Abstract
Background
The purpose of this work was to study the performance and reliability of a test of fast alternating forearm movements and its relationship with measures of lower extremity function in older women with dementia.
Methods
Fast alternating movements was studied in 26 female patients (mean age 81.7 ± 5.9 years) with dementia and 34 controls (mean age 87.5 ± 4.7 years). Subgroup analyses for those aged 80–89 years were performed due to significant differences in the mean ages of the study groups. Test–retest reliability for alternating forearm movements was studied in 11 patients (mean age 80.3 ± 6.7 years) and 10 controls (mean age 87.4 ± 1.6 years). Pulses generated were transformed to an analog signal shown on a modified electrocardiogram. Numbers of cycles at 10 and 15 seconds were calculated for the right and left hand. Walking 2 × 15 m and the Get-Up-and Go (GUG) test were performed at self-selected and maximal speed. Associations between tests of upper and lower extremity function were sought in eight patients (mean age 85 ± 2.7 years) and 16 controls (mean age 85.1 ± 2.8 years) and also according to types of dementia in nine patients with probable Alzheimer’s disease and 10 patients with other types of dementia.
Results
Patients with dementia could perform the test and had significantly fewer cycles (P = 0.02–0.006) at both 10 and 15 seconds compared with controls after age adjustment. A higher number of cycles was associated with higher self-selected walking speeds in patients (r = −0.79). Test–retest reliability for alternating forearm movements was high for both patients (intraclass correlation 0.88–0.94) and controls (intraclass correlation 0.74–0.94).
Conclusion
Alternating forearm movements at fast speed can be used as a reliable test in both patients with dementia and healthy older subjects. The test can be used as a measure of bradykinesia and might be useful as a proxy for lower extremity function in older persons with dementia when testing of the lower extremities is not applicable due to walking disability.
Introduction
Complex motor functions such as alternating hand movements are affected early in the process of cognitive decline.Citation1 Slowing of movement and decreased performance of alternating hand movements on clinical testing have been found in subjects with mild cognitive impairment and mild Alzheimer’s disease (AD).Citation2–Citation5 In moderately to severely disabled community-living older women, upper extremity measures declined in parallel, albeit somewhat less than lower extremity measures over a 3-year period.Citation6
Gait and cognitive functioning are closely related, both in normal aging and age-associated dementias.Citation7,Citation8 Decreased walking speed has been shown to precede the onset of cognitive impairment,Citation9 and is predictive of persistent cognitive deficitsCitation10 and incident dementia.Citation11 Limitations in walking ability are common among the elderly, and average prevalence rates of 6%–30% have been reported.Citation12 In ambulatory nursing home residents with middle-stage dementia, the incidence of walking disability within one year was observed to be 40.8%.Citation13 Gait dysfunction increases with severity of dementia.Citation14
The observed association between gait and cognition has also been observed for hand motor function.Citation15 Hebert et al,Citation16 using composite scores, found declines in physical performance scores over time for both upper and lower extremity function in community-living persons with AD.Citation16 Both lower and upper extremity motor performance has been found to be predictive of, and to contribute to, functional impairments in basic activities of daily living, mobility, and range of motion in persons with AD, independent of cognitive performance.Citation17 The finding by Hebert et alCitation16,Citation17 that upper and lower extremity function declines in parallel suggests that testing of motor characteristics of the upper extremities could be used to identify individuals who are at risk of becoming functionally dependent due to impaired mobility and declining motor function when gait assessments are not applicable due to walking disability, comorbidity, adverse drug effects, and environmental factors.Citation13 Information on declining motor function could be useful and important in the care of patients with dementia to reduce risk of falls and fractures. To our knowledge, information is scarce on the relationship between physical performance measures of upper and lower extremity motor function in older individuals with cognitive impairment. Therefore, it was of interest to explore the association between separate measurements of upper and lower extremity motor function in older women with and without dementia.
The aims of this study were to investigate if a quantitative test of fast alternating forearm movements could be useful in older women with dementia and if upper extremity performance was associated with lower extremity function. An additional aim was to describe the reliability of the test of rapid alternating forearm movements.
Materials and methods
Patients
Thirty-four female patients were recruited from a neuropsychiatric and geriatric unit providing short-term care. Inclusion criteria were age older than 64 years, fulfilment of the DSM-III-R (Diagnostic and Statistical Manual of Mental Disorders, Third Edition) criteriaCitation18 for dementia, and ability to understand and perform simple acts on verbal command and ability to stand up and walk independently. Exclusion criteria were signs of metabolic disturbances, renal or hepatic disease, neurological disease other than dementia, malignant tumors, major depression, rheumatoid arthritis or a history of musculoskeletal disorder, pathological cobalamin values, diabetic mellitus, and alcohol abuse. Eight patients were excluded because of neurological disease (n = 1), pathological cobalamin values (n = 2), rheumatoid arthritis or a history of musculoskeletal disorder (n = 3), alcohol abuse (n = 1), and unclear diagnosis on clinical examination (n = 1). Thus, 26 patients of mean age 81 (72–91) years were included in the study.
Controls
Thirty-four controls of mean age 87 (79–99) years were recruited from a group of 49 older community-dwelling women who were taking part in a longitudinal 5-year follow-up study.Citation19 These women were originally recruited from a random selection of women living in their own homes in the city of Malmö, using the population registry at the city council. The inclusion and exclusion criteria were the same as for the patients in our study, except for the absence of a diagnosis of dementia. Fifteen of 49 women were excluded for the following reasons. Ten subjects fulfilled the DSM-III-R criteria for dementia and/or had cognitive and/or spatial disturbances, two had rheumatoid arthritis, and one subject was blind. Two women were not able to undergo the examination and were excluded because of missing data.
There was a significant (P < 0.0001) difference in age between the patients and the controls (). Therefore, two comparable subgroups, which consisted of 13 patients and 23 controls (aged 80–89 years) were created (). The age-stratified data are presented to enable comparisons of mean values in the same age groups. To avoid the influence of gender,Citation20,Citation21 we included only women in our study.
Table 1 Comparison of alternating forearm movements at 10 and 15 seconds in female controls versus females with dementia
Procedure
A letter with information about the study was handed out to the nurses in charge of patient care, who selected the patients based on the inclusion criteria. The patients were asked if they wanted to participate and after an oral or written consent to participate from the patient or a relative (or both if necessary) was obtained, the selected patients were entered into the study, which was approved by the local ethics committee at Lund University.
All 26 patients underwent a thorough clinical examination, including medical history, and a physical, psychiatric, and neurological examination. Screening laboratory blood tests, electrocardiography, chest radiography, and electroencephalography were done according to clinical indication. Computerized tomography (n = 14), psychometric testing (n = 14), and cerebral blood flow (n = 18) measured using either single photon emission computerized tomography or a 133Xenon inhalation techniqueCitation22 were also included.
Eleven patients fulfilled the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteriaCitation23 for probable AD, two patients fulfilled the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-ARIEN)Citation24 for vascular dementia, six patients had a diagnosis of mixed dementia (AD and vascular dementia), five patients had dementia of unspecified type, and two patients fulfilled the Lund–Manchester criteria for frontal lobe dementia.Citation25 Type of dementia was dichotomized into two subgroups, ie, patients with probable AD (n = 11) and those with other types of dementia (n = 15). Severity of dementia was evaluated by a senior neuropsychiatrist according to clinical course, medical records, and the Mini Mental State Examination (MMSE).Citation26 Severity was categorized as mild, moderate, or severe, corresponding, respectively, to MMSE levels ≥ 19 (n = 5), 18–13 (n = 13), and 12–0 points (n = 8). The median duration of dementia was 5 (1–10) years.
In the group aged 80–89 years, the association between upper and lower extremity function was studied in 16 controls (85.1 ± 2.8 years) and eight patients (85 ± 2.7 years) who performed tests of both fast alternating forearm movements and tests of lower extremity function.
The association between upper and lower extremity function, according to type of dementia, was also studied in nine patients with probable AD (mean age 78.7 ± 4.6 years) and 10 patients with other types of dementia (mean age 82.2 ± 6.1 years).
Two physical performance tests, used earlier in women with dementiaCitation27 and related to lower extremity function, were performed. The subjects were given verbal instructions and a practical demonstration if necessary. The two tests were performed first once at self-selected speed and then once at maximal speed, with a rest of about 2 minutes in between. Only women who were not dependent on walking aids were included.
To study the test–retest reliability of the test of fast alternating forearm movements, 11 women with dementia (mean age 80.3 ± 6.7 years) and 10 healthy controls (mean age 87.4 ± 1.6 years) were consecutively recruited from the total sample during the course of the study. The subjects performed two trials, first with the right hand and then with the left hand.
To describe the reliability of assessment of alternating forearm movements, the intrarater reliability was studied in a separate study sample of ten healthy younger women (mean age 62 [57–66] years) in three sessions over a 3-week period. The women recruited were staff members from the geriatric department who volunteered for the reliability study. The second test took place after 2 weeks and the third after a further week. The women performed one trial first with the right hand and then another with the left hand. The instructions for the healthy younger women were the same as for the study population.
All sessions for testing of alternating forearm movements and gait for both patients and controls were conducted by a skilled physiotherapist with more than 20 years of experience in neurological and geriatric rehabilitation, and who had no information about the type and degree of dementia of the patients at the time of the test.
Alternating forearm movements
Alternation of forearm movements (pronation and supination) as fast as possible was measured with a special apparatus constructed at the Department of Biomedical Engineering, Malmö University Hospital, Sweden. An easily movable handle was connected to a digital resolver, Hewlett-Packard HEDS 5701, giving 512 pulses per revolution. The pulses, generated when the handle was turned, were counted by an electronic calculator and transformed to an analog signal. The signal amplitude was proportional to the number of pulses and was shown on a pen recorder, a modified electrocardiograph (Cardiofax, model ECG-6511, Nihon Kohden Co, Tokyo, Japan), with a millimeter scaled paper slip running at a velocity of 25 mm per second.
At the beginning of the test, the subject held the handle in a vertical position and the calculator was set to zero. This position was the reference level for each subject, around which a pendulum curve was produced when the handle was moved back and forth into pronation and supination. The curve made it possible to identify the direction of the turning movement, and the frequency of turnings could be calculated, disregarding the amplitude. One cycle was defined as one pronation and supination movement. Calculation of the numbers of performed cycles at 10 and 15 seconds was done manually.
The test took place in a separate room with no disturbances present in the surroundings. The equipment was placed on an adjustable table. The subjects were seated in front of the equipment on a wooden stool (45 × 45 × 45 cm) with feet on the floor. The wheels on the stool facilitated adjustment of right and left arm position in relation to the handle. The subjects were instructed to grip the handle, with the shoulder adducted, the elbow at an angle at approximately 90°, and the unsupported forearm in a neutral position. The subjects were instructed to perform as many alternating forearm movements as fast as possible during 15 seconds on a given command. The supervisor ensured that the movement was performed primarily at the elbow joint.
Each subject was given verbal instructions, a demonstration of the procedure was performed by the examiner (EBR), demonstrating the amplitude of a full pronation and supination movement, and the subject was allowed to perform a practice trial before the actual test. Those who wanted to abandon the test were allowed to do so. Each subject was tested once, performing one trial with first the right and then the left hand, with a 2-minute rest in between. A standard digital stopwatch was used to control the duration of the test. All subjects reported being right-handed, except for two of the control subjects.
Walking 2 × 15 m, including a 180° turn
The subjects were instructed to walk 15 m from a standing start, turn at a marker, and return to and pass the starting point before stopping. The time taken to complete the task was registered. The reliability of this test has been shown to be very high in elderly women (intraclass coefficient [ICC] 0.95–0.98).Citation28
Get-Up and Go test
The Get-Up and Go test (GUG) test involves rising from sitting in a chair, walking 3 m towards a wall, turning without touching the wall, and returning to sitting.Citation29 A chair with armrests and a seat height of 45 cm was used. The subjects were allowed to rise according to their personal preference. Time from leaving the seat until being seated again was measured. The GUG test was developed originally as a clinical measure of balance in elderly people and was scored on an ordinal scale.Citation29 Podsiadlo and RichardsonCitation30 modified the test by timing the task and letting the participants pass over a line before turning instead of turning in front of a wall. High test–retest reliability (ICC3.1 = 0.76) for this test has be found in patients with AD.Citation31 In elderly people, high test–retest reliability (ICC2.1 = 0.97) within the same session has been reported.Citation32 Excellent intrarater and inter-rater reliability (ICC = 0.99) has been described.Citation30 These tests have been able to discriminate between healthy female controls and female patients with dementia.Citation27
Statistical analysis
The Student’s t-test was used to compare the performance of the controls and patients after a test of normality had been done. Analysis of covariance was used to investigate the association between alternating forearm movements and dementia, using the nondemented subjects as controls. The model was also adjusted for age. The level of significance was set to P < 0.05. To describe the test–retest and intrarater reliabilities, ICC2.1, and the 95% confidence intervals were calculated. The Spearman correlation coefficient was used for analyses of association between number of cycles and tests of mobility. All calculations were performed using the SPSS software version 11.0 (SPSS Inc, Chicago, IL, USA).
Results
Most of the 34 patients and all controls were able to perform the test of fast alternating forearm movements, with a few exceptions. One patient was not able to sustain the alternating pronation and supination movements for 15 seconds with the right arm, and three patients were not able to complete the test with the left arm because of pain or an earlier arm fracture.
In the total sample, the patients, although younger than the controls, were significantly slower and performed fewer numbers of cycles at both 10 and 15 seconds compared with controls, with the greatest difference seen at 15 seconds (). In the groups aged 80–89 years, with the same mean age, patients remained slower (). The healthy controls were significantly faster and completed a larger number of cycles at both 10 and 15 seconds. A regression model including the total sample showed the same age-adjusted results, namely that demented patients were slower in performing forearm movements than controls ().
Table 2 Association between alternating forearm movements and dementia, adjusted for age and dementia in a linear regression model
In the group aged 80–89 years, there was a significant negative association between number of cycles and time taken to walk 2 × 15 m and perform the GUG test at a self-selected speed for the patients but not for the controls (). Thus, performing fewer cycles (because of slow movement speed) during the test of fast alternating forearm movements was associated with slower walking speed among the patients. The correlation between time taken to perform the physical tests related to lower extremity function and number of cycles at 10 seconds are exemplified in . In the controls, there were a significant positive correlation between time taken to walk 2 × 15 m at a self-selected speed and the test of fast alternating forearm movements ().
Table 3 Correlation between measures of alternating forearm movements, expressed as number of cycles at 10 and 15 seconds, and lower extremity function in female controls versus females with dementia
Figure 1 Association between physical performance in upper extremities (number of cycles, left hand) and lower extremities (Get Up and Go and walking 2 × 15 m, self-selected speed) in 80–89-year-old women with dementia.
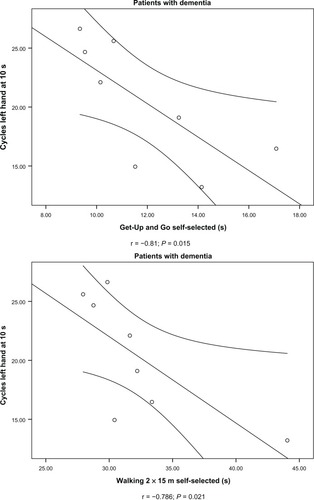
In the subanalysis of patients according to subtype of dementia, there was a significant negative association between numbers of cycles and time taken to walk 2 × 15 m at both speeds and for the GUG test at maximal but not at a self-selected speed in patients with probable AD but not for patients in the group with other types of dementia. Thus, in patients with probable AD, slowing of the upper extremities (lower number of cycles) is associated with slower walking speeds ().
Table 4 Correlation between measures of alternating forearm movements, expressed as number of cycles at 10 and 15 seconds, and lower extremity function in women with probable AD and other types of dementia
In the test–retest at one session, the ICC ranged from 0.74 to 0.93 for controls and from 0.88 to 0.94 for patients (). Intrarater reliability in ten healthy younger women, over three different sessions, showed ICCs ranging from 0.70 to 0.79 between the first and second session and from 0.75 to 0.94 between the second and third session for both hands. The ICCs were somewhat lower between the first and third session, ranging from 0.41 to 0.56. However, there were no differences in the mean number of cycles between the three test sessions for the right hand at 10 seconds (29.1 versus 29.8 versus 31.0) and 15 seconds (42.3 versus 43.9 versus 45.1). Corresponding values for the left hand were 25.3 versus 26.9 versus 27.6 at 10 seconds and 36.5 versus 39.2 versus 39.7 at 15 seconds, respectively.
Table 5 Test–retest of alternating forearm movements in older female controls versus females with dementia
Discussion
This study shows that a quantitative test of fast alternating forearm movements is feasible in female patients with dementia who can understand and follow simple verbal commands. Almost all the 26 patients, in spite of their advanced age and cognitive decline, were able to perform the test. The patients showed impairment in ability to perform rapidly alternating forearm movements and were slower than healthy women, adjusted for age. Cognitive and presumably behavioral factors could have influenced the test situation. However, the limiting factor for the few patients who could not complete the test seemed to be related more to physical problems than to cognition. The finding that the demented patients generally cooperated well and had no difficulty with comprehension and execution of a test of movement speed and limb coordination is in agreement with that reported by others.Citation2,Citation4 Kischka et al,Citation2 using electrophysiological techniques, found that tasks related to bradykinesia in the upper extremities could be completed by all but the most severely demented AD patients. Franssen et al,Citation4 using timed clinical measurements, found participants with mild AD to cooperate well but to be more distractible and to have some mild motor impersistence.
Our demented patients were slower than controls when performing alternating forearm movements, which is in agreement with other studies.Citation3,Citation4 Franssen et alCitation4 used timed trials of palmar to dorsal turns of the hand at maximum speed and found significantly greater prevalence of decreased performance in subjects with mild cognitive impairment and patients with mild AD compared with cognitively intact subjects. Kluger et al,Citation3 using the number of correct turns in 10 seconds of alternating hand movements, found the mean value of correct turns to be lower in mildly cognitively impaired patients and patients with mild AD compared with healthy subjects. The reduction in frequency (lower number of cycles) found in our test of fast alternating forearm movements is interpreted as a reduced ability to execute rapid alternating movements with the limb, ie, a slowing of motor performance in the upper extremities.
Hemsdorfer et alCitation20 found a difference between right and left hand performance, which is in agreement with our findings. The mean values for number of cycles performed by both patients and controls show that they completed a larger number of cycles with the right hand, and thus were faster with this hand compared with the left. All subjects were asked about hand preference and almost all, except two of the older female controls, reported being right-handed. No separate test for assessing hand dominance was performed. Fast alternating forearm movements represent a complex motor task that demands attention, timing, sequencing, and self -monitoring of motor behavior while changing the direction of movement during performance. A possible explanation for right hand performance being better than left hand performance might be that performing the test with the nondominant hand is less automatic and therefore more cognitively demanding.
In the test of intrareliability, we found a mean value for the number of cycles at three different sessions for the right hand and left hand in younger healthy females to be in good agreement with other reports.Citation3,Citation33 Our subjects were somewhat slower with the left hand, which is in agreement with the finding by others of a difference in right and left arm performance.Citation20,Citation33 Beuter et alCitation33 found a mean frequency of 3.1 Hz for the right hand and 3.0 Hz for the left hand in their controls (mean age 54 ± 4 years). Kluger et al,Citation3 using a clinical test of alternating hand movements as part of a test battery, found a mean value of number of correct turns in 10 seconds to be 29.1 in healthy men and women (mean age 69.9 ± 8.6 years). The slight difference in frequencies, compared with our study, might be due to the different methodology used and age and gender differences.
Our findings of a somewhat lower intrarater reliability coefficient over time in younger women are in agreement with the findings of Hermsdorfer et al.Citation20 They found the reliability coefficient to be r = 0.65 (P < 0.001) when studying test–retest reliability in the dominant hand about 4 weeks after the first examination. Their subjects were tested only twice, whereas our subjects were tested on three occasions. We found a slight increase in the mean numbers of cycles in three trials over a 3-week period, which might indicate a learning effect of frequent testing over time.
We found test–retest reliability at one session to be high for both the older female patients and the controls. Franssen et alCitation4 also found high reliability when testing alternating pronation and supination of the forearm at maximum speed in a clinical setting. In a group of older subjects at stages 1–6 of the Global Deterioration Scale (representing stages from no memory loss through to moderately severe dementia), high ICC coefficients were found for both interrater reliability (0.72) and intrarater reliability (0.97). Our findings and those of others indicate that quantification of forearm pronation and supination is a reliable method and can be recommended as a test of upper extremity function both in older patients and healthy subjects. However, in this study, intrarater reliability was not assessed in the elderly female subjects due to low compliance and lack of willingness to come back several times.
In the group aged 80–89 years, we found associations between movement speed in the upper and lower extremities for patients but not for controls. We found the correlation coefficients between alternating forearm movements and self-selected walking speed to be higher than the coefficients for maximal walking speed in the total sample. There is probably an increased variation in the intersubject maximal walking speed that would explain the reduced correlations with alternating forearm movements compared with self-selected walking speed.
When the association was studied according to type of dementia, a high correlation coefficient was found between alternating forearm movements and lower extremity function, but only in the group of patients with probable AD. No associations were found in the group with other types of dementia. The explanation for this observation is probably that the group was heterogeneous, and consisted of patients with various types of dementia other than AD.
Correlations of forearm movements and measures of cognition or dementia severity were not analyzed in this study, because of its small sample size and thus low power. Another limitation is that we only included women. Men have been found to be faster than women on performing alternating forearm movements,Citation20 and Beuter et alCitation21 found an effect of both age and gender.
Motor function differs between healthy older adults and adults with cognitive impairment and dementia.Citation8 Complex motor functions as well as motor functions associated with executive function are affected early in the process of cognitive decline, and patients with dementia perform worse than healthy older subjects.Citation8 Our finding that motor slowing affects both the upper and lower extremities in patients with dementia is in agreement with reports from others.Citation8,Citation16,Citation34,Citation35 Our interpretation of this association is that a test of alternating forearm movements might be useful as a proxy for assessment of lower extremity function, given that walking disability is common among demented patients.Citation13 We acknowledge that bradykinesia is only one of many causes (eg, pain, fractures, arthritis) of impaired gait and impaired mobility. However, alternating forearm movements, as a marker for bradykinesia, could provide additional information on decline in motor function in general and be a proxy for lower extremity function when lower extremity testing is not possible. This research device, assessing alternating forearm movements, has produced promising results, and is primarily recommended for use in longitudinal and interventional research. Future studies will reveal if it is usable in clinical settings.
The proportion of elderly subjects has been small or lacking in previous studies.Citation3,Citation4,Citation20,Citation21 To our knowledge, no one has studied rapid alternating forearm movements in women with the same advanced age as our subjects, both healthy and demented, or in regard to right and left hand performance. Only a few earlier studies have shown that alternating forearm movements can be used in subjects suffering from moderate to severe dementia. This study confirms that alternating forearm movements are applicable in patients with dementia. This is demonstrated by data both from test–retest with high ICCs, thus showing the test to be reliable, and in the significant differences found between patients and controls, showing that the test can discriminate. Patients with dementia are frail and have impaired motor function. We have shown that impaired motor function can be assessed. This information is important in patient care to avoid falls and fractures.
Earlier clinical studiesCitation3,Citation4 using rapid alternating hand movements did not report results separately for the right and left hand, which might be questioned, because othersCitation20,Citation33,Citation36 have found a difference between left and right hand performance. We found significant associations between number of cycles and mobility tests for the left hand but not for the right hand. The associations for the right hand are in the same direction though, and lack of significance might be due to a lack of statistical power. We found the same type and magnitude of association for number of cycles at 10 seconds as well as 15 seconds in both controls and patients. Due to information about motor impersistence,Citation4 and the fact that fatigue might affect test performance in demented patients, a testing duration of 10 seconds would be recommended.
Conclusion
Bradykinesia in the upper extremities can be evaluated in women with dementia by a quantitative measure of alternating forearm pronation and supination at fast speed. Poorer performance on the test of alternating forearm movements is associated with slower movement speed and longer time taken to perform tests related to lower extremity function. In demented patients who are unable to walk, it may be useful to study movement speed in the arms as a functional parameter of bradykinesia and as a possible proxy for lower extremity function.
Acknowledgements
The authors want to thank Styrbjörn Lindberg, Electronic Engineering, Lund University, Lund, Sweden, for offering support and making a major contribution to the development of the technical equipment. The study was supported by the Medical Faculty, Lund University, Sweden.
Disclosure
The authors report no conflicts of interest in this work.
References
- KlugerAGianutsosJGGolombJWagnerAJrWagnerDScheurichSClinical features of MCI: motor changesInt Psychogeriatr2008201323918072982
- KischkaUMandirASGhikaJGrowdonJHElectrophysiologic detection of extrapyramidal motor signs in Alzheimer’s diseaseNeurology1993433 Pt 15005058450990
- KlugerAGianutsosJGGolombJPatterns of motor impairement in normal aging, mild cognitive decline, and early Alzheimer’s diseaseJ Gerontol B Psychol Sci Soc Sci1997521P28P399008673
- FranssenEHSourenLETorossianCLReisbergBEquilibrium and limb coordination in mild cognitive impairment and mild Alzheimer’s diseaseJ Am Geriatr Soc199947446346910203123
- EconomouAPapageorgiouSGKarageorgiouCVassilopoulosDNonepisodic memory deficits in amnestic MCICogn Behav Neurol20072029910617558253
- OnderGPenninxBWLapuertaPChange in physical performance over time in older women: the Women’s Health and Aging StudyJ Gerontol A Biol Sci Med Sci2002575M289M29311983722
- ScherderEEggermontLSwaabDGait in ageing and associated dementias; its relationship with cognitionNeurosci Biobehav Rev200731448549717306372
- BottiggiKHarrisonAThe association between change in motor function and cognition in older adults: a descriptive reviewPhys Ther Rev200813291101
- BuracchioTDodgeHHHowiesonDWassermanDKayeJThe trajectory of gait speed preceding mild cognitive impairmentArch Neurol201067898098620697049
- MarquisSMooreMMHowiesonDBIndependent predictors of cognitive decline in healthy elderly personsArch Neurol200259460160611939895
- WaiteLMGraysonDAPiguetOCreaseyHBennettHPBroeGAGait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons StudyJ Neurol Sci2005229–2308993
- van GoolCHPicavetHSDeegDJTrends in activity limitations: the Dutch older population between 1990 and 2007Int J Epidemiol20114041056106721324941
- SlaughterSEEliasziwMMorganDDrummondNIncidence and predictors of excess disability in walking among nursing home residents with middle-stage dementia: a prospective cohort studyInt Psychogeriatr2011231546420199700
- SheridanPLHausdorffJMThe role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s diseaseDement Geriatr Cogn Disord200724212513717622760
- ScherderEDekkerWEggermontLHigher-level hand motor function in aging and (preclinical) dementia: its relationship with (instrumental) activities of daily life – a mini-reviewGerontology200854633334118997468
- HebertLEScherrPAMcCannJJBieniasJLEvansDAChange in direct measures of physical performance among persons with Alzheimer’s diseaseAging Ment Health200812672973419023724
- HebertLEBieniasJLMcCannJJScherrPAWilsonRSEvansDAUpper and lower extremity motor performance and functional impairment in Alzheimer’s diseaseAm J Alzheimers Dis Other Demen201025542543120484749
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM III-R)Washington, DCAmerican Psychiatric Association1987
- ElmstahlSRosenIPostural hypotension and EEG variables predict cognitive decline: results from a 5-year follow-up of healthy elderly womenDement Geriatr Cogn Disord1997831801879137897
- HermsdorferJMarquardtCWackSMaiNComparative analysis of diadochokinetic movementsJ Electromyogr Kinesiol19999428329510437981
- BeuterAEdwardsRdeGeoffroyAMerglerDHundnellKQuantification of neuromotor function for detection of the effects of manganeseNeurotoxicology1999202–335536610385896
- ObristWDThompsonHKJrWangHSWilkinsonWERegional cerebral blood flow estimated by 133-xenon inhalationStroke1975632452561154462
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s DiseaseNeurology19843479399446610841
- RomanGCTatemichiTKErkinjunttiTVascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International WorkshopNeurology19934322502608094895
- BrunAEnglundEGustafsonLConsensus statement: clinical and neuropathological criteria for fronto-temporal lobe dementiaJ Neurol Neurosurg Psychiatry1994574164188163988
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state”. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res19751231891981202204
- Bramell-RisbergEJarnloGBMinthonLElmstahlSLower gait speed in older women with dementia compared with controlsDement Geriatr Cogn Disord200520529830516166777
- JarnloGBNordellEReliability of the modified figure of eight – a performance test for elderly womenPhysiother Theory Pract2003193543
- MathiasSNayakUSIsaacsBBalance in elderly patients: the “get-up and go” testArch Phys Med Rehabil19866763873893487300
- PodsiadloDRichardsonSThe timed “Up and Go”: a test of basic functional mobility for frail elderly personsJ Am Geriatr Soc19913921421481991946
- SuttanonPHillKDDoddKJSaidCMRetest reliability of balance and mobility measurements in people with mild to moderate Alzheimer’s diseaseInt Psychogeriatr20112371152115921489342
- SteffenTMHackerTAMollingerLAge- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up and Go Test, and gait speedsPhys Ther200282212813711856064
- BeuterAMerglerDde GeoffroyADiadochokinesimetry: a study of patients with Parkinson’s disease and manganese exposed workersNeurotoxicology19941536556647854603
- OttBRElliasSALannonMCQuantitative assessment of movement in Alzheimer’s diseaseJ Geriatr Psychiatry Neurol19958171757710652
- GoldmanWPBatyJDBucklesVDSahrmannSMorrisJCMotor dysfunction in mildly demented AD individuals without extrapyramidal signsNeurology199953595696210496252
- HermsdorferJGoldenbergGIpsilesional deficits during fast diadochokinetic hand movements following unilateral brain damageNeuropsychologia200240122100211512208006