation of geriatric asthma, including the partial loss of reversibility and of the allergic component, contributes to this misconception. Aging induces structural changes in the airway wall that contribute to the phenomenon of remodeling, and is associated with modifications of the immune system, defined as immunosenescence, which can influence the atopic state in the geriatric age group. Initial observations concerning atopy in the geriatric population, derived from longitudinal studies in the general population, led to the notion that the prevalence of atopy decreases with aging, reinforcing the concept that allergic asthma is a rare nosologic entity in the elderly. Today, asthma in the elderly is a well recognized condition, with unique features reflecting the coexistence of other diseases, use of multiple respiratory and nonrespiratory drugs, individual responsiveness to treatment, and differences in the perception of symptoms. The prevalence of asthma in older adults has been demonstrated to be not different from that of younger adults, ranging from 6% to 10%.Citation1 The burden of senile asthma should be weighed in the perspective of the progressive aging of the population.Citation2 In addition, the senescence-associated limitations in functioning and in local and systemic defenses, along with long-term exposure to noxious environmental stimuli, expose the elderly asthmatic to the risk of more frequent and more intense attacks. Taken together, these observations allow us to predict that asthma in the elderly will have a markedly increased impact on public health in the upcoming years. In this context, Bellia et al demonstrated that asthma in the elderly was associated with a higher mortality rate.Citation3
In the current review, we describe the contribution of the leukotriene antagonists (LTRAs) in the management of “geriatric” asthma, focusing on the safety and efficacy profiles of the drugs available. To this end, studies specifically addressing the role of leukotriene modifiers in the most advanced age groups are analyzed.
Asthma in the elderly
The “elderly” are commonly identified as subjects 65 years of age and over; however, for the purposes of the present review, we decided to incorporate studies that refer to populations of different ages, such as 50 years and over, 60 years and over, or 80 years and over. Asthma in the elderly can be distinguished in terms of early or late onset. This identifies two patterns that may have some clinical and functional differences; in particular, “late” identifies those patients who develop asthma at age older than 65 years.Citation4 As discussed below, atopy seems more frequent in patients with early-onset asthma than in those with late-onset asthma. Late-onset asthma appears to be a peculiar condition,Citation4 perhaps linked with age-associated loss of beta-2 receptors. Older asthmatics seem to complain less about their symptoms, and Connolly et alCitation5 demonstrated that elderly patients were less aware of experimentally induced bronchoconstriction than younger controls. This observation was confirmed by other studies,Citation6,Citation7 supporting the notion that elderly asthmatics can be considered “poor perceivers” of their symptoms. From a clinical perspective, asthma maintains its clinical characteristics in the elderly, although some overlap with features of chronic obstructive pulmonary disease may occur as the patient ages. It should be noted that age per se may lead to cognitive impairment and mood changes, such as anxiety and depression, which may have considerable impact on the occurrence of symptoms. The functional condition of reversible airflow limitation may not be applicable in elderly asthmatics, due to airway remodeling changes that modify the characteristics of airway obstruction towards nonreversible features.
Allergic component
Allergies have been traditionally considered to be conditions affecting children and young adults. The immune system undergoes an involutional process with a consequent decline in production of immunoglobulins, including IgE. This has led us to consider asthma in the elderly as a disease of non-allergic pathogenesis. As a consequence, atopy has never been taken into consideration in the clinical assessment and management of the geriatric respiratory patient. The decrease in prevalence of allergen sensitization with increasing ageCitation8,Citation9 has been interpreted to mean that allergic patients tend to outgrow their allergy as they get older. Although the prevalence of atopy is lower in the elderly compared with the younger population, the clinical significance of this condition and its impact on the comprehensive management of asthma in this age group is still a matter of debate. It is commonly accepted that allergic reactions may have the same or even worse manifestations in the elderly than in younger individuals; however, due to the aging decline in function of various systems, such as the cardiovascular system, older patients may not be able to compensate for the consequences of an allergic reaction. In a recent review of atopy in elderly asthmatics,Citation10 we concluded that the prevalence of allergic sensitization in the elderly population with respiratory symptoms is substantial enough to warrant assessment of atopy and the evaluation of the atopic condition. In particular, we suggested that assessment of the allergic condition in this age group could still contribute to improved diagnosis and specific treatment.
Airway inflammation
Airway inflammation in asthma is a multicellular process involving mainly eosinophils, CD4 T-lymphocytes, and mast cells. Age-related changes in eosinophil function and their potential implications for asthma were investigated in a study by Mathur et alCitation11 showing that peripheral blood eosinophil degranulation in response to interleukin-5 stimulation was significantly decreased in the older age group, whereas eosinophil adhesion, eosinophil chemotaxis, lung function, and the percentage of sputum eosinophils were similar in young and older adult asthmatics. The clinical and functional similarities with chronic obstructive pulmonary disease do not necessary imply that the two pathologic conditions also share similar inflammatory patterns. In this regard, Fabbri et alCitation12 clearly demonstrated that asthma in the elderly has a pathology which is markedly different from that of chronic obstructive pulmonary disease, even in the presence of irreversible airway obstruction. Indeed, persistent airflow limitation can develop in nonsmoking patients with asthma and is associated with older age, a longer duration of asthma, sputum eosinophilia, and airway hyperresponsiveness.Citation13 Fabbri et alCitation12 showed that asthmatic airway inflammation does not lose its cytologic and histochemical peculiarities with the development of fixed airflow obstruction. These findings were confirmed by another study addressing the inflammatory characteristics of the airways in elderly patients with asthma or chronic obstructive pulmonary disease not differing for lung function.Citation14 Taken together, these observations lead us to assume that the inflammatory cascade characterizing the processes occurring in the asthmatics airways is not profoundly affected by age. In the sequence of inflammatory events, several mediators are involved and, among them, a specific role is attributed to the leukotrienes, which are produced by a number of cell types, particularly mast cells, eosinophils, basophils, macrophages, and monocytes. Soon after their characterization, the leukotrienes were described as being implicated in the pathogenesis of asthma, thus becoming targets for therapeutic modulation.
Role of leukotrienes in the inflammatory cascade
Leukotrienes derive from arachidonic acid, a component of the lipid bilayer of the cell and nuclear membranes. The cytosolic enzyme, phospholipase A2, cleaves arachidonic acid from the lipid bilayer, generating free arachidonic acid. The 5-lipoxygenase enzyme acts upon free arachidonic acid, generating leukotriene A4. Leukotriene A4 is then converted to leukotriene C4 by its synthase, and is then transported into the extracellular microenvironment, where it is converted to leukotriene D4. Finally, this is converted to leukotriene E4. Leukotriene C4, leukotriene D4, and leukotriene E4 all contain cysteine, and are therefore collectively known as the cysteinyl leukotrienes. There are two known receptors to which cysteinyl leukotrienes bind: CysLT-1, which binds to all three cysteinyl leukotrienes and is found on eosinophils, monocytes, airway smooth muscle cells, neutrophils, B cells, plasma cells, and tissue macrophages, and CysLT-2, which has higher affinity for leukotriene C4 and leukotriene D4, and is detected in many tissues of the body, including the cardiovascular system, adrenal glands, the nasal epithelium, and mucus glands.Citation15 There is evidence for the presence of cysteinyl leukotriene receptors (CysLT1 and CysLT2) in the human lung; furthermore, Back et al suggested the possible existence of a third type of cysteinyl leukotriene receptor (CysLT3) in the human pulmonary artery.Citation16
As stated above, cysteinyl leukotrienes are produced by many inflammatory cells (eosinophils, macrophages, basophils, and mast cells) and may be considered as pivotal inflammatory lipid mediators that contribute to the pathogenesis of asthma. In fact, they are able to promote eosinophil recruitment and activation, increase microvascular permeability, and cause secretion of mucus, smooth muscle constriction, and proliferation.Citation17 Their bronchospastic effect is greater than that of histamine or methacholine in human airways, both in vivo and in vitro.
Contribution of leukotriene modifiers to asthma treatment
Based on the above-described role of leukotrienes as main inflammatory mediators, leukotriene modifiers have been developed for asthma treatment. These include CysLT1 receptor antagonists (montelukast, pranlukast, and zafirlukast), and a 5-lipoxigenase inhibitor (zileuton). These medications, although with different modes of action, result in prevention of leukotriene activity. Several clinical studies have demonstrated that leukotriene modifiers are capable of protecting inflamed airways against constriction induced by different spasmogens, reducing asthma symptoms and exacerbations, as well as the need for use of rescue bronchodilators, and decreasing the level of airway inflammation.Citation18,Citation19 The latter is of great importance, given that leukotriene production is not completely suppressed by treatment with corticosteroids alone. Studies have been conducted on the combined effect of leukotriene modifiers, and the efficacy of this therapeutic modality has been largely recognized.
Pranlukast was the first orally active LTRA available for the treatment of asthma, and is capable of blunting the bronchoconstriction induced by leukotriene D4. In a multicenter European study addressing the safety and tolerability of pranlukast, Barnes et al demonstrated the efficacy of the LTRA in patients with chronic asthma for the first time, as well as its safety profile.Citation18 In 2003, Horiguchi et alCitation20 assessed the efficacy of long-term administration of pranlukast in adult asthmatics who were poorly controlled in spite of treatment with an inhaled corticosteroid. Their study showed significant improvement in lung function and asthma control, accompanied by a decrease in the peripheral eosinophil count and serum eosinophil cationic protein levels, suggesting that pranlukast further reduces the degree of airway inflammation by specifically acting on eosinophils. One of the conclusions from these studies was that the anti-inflammatory effects of inhaled corticosteroids and LTRAs may be complementary.
Randomized controlled trials have confirmed the efficacy of LTRAs when used as monotherapy or added to inhaled corticosteroids for improving asthma outcomes, including lung function, symptoms, asthma exacerbations, health-related quality of life, rates of hospitalization, and death due to asthma attacks.Citation21–Citation24 In a recent study, Yasui et alCitation25 showed that addition of oral pranlukast to an inhaled corticosteroid or an inhaled corticosteroid plus a long-acting beta-2 adrenergic agent in stable asthmatics provided additional clinical benefit. Interestingly, add-on oral pranlukast was found to significantly reduce the levels of alveolar nitric oxide, a parameter of small airway inflammation, suggesting that the additional clinical effect of oral pranlukast could be mediated by reduction in peripheral airway inflammation.
Several clinical studies have confirmed the effectiveness of montelukast in adults with both asthma and allergic rhinitis. To date, according to Global Initiative for Asthma guidelines,Citation26 LTRAs are recommended as alternative controller medications for adults with mild persistent asthma (step 2). Moreover, patients with aspirin-sensitive asthma seem to respond well to LTRAs.Citation27 The use of LTRAs as a steroid-sparing treatment is also encouraged in clinical practice. When used as add-on therapy, LTRAs may reduce the dose of inhaled corticosteroids required by patients with moderate to severe asthma and may improve asthma control in individuals whose asthma is not controlled with a low dose or high dose of inhaled corticosteroids.
Finally, because current treatments for acute asthma provide inadequate benefit for some patients, the efficacy of intravenous montelukast as adjunctive therapy for acute asthma was evaluated. In this regard, addition of intravenous montelukast to standard care produced a more rapid improvement in forced expiratory volume in one second (FEV1) in acute asthma.Citation28 More recently, the acute effect of montelukast was investigated by the same groupCitation29 in 600 adult asthmatics with FEV1 <50% predicted, randomly allocated to intravenous montelukast 7 mg or placebo in addition to standard care. This randomized, double-blind, placebo-controlled study showed that intravenous montelukast, when added to standard therapy, provided significant, rapid, and sustained benefit in acute asthma. Montelukast was generally well tolerated, and its safety profile was comparable with that of placebo.
Leukotriene modifiers in the elderly
Inhaled corticosteroids are recommended in many clinical practice guidelines for asthma managementCitation26 as first-line anti-inflammatory control agents, whereas LTRAs are second-choice drugs. These guidelines do not include specific sections for the management of the disease in the elderly, thus implying that drugs indicated for asthma treatment are effective across the age groups, as suggested by the National Asthma Education and Prevention Program Working Group Report on asthma in the elderly.Citation30 However, these age-specific guidelines were published in 1996 and never updated. It should be emphasized that advanced age is one of the main exclusion criteria for randomized controlled trials involving asthmatics. It is logical to assume that, in real-life settings, elderly asthmatic subjects are currently treated by protocols based on results of randomized controlled trials for which they would have been ineligible.
It is known that the real clinical benefit provided by inhaled therapy can be influenced by several factors, mostly related to the patient rather than to the medication itself. Amongst these, adherence plays a major role, particularly in elderly patients. In elderly patients, unintentional nonadherence with inhalation therapy represents a complex problem that may lead to significant impairment of asthma symptom control.Citation31–Citation33 Elderly patients often suffer from multiple chronic diseases, such as cognitive impairment, hearing or visual problems, or other physical disabilities (arthritis, tremor, and low coordination) that significantly affect their ability to inhale the drug properly. In this context, the simpler route of administration of LTRAs, compared with the inhaled agents, could provide a valid strategic option in asthma therapy.
Among the products derived from the lipoxygenase pathway of arachidonic acid metabolism, cysteinyl leukotrienes have proinflammatory functions, whereas lipoxin carry potent anti-inflammatory signals.Citation34 Lipoxins, especially LXA4, antagonize a number of proinflammatory mediators, resulting in inhibition of leukocyte-dependent inflammation.Citation34 Given that aging is characterized by an increased incidence of diseases with a marked inflammatory component, Gangemi et alCitation34 tested the hypothesis that aging is associated with impairment of resolution mechanisms by measuring urinary levels of anti-inflammatory LXA4 in healthy subjects of different ages, including centenarians. They demonstrated that the urinary concentrations of LXA4 decrease with age, whereas cysteinyl leukotriene concentrations increase with age. As a consequence, the ratio of LXA4 to cysteinyl leukotrienes, which was proposed as an inflammation protection index, was significantly lower in the elderly.Citation34 These results support the hypothesis that the endogenous host defense barrier against inflammatory insults tends to become impaired with age and they are in line with observations in bronchial asthma, where it has been demonstrated that adult subjects with moderate or severe asthma had lower LXA4 concentrations in the supernatants of induced sputum than those with mild asthma.Citation35
Older evidence suggests that zafirlukast is able to improve pulmonary function and asthma symptoms in adolescents, adults, and the elderly.Citation36 However, this study has several limitations, including its open-label design, lack of a control group, predominance of white female patients, and a relatively short trial duration (4 weeks). Moreover, improvements in elderly patients were less dramatic compared with the other two age groups. In the elderly group, the more severe the asthma, the greater the percentage of patients with a reduction in symptoms, whereas the inverse association occurred in adolescents.Citation36 Of note, zafirlukast was well tolerated by patients in all age groups, with treatment-related adverse events occurring in 4.2% of adolescents, 7.4% of adults, and 8.1% of elderly patients, respectively. Elderly patients had the highest incidence of headache, abdominal pain, diarrhea, and nausea, and the lowest incidence of infection and sinusitis.Citation36 In contrast with those results, a retrospective analysisCitation37 of five published clinical trials, in which fluticasone was compared with zafirlukast, showed that treatment with zafirlukast in asthmatic patients aged 50 years and over resulted in small improvements in asthma symptoms, minimal or no improvement in lung function, and increased exacerbations. The authors were concerned that zafirlukast could mask the deterioration of asthma in this aged population. With regard to the safety profile, it is reassuring that adverse events (headache, nausea, diarrhea, and heartburn) occurred in less than 2% of the older patients, similar to what is observed in younger asthmatics.Citation37 The strength of this review is its evaluation of studies with a randomized and double-blind design. Taken together, these observations suggest caution in using zafirlukast in adults aged 50 years and over.
A possible explanation for these negative results could be provided by a study that aimed at characterizing asthma at a cellular and molecular levelCitation38 by comparing production of proinflammatory lipid mediators, ie, LTB4 and cysteinyl leukotrienes, both in vitro and in vivo in young and older asthmatics. In this pilot study, adult/elderly subjects (aged 50–70 years) with mild to moderate asthma showed lower levels of LTB4 and cysteinyl leukotrienes in induced sputum, with no difference in eosinophil or neutrophil counts in the airways. Moreover, in the older asthmatics, lipopolysaccharide stimulation of peripheral blood mononuclear cells resulted in less production of granulocyte macrophage colony-stimulating factor (GM-CSF), which in turn is a stimulus to produce leukotrienes. Therefore, the authorsCitation38 proposed that a potential explanation for the age-related differences in sputum leukotriene levels could be less GM-CSF in the airways of older asthmatics. The clinical implication of these results is that the lower levels of cysteinyl leukotrienes in the airways of older asthmatics may have an impact on the effectiveness of the therapy with LTRAs. These results appear to be in contrast with those of Gangemi et al, that have been previously discussed.Citation34 However, the two studies are not comparable because of their different study populations. Indeed, Nyenhuis et alCitation38 incorporated a very small number of “elderly” asthmatics, whereas Gangemi et al studied healthy subjects and included centenarians. In this respect, they found the apparent paradox of elevated inflammatory indices in extreme longevity, since centenarians displayed the lowest LXA4 levels and LXA4 to cysteinyl leukotriene ratio. This observation suggests that additional factors may compensate for the fall in anti-inflammatory mediators to prevent development of disease in extreme longevity.
Compliance with medication was found to be higher in patients taking pranlukast than in those taking inhaled fluticasone (about 93% versus 81%),Citation39 and both drugs achieved similar reductions in the use of rescue medications, serum and sputum concentrations of eosinophil cationic protein, and similar improvements in lung function, as assessed by FEV1, compared with baseline values after one and 4 weeks. A randomized crossover trialCitation25 with an open-label and uncontrolled design in a small number of controlled asthmatics of median age 66 (range 24–86) years showed that additional administration of oral pranlukast to stable asthmatics on inhaled therapy (corticosteroids alone or in combination with long-acting beta-adrenergic agents) had clinical benefit in terms of asthma symptoms, lung function, and inflammatory markers of proximal and peripheral airways. Since only half of this small sample included elderly patients, the use of pranlukast in elderly patients with controlled asthma requires caution.
In a multicenter Spanish study, 639 patients aged 18–79 years were randomized to receive montelukast 10 mg (n = 326) or placebo (n = 313) once daily for 16 weeks, on top of regular treatment with budesonide 400–1,600 μg/day throughout the study.Citation21 Treatment with montelukast produced a significant reduction of 35% in the percentage of asthma exacerbation days compared with placebo, with a similar incidence of adverse events in both groups. No post hoc analysis was performed to evaluate the influence of age. Therefore, we can only speculate that age per se did not have any relevant impact on the outcomes. In this respect, several studiesCitation40–Citation43 of montelukast share similar characteristics in terms of age and data analysis, ie, inclusion of patients over 65 years of age is generally limited to the smallest proportion of the sample, and no age-specific subanalysis is performed. Therefore, to establish whether older patients show similar responses compared with younger patients in terms of the efficacy and safety of montelukast may be a difficult task. This severely limits the ability to draw definite conclusions on the implementation of montelukast in elderly asthmatics.
In a double-blind, placebo-controlled, crossover, randomized add-on study, montelukast 10 mg was added for 14 days in a total of 72 adult asthmatics (age range 22–79 years), who were symptomatic despite treatment with inhaled corticosteroids.Citation44 In this study, the additional treatment in an outpatient setting did not result in improvement of lung function or symptom control. A more recent studyCitation22 comparing the efficacy of montelukast as an add-on therapy to inhaled corticosteroids in elderly patients with severe asthma to improve symptom control showed that montelukast plus inhaled corticosteroids for 24 months increased days without asthma symptoms, reduced days with beta-agonist use, and improved the mean asthma control test score. The authors suggested that the greater adherence to therapy observed with montelukast as compared with inhaled therapy may have contributed to the observed improvements. These results were novel, since no similar observations in a homogenous group of severe asthmatics over 60 years of age had ever been performed.
With the aim of assessing the effectiveness and tolerability of montelukast in the treatment of asthma in everyday clinical practice in representative patient groups, a retrospective cross-sectional survey of routinely collected clinical information was undertaken in 56 centers throughout the United Kingdom (20 general practice centers and 36 hospitals).Citation23 A total of 1,429 asthmatic patients on regular treatment with montelukast were included in the analysis. Subjects ranged in age from one to 88 years, making it possible to extrapolate data for older subjects. Overall, montelukast was demonstrated to be effective across all age groups, and adverse events possibly related to montelukast occurred in a proportion similar to those seen in the montelukast and placebo groups in randomized controlled trials. However, logistic regression analysis showed a significant association between age and efficacy, meaning that an older individual was less likely to be a responder. The efficacy and safety of add-on montelukast to existing controller medications were also assessed in a real-life settingCitation45 in a total of 5,855 individuals with asthma, whose age range was 16–96 years. Following addition of 10 mg montelukast, the vast majority of patients reported a strong or marked improvement in day-time and night-time asthma symptoms. Similar improvement was detected for all nasal symptoms. Use of asthma and rhinitis medication was also reduced. However, a subgroup analysis specific for the elderly was not performed. Overall, montelukast was safe and well tolerated by all patients. We can infer that the evidence of a beneficial effect for montelukast in elderly asthmatics is not sufficient to suggest its use as a first-line option. However, its safety profile encourages the use of montelukast in the context of reducing t use of corticosteroids, which may have detrimental effects when taken regularly for long periods by elderly asthmatics who suffer from comorbid conditions.
No clinical trial including subjects at advanced ages shows any age-related adverse effect. This reinforces the use of LTRAs at all ages, with no age restrictions. However, it is worth noting that cases of severe adverse events, such as acute hepatitisCitation46 or Churg-Strauss syndrome,Citation47 have been described even after a latency of years.Citation46 Whether the elderly are more prone to adverse events, as suggested by Russmann et alCitation46 and by Tuggey et al,Citation47 opened new perspectives with suggested new indications.
In the last few years, experimental studies have opened new perspective for the LTRAs, since it has been shown that these drugs inhibit development of atherosclerosis, reduce intimal hyperplasia after vascular injury, and exert protective effects after cerebral ischemia and reperfusion.Citation48 These observations suggest that LTRAs could potentially inhibit the inflammatory response associated with atherosclerosis, beyond their actions in asthma. On this basis, Ingelsson et alCitation49 evaluated the effects of montelukast on the incidence and recurrence of either myocardial infarction or ischemic stroke. The results demonstrated, in a large sample of patients, that montelukast was associated with a lower risk of recurrent myocardial infarction in males and it use was associated with a lower risk of recurrent stroke in both genders.Citation49 This provides evidence for the protective effects of montelukast in secondary prevention. These results could suggest a new indication for LTRAs in elderly asthmatics, given that cardiovascular disease has a higher prevalence in this age group. Indeed, if these data can be confirmed by randomized controlled trials, the LTRAs could become first-line drugs for elderly asthmatics with a history of cardiovascular events.
Recently, LTRAs have been proposed for use in elderly individuals with asthma which has become difficult to treat because of steroid resistance.Citation50 This could be an interesting field for use of LTRAs in the elderly, who are particularly at risk of severe asthma. However, a study conducted in 2005 by Jayaram et alCitation51 failed to show an additive effect of montelukast over placebo for reducing sputum eosinophilia in 14 asthmatics (mean age 61.1 ± 10.2 years) with relative corticosteroid resistance.
In conclusion, the data available concerning the role of montelukast in the treatment of elderly asthmatics do not appear to indicate a specific role for this drug in this age group. Studies testing montelukast in the elderly population are very fewCitation22,Citation36–Citation39 and have several limitations. summarizes the main limitations as well as the favorable aspects of using LTRAs in the elderly. Antileukotrienes may have advantages over inhaled long-acting beta-2 agonists as additive treatment with corticosteroids, given that long-acting beta-2 agonists are often contraindicated in older populations due to comorbidity, such as cardiovascular disease. Another important advantage, which is amplified in older populations, is the opportunity to achieve better adherence with oral medication. This has been demonstrated not to be a trivial issue in older patients with chronic disease. Further, the safety profile of montelukast is reassuring for physicians considering use of this drug in elderly patients. However, as indicated in , some of the positive evidence for the effect of montelukast in the elderly appears to come from open-label studies,Citation36,Citation39 whereas double-blind, placebo-controlled studies show little or no effect, or were not superior to inhaled corticosteroids or combination inhaled treatments.Citation44
Figure 1 Description of the main advantages and limitations of using leukotriene modifiers in elderly asthmatics.
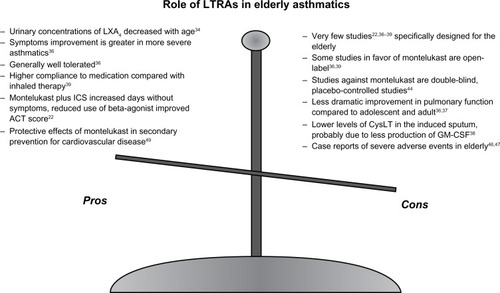
Taken together, these observations are reassuring regarding safe use of montelukast in the management of asthma at advanced ages, as an add-on treatment to inhaled combination therapy to control the disease, or as an alternative to inhaled corticosteroids or long-acting beta-2 agonists when these are contraindicated in the elderly population. Specifically designed studies to test the anti-inflammatory role of montelukast in diseases other than asthma are advocated in elderly asthmatics suffering from comorbid conditions.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZureikMOrehekJDiagnosis and severity of asthma in the elderly: results of a large survey in 1,485 asthmatics recruited by lung specialistsRespiration200269322322812097765
- World Health OrganizationAgeing and life course2013 Available from: http://www.who.int/ageing/en/Accessed July 25, 2013
- BelliaVPedoneCCatalanoFAsthma in the elderly: mortality rate and associated risk factors for mortalityChest200713241175118217890479
- BramanSSKaemmerlenJTDavisSMAsthma in the elderly. A comparison between patients with recently acquired and long-standing diseaseAm Rev Respir Dis199114323363401990949
- ConnollyMJCrowleyJJCharanNBNielsonCPVestalREReduced subjective awareness of bronchoconstriction provoked by methacholine in elderly asthmatic and normal subjects as measured on a simple awareness scaleThorax19924764104131496497
- CuttittaGCibellaFBelliaVChanges in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyperresponsiveness and agingChest200111961685169011399691
- EkiciMApanAEkiciAErdemogluAKPerception of bronchocon-striction in elderly asthmaticsJ Asthma200138869169611758898
- LawMMorrisJKWaldNLuczynskaCBurneyPChanges in atopy over a quarter of a century, based on cross sectional data at three time periodsBMJ200533075011187118815833748
- LinnebergAGislumMJohansenNHusemoenLLJorgensenTTemporal trends of aeroallergen sensitization over twenty-five yearsClin Exp Allergy20073781137114217651142
- ScichiloneNAugugliaroGTogiasABelliaVShould atopy be assessed in elderly patients with respiratory symptoms suggestive of asthma?Expert Rev Respir Med20104558559120923338
- MathurSKSchwantesEAJarjourNNBusseWWAge-related changes in eosinophil function in human subjectsChest2008133241241918252914
- FabbriLMRomagnoliMCorbettaLDifferences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167341842412426229
- ten BrinkeAZwindermanAHSterkPJRabeKFBelEHFactors associated with persistent airflow limitation in severe asthmaAm J Respir Crit Care Med2001164574474811549526
- Di LorenzoGMansuetoPDittaVSimilarity and differences in elderly patients with fixed airflow obstruction by asthma and by chronic obstructive pulmonary diseaseRespir Med2008102223223818006291
- GraysonMHKorenblatPETreating asthma in the older patient: is there a place for leukotriene modifiers?Drugs Aging200623645145916872230
- BackMKumlinMCotgreaveIADahlenSEAn alternative pathway for metabolism of leukotriene D(4): effects on contractions to cysteinyl-leukotrienes in the guinea-pig tracheaBr J Pharmacol200113371134114411487525
- MechicheHNalineECandenasLEffects of cysteinyl leukotrienes in small human bronchus and antagonist activity of montelukast and its metabolitesClin Exp Allergy200333788789412859443
- BarnesNCPujetJCPranlukast, a novel leukotriene receptor antagonist: results of the first European, placebo controlled, multicentre clinical study in asthmaThorax19975265235279227718
- BarnesNCMillerCJEffect of leukotriene receptor antagonist therapy on the risk of asthma exacerbations in patients with mild to moderate asthma: an integrated analysis of zafirlukast trialsThorax200055647848310817796
- HoriguchiTTachikawaSKondoRShigaMHiroseMItoTClinical effects of long-term administration of pranlukast, a leukotriene receptor antagonist, on adult patients with bronchial asthmaArzneimittelforschung2003531071472114650364
- VaquerizoMJCasanPCastilloJEffect of montelukast added to inhaled budesonide on control of mild to moderate asthmaThorax200358320421012612294
- BozekAWarkocka-SzoltysekBFilipowska-GronskaAJarzabJMontelukast as an add-on therapy to inhaled corticosteroids in the treatment of severe asthma in elderly patientsJ Asthma201249553053422551116
- BarnesNThomasMPriceDTateHThe national montelukast surveyJ Allergy Clin Immunol20051151475415637546
- OhshimaNMatsuiHMatsuiYAddition of leukotriene receptor antagonists to inhaled corticosteroids improved QOL of patients with bronchial asthma surveyed in suburban Tokyo, JapanAllergol Int201160447348121681017
- YasuiHFujisawaTInuiNImpact of add-on pranlukast in stable asthma; the additive effect on peripheral airway inflammationRespir Med2012106450851422265857
- Global Initiative for AsthmaGlobal Strategy for Asthma Management and Prevention2012 Available from: http://www.ginasthma.org/Accessed August 27, 2013
- DahlenSEMalmstromKNizankowskaEImprovement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trialAm J Respir Crit Care Med2002165191411779723
- CamargoCAJrSmithlineHAMaliceMPGreenSAReissTFA randomized controlled trial of intravenous montelukast in acute asthmaAm J Respir Crit Care Med2003167452853312456380
- CamargoCAJrGurnerDMSmithlineHAA randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthmaJ Allergy Clin Immunol2010125237438020159247
- National Asthma Education and Prevention ProgramConsiderations for diagnosing and managing asthma in the elderly NIH publication 1996; No 96-3662. Available from: http://msdh.ms.gov/msdhsite/_static/resources/2107/pdfAccessed July 26, 2013
- DolovichMBAhrensRCHessDRDevice selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and ImmunologyChest2005127133537115654001
- LaforestLDenisFVan GanseECorrelates of adherence to respiratory drugs in COPD patientsPrim Care Respir J201019214815420094689
- ScichiloneNSpataforaMBattagliaSArrigoRBenfanteABelliaVLung penetration and patient adherence considerations in the management of asthma: role of extra-fine formulationsJ Asthma Allergy20136112123378776
- GangemiSPescaraLD’UrbanoEAging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levelsExp Gerontol200540761261415935589
- VachierIBonnansCChavisCSevere asthma is associated with a loss of LX4, an endogenous anti-inflammatory compoundJ Allergy Clin Immunol20051151556015637547
- KorenblatPEKempJPSchergerJEMinkwitzMCMezzanotteWEffect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT)Ann Allergy Asthma Immunol200084221722510719780
- CreticosPKnobilKEdwardsLDRickardKADorinskyPLoss of response to treatment with leukotriene receptor antagonists but not inhaled corticosteroids in patients over 50 years of ageAnn Allergy Asthma Immunol200288440140911991558
- NyenhuisSMSchwantesEAMathurSKCharacterization of leukotrienes in a pilot study of older asthma subjectsImmun Ageing20107820602786
- HoriguchiTTachikawaSKondoRComparative evaluation of the leukotriene receptor antagonist pranlukast versus the steroid inhalant fluticasone in the therapy of aged patients with mild bronchial asthmaArzneimittelforschung2007572879117396618
- WilliamsBNoonanGReissTFLong-term asthma control with oral montelukast and inhaled beclomethasone for adults and children 6 years and olderClin Exp Allergy200131684585411422148
- RandCBilderbackASchillerKEdelmanJMHustadCMZeigerRSAdherence with montelukast or fluticasone in a long-term clinical trial: results from the mild asthma montelukast versus inhaled corticosteroid trialJ Allergy Clin Immunol2007119491692317349681
- StormsWMicheleTMKnorrBClinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged ≥6 yearsClin Exp Allergy2001311778711167954
- PearlmanDSWhiteMVLiebermanAKFluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthmaAnn Allergy Asthma Immunol200288222723511868930
- RobinsonDSCampbellDBarnesPJAddition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trialLancet200135792732007201111438132
- VirchowJCBachertCEfficacy and safety of montelukast in adults with asthma and allergic rhinitisRespir Med2006100111952195916626955
- RussmannSIselinHUMeierDAcute hepatitis associated with montelukastJ Hepatol200338569469512713887
- TuggeyJMHoskerHSChurg-Strauss syndrome associated with montelukast therapyThorax200055980580610950903
- BackMLeukotriene signaling in atherosclerosis and ischemiaCardiovasc Drugs Ther2009231414818949546
- IngelssonEYinLBackMNationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular diseaseJ Allergy Clin Immunol20121293702707. e70222244598
- HananiaNAKingMJBramanSSAsthma in the elderly: Current understanding and future research needs – a report of a National Institute on Aging (NIA) workshopJ Allergy Clin Immunol2011128Suppl 3S4S2421872730
- JayaramLDuongMPizzichiniMMFailure of montelukast to reduce sputum eosinophilia in high-dose corticosteroid-dependent asthmaEur Respir J2005251414615640321