Abstract
Background
Considerable controversy exists regarding the contribution of mineral/bone metabolism abnormalities to the association between cardiovascular diseases (CVDs) and osteoporotic fractures.
Aims and methods
To determine the relationships between mineral/bone metabolism biomarkers and CVD in 746 older patients with hip fracture, clinical data were recorded and serum concentrations of parathyroid hormone (PTH), 25-hydroxyvitamin D, calcium, phosphate, magnesium, troponin I, parameters of bone turnover, and renal, liver, and thyroid functions were measured.
Results
CVDs were diagnosed in 472 (63.3%) patients. Vitamin D deficiency was similarly prevalent in patients with (78.0%) and without (82.1%) CVD. The CVD group had significantly higher mean PTH concentrations (7.6 vs 6.0 pmol/L, P < 0.001), a higher prevalence of secondary hyperparathyroidism (SPTH) (PTH > 6.8 pmol/L, 43.0% vs 23.3%, P < 0.001), and excess bone resorption (urinary deoxypyridinoline corrected by creatinine [DPD/Cr] > 7.5 nmol/μmol, 87.9% vs 74.8%, P < 0.001). In multivariate regression analysis, SHPT (odds ratio [OR] 2.6, P = 0.007) and high DPD/Cr (OR 2.8, P = 0.016) were independent indictors of CVD. Compared to those with both PTH and DPD/Cr in the normal range, multivariate-adjusted ORs for the presence of CVD were 17.3 (P = 0.004) in subjects with SHPT and 9.7 (P < 0.001) in patients with high DPD/Cr. CVD was an independent predicator of SHPT (OR 2.8, P = 0.007) and excess DPD/Cr (OR 2.5, P = 0.031). CVD was predictive of postoperative myocardial injury, while SHPT was also an independent predictor of prolonged hospital stay and in-hospital death.
Conclusion
SHPT and excess bone resorption are independent pathophysiological mediators underlying the bidirectional associations between CVD and hip fracture, and therefore are important diagnostic and therapeutic targets.
Introduction
There is little doubt that cardiovascular diseases (CVDs) and osteoporotic fractures are two important public health-care problems worldwide with a high impact on morbidity and mortality and increasing prevalence as the population ages. Although there are similarities in bone formation and vascular calcification and both disorders share several common risk factors (aging, female sex, low physical activity, smoking, alcohol overuse, renal and metabolic diseases),Citation1 traditionally they are regarded as separate but coexisting diseases.Citation2 In the last decade, emerging data suggest a positive association between CVD and osteoporosis beyond these factors and indicate that this link may have common underlying biological mechanisms.Citation3–Citation5 However, there is still a considerable amount of controversial literature on this important topic. Many studies report that low bone mineral density (BMD) or bone mass, independently of age and classical cardiovascular risk factors, is related to increased cardiovascular morbidity,Citation4,Citation6–Citation8 especially vascular calcification,Citation5,Citation9,Citation10 as well as increased cardiovascular and all-cause mortality.Citation11,Citation12 Conversely, bone loss and fracture risk is significantly increased in subjects with CVD.Citation13–Citation16 In other studies, however, no significant associations between BMD and CVD or mortality were observed.Citation17,Citation18 Peripheral arterial disease was not associated with risk of hip fracture (HF) during a 21-year follow-up.Citation19
Among several potential pathophysiological mechanisms linking CVD and osteoporotic fractures, dysregulation in mineral and bone metabolism appears as the most important, but published data are conflicting. Disturbances in mineral and bone metabolism well established in osteoporosis have been implicated in the pathogenesis of CVD. These include vitamin D deficiency,Citation20–Citation26 abnormalities in serum levels of parathyroid hormone (PTH),Citation27–Citation34 calcium and phosphorus homeostasis,Citation35–Citation40 and in bone-turnover markers.Citation41–Citation46 Other researchers, however, did not support these findings and concluded that the evidence for association of these parameters and CVD is currently insufficient.Citation35,Citation40,Citation41,Citation47–Citation50 In one study, for example, the development of incident coronary artery disease (CAD) in men without chronic kidney disease (CKD) was not associated with PTH, phosphorus, or fibroblast growth factor 23 (FGF23).Citation49 The contribution and relative importance of specific abnormalities in mineral/bone metabolism in the genesis of CVD remain unclear.
The relationship between CVD and HF, the most devastating consequence of osteoporosis, remains uncertain. Although CVDs are the main causes of morbidity and death after HF surgery,Citation51–Citation53 their prevalence in patients with HF has not been reported. Previous studies have focused mostly on individual biomarkers of mineral or bone metabolism, and were often limited to women or patients with HF, or subjects with CVD. To our knowledge, no previous investigation has simultaneously evaluated parameters of mineral and bone metabolism in older HF patients with respect to CVDs.
The aims of this study of a cohort of consecutive older patients with osteoporotic HF were (1) to examine the prevalence of CVDs, (2) to compare the markers of mineral and bone metabolism in those with and without CVD, (3) to determine whether these factors explain similarities and differences between the two groups, and (4) to estimate the contribution of CVD and mineral/bone biomarkers to short-term outcomes.
Materials and methods
Study patients
A total of 847 consecutive patients aged 60 years and older who were admitted to the Canberra Hospital with low-trauma HF and underwent surgery were investigated. After the exclusion of 86 patients with pathological HF (metastatic bone cancer, multiple myeloma, Paget’s disease) or primary hyperparathyroidism and 15 patients in whom data were incomplete, a total of 746 patients (536 women and 210 men) were included in the analysis.
All patients or their guardians gave informed consent to undergo examination and surgical treatment. The study was conducted according to the Helsinki Declaration and approved by the regional Human Research Ethics Committee.
Clinical data collection
In all patients, a detailed medical history and full physical examination was performed. The presence of comorbidities with a focus on CVD (hypertension, CAD, previous myocardial infarction, stroke or transient ischemic attack, atrial fibrillation, peripheral vascular disease, and congestive heart failure) was identified based on clinical manifestations, review of hospital medical records, physicians’ progress notes/letters, and medications prescribed. The data collected prospectively included medical, demographic, lifestyle, and residential characteristics, as well as in-hospital management, perioperative complications, and short-term outcomes recorded.
Outcome measures included (1) postoperative myocardial injury as defined by cardiac troponin I (cTnI) rise, (2) prolonged length of stay (LOS) (≥20 days), (3) discharge to a permanent residential care facility for patients who were admitted from home, and (4) all-cause in-hospital death.
Sample collection and laboratory analyses
In each patient, venous blood and second morning urine samples were collected under standardized conditions after a 12-hour overnight fast, usually within 24 hours after arrival at the emergency department. After centrifugation of blood, one serum sample as well as the urine sample were frozen and stored at −70°C until further analysis. The following biochemical parameters of mineral and bone metabolism were determined: serum concentrations of 25-hydroxyvitamin D (25[OH]D), intact PTH, total calcium, phosphate, magnesium, osteocalcin (OC), and bone-specific alkaline phosphatase (BAP) as markers of bone formation and urinary concentrations of deoxypyridinoline (DPD/Cr), and cross-linked N-telopeptide of type 1 collagen (NTx) as markers of bone resorption (both corrected for urinary creatinine concentrations in the same sample).
Serum 25(OH)D was measured by radioimmunoassay kit (Dia Sorin, Stillwater, MN, USA) and intact PTH by two-site chemiluminescent enzyme-linked immunoassay on DPC Immulite 2000 (Diagnostic Products, Los Angeles, CA, USA). Serum OC was determined by electrochemiluminescent immunoassay (Elecsys 1010; Roche Diagnostics, IN, USA), serum BAP by Metra BAP enzyme-linked immunosorbent assay (Quidel, San Diego, CA, USA), urinary cross-linked N-telopeptide of type I collagen (NTx) by enzyme-linked immunosorbent assay (Wampole Labs, Princeton, NJ, USA), and urinary DPD by two-site chemiluminescent enzyme-labeled immunoassay (DPC Immulite 2000; Diagnostic Products). All samples were analyzed with commercially available kits of the same lot number according to the manufacturer’s protocol, and blind to any clinical information. In these methods, both the intra-and interassay coefficient of variations (CVs) ranged from 2.1% to 12.7%.
On the day of arrival and blood sampling, the following routine laboratory tests also were performed: full blood count, creatinine, urea nitrogen, albumin, liver and thyroid function tests (by standard automated methods), and serum cTn, by two-step chemiluminescent microparticle immunoassay (Chemiflex; Abbott Labs, Mississauga, ON, Canada). Full blood count and serum cTnI were also assessed within 24 hours postoperatively and subsequently if clinically indicated. Serum calcium concentrations were corrected for serum albumin. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation.Citation54
For the analyses, deficiency of vitamin D was defined as 25(OH)D < 50 nmol/L and moderate–severe deficiency as 25(OH)D < 25 nmol/L, based on current guidelines. Secondary hyperparathyroidism (SHPT) was defined as elevated serum PTH (>6.8 pmol/L, the upper limit of the laboratory reference range). For levels of bone-turnover markers, we used the standard laboratory reference ranges and data provided by the manufacturer.
Statistics
Stata software version 10 (StataCorp, College Station, TX, USA) was used for all statistical analyses. Descriptive statistics included means (± standard deviation of mean) for continuous variables or percentages for categorical variables. Comparisons between groups were performed using analysis of variance and Student’s t-test (for continuous variables) and χ2 test (for categorical variables). Pearson’s correlation coefficient with log-transformed data (to achieve normal distribution) was used to study the correlation between variables; Bonferroni and Sidak adjustments for multiplicity were performed. Univariate and multivariate linear regression analyses were performed to determine the associations between different parameters of mineral and bone metabolism and presence of CVD as well as short-term outcomes; all potential confounding variables (demographic, clinical, biochemical) with statistical significance ≤ 0.15 on univariate analyses were included in multivariate analysis. To quantify the significance of multicollinearity phenomena in regression analysis, the variance inflation factor was calculated. Two-sided P < 0.05 values were considered statistically significant.
Results
General characteristics of study patients
Demographic and clinical characteristics of the study patients are shown in . A history of CVD was present in 472 (63.3%) of 746 patients included in the study. This group was older (on average 2.2 years) and had a higher proportion of patients with American Society of Anesthesiologists (ASA) score ≥ 3 (+22%) and renal impairment (CKD ≥ 3, +10.9%). No significant differences between patients with and without CVD were observed in regard to sex, residential status, use of walking device, current and former smoking status, alcohol consumption, dementia, type 2 diabetes mellitus, chronic obstructive pulmonary disease, Parkinson’s disease, thyroid dysfunction, anemia, or hypoalbuminemia.
Table 1 Socio-demographic and clinical characteristics of older patients with hip fracture included in the study (n = 746)
The group with CVD comprised 337 (45.2% of the total cohort) patients with hypertension, 171 (22.9%) with CAD, including 39 (5.2%) with a previous myocardial infarction, 100 (13.4%) with history of stroke, 55 (7.4%) with a history of transient ischemic attack, and 99 (13.2%) with atrial fibrillation (AF). Among the patients with CVDs, one condition was present in 290 (61.4%), two in 121 (25.6%) and three or more in 66 (14.0%) subjects. Chronic heart failure was diagnosed in 384 (59.5% of the total cohort, 81.4% of patients with CVD). Hypertension was associated with CAD (Pearson correlation r = 0.146, P = 0.014) and history of stroke (r = 0.186, P = 0.002). CAD was also associated with history of stroke (r = 0.221, P = 0.033) and AF (r = 0.221, P < 0.001).
Among patients with CVD, angiotensin-converting enzyme (ACE) inhibitors were used by 26.5%, angiotensin 2-receptor blockers (ARBs) by 23.3%, beta blockers by 27.4%, calcium-channel blockers by 12.7%, and statins by 21.5%. Antiosteoporotic medications were used as follows: vitamin D supplement by 19.1% of patients with CVD and 17.7% without CVD, calcium supplement by 22.5% and 19.9%, bisphosphonate by 13.5% and 12.3%, and raloxifene by 1.12% and 0.72%, respectively.
Parameters of mineral and bone metabolism in patients with and without cardiovascular disease
The mean values of serum 25(OH)D concentrations in the group of patients with CVD in total and with each analyzed cardiovascular condition separately were low and did not differ from patients without CVD (). In contrast, serum PTH levels were significantly higher in all groups with CVD compared to non-CVD patients. Patients with hypertension and CAD also had higher serum phosphate levels, and subjects with CAD, history of stroke, and AF demonstrated higher magnesium levels.
Table 2 Parameters of mineral and bone metabolism in older hip-fracture patients with and without cardiovascular disease
The prevalence of vitamin D deficiency (25[OH] D < 50 nmol/L) was high in both patients without CVD and in the total group with CVD (82.1% and 78.0%, respectively), but slightly lower in patients with CAD (73.1%, P = 0.032) and history of stroke (68.0%, P = 0.006). The percentage of patients with moderate–severe vitamin D deficiency (25[OH] D < 25 nmol/L) did not differ between groups either: 28.9% in the non-CVD group and 31.2% among subjects with CVD, including those with hypertension (34.0%), CAD (33.3%), history of stroke (27.0%), and AF (34.5%).
Conversely, compared to patients without CVD, the proportion of subjects with elevated PTH levels (>6.8 pmol/L) indicating SHPT was about two times higher among the patients with CVD (43% vs 23.3%, P < 0.001), and the highest percentages were observed in patients with CAD (53.8%) and history of stroke (51.0%), despite the lower prevalence of vitamin D deficiency in these two groups. Among patients with both vitamin D deficiency and elevated PTH, there was a higher prevalence of subjects with CVD (35.5% vs 21.1%, P = 0.024), including those with hypertension (37.7%, P = 0.012), CAD (46.4%, P = 0.003), history of stroke (37.8%, P = 0.0.13), and AF (35.5%, P = 0.041). Of 267 patients (35.8% of the total cohort) with SHPT, 203 (76.03%) had CVD. Subjects with CVD and SHPT compared to those with normal PTH status (12.3 ± 1.8 vs 4.0 ± 1.6 pmol/L) had a lower level of serum calcium (2.24 ± 0.12 vs 2.30 ± 0.12; P = 0.002) and eGFR (54.4 ± 24.6 vs 68.1 ± 20.0 mL/minute/1.73 m2; P < 0.001), but there were no differences in age or other biochemical and clinical parameters, except a higher proportion of patients with ASA ≥ 3 among the first group (88.3% vs 73.9%; P = 0.038).
The mean serum levels of both bone-formation markers (OC and BAP) and the OC/BAP ratios (indicator of osteoblastic differentiation) did not differ among the groups with and without CVD. Low OC levels (<14 ng/mL, below reference range) were observed in approximately half of the patients (53.1% without CVD and 51.7% with CVD), while low BAP levels (<14 IU) occurred in 9.7% (13.3% and 7.7%, respectively). The different behaviors of OC and BAP, each of which reflects different aspects of bone metabolism and different stages of osteoblastic differentiation, have previously been observed in other clinical settings.Citation55
The urinary excretion of DPD adjusted for creatinine (DPD/Cr), a bone-resorption marker, was significantly higher in patients with CAD (31.9%), history of stroke (27.6%), atrial fibrillation (17.2%), and in the total group with CVD (14.7%). However, the association between CVD and urinary NTx/Cr, the collagen product which resembles DPD, was not significant. In other studies, these two bone-resorption markers have also shown clinically distinct properties.Citation56–Citation58 The prevalence of elevated DPD/Cr (>7.5 nmol/μmol) was significantly higher in the group with CVD (87.9% vs 74.8%; P < 0.001), including those with hypertension (87.8%, P < 0.001), CAD (91.8%, P < 0.001), history of stroke (94%, P < 0.001), and AF (90%, P = 0.002). Among patients with CVD and high DPD/Cr excretion compared to those with DPD/Cr in the normal range, the proportion of subjects with SHPT (40.9% vs 66.7%; P = 0.038) and low serum OC (49.6% vs 83.3%; P = 0.007) was lower.
With an increasing number of CVDs, there was a gradient increase in mean levels of serum PTH (in subjects with one CVD 7.4 pmol/L, with two CVDs 9.1 pmol/L, and with three or more CVDs 10.2 pmol/L; P for trend = 0.001), as well as in the prevalence of secondary hyperparathyroidism (35.5%, 45.5%, and 60.0%, respectively; P for trend = 0.001). Similarly, increasing the number of CVDs increased the mean levels of urinary DPD/Cr (12.1, 14.3, and 15.8 nmol/μmol, respectively; P for trend = 0.030) and the proportion of patients with abnormally high bone-resorption status (83.8%, 88.0%, and 95.2%, respectively; P for trend = 0.016).
Correlations between biomarkers of mineral and bone metabolism by cardiovascular disease
To evaluate further the associations between CVD and altered parameters of mineral and bone metabolism in HF patients, Pearson correlation coefficients were estimated separately in subjects with and without CVD. Values for PTH, 25(OH)D, mineral-and bone-metabolism parameters, and eGFR were logarithmically transformed before analysis. This analysis revealed similarities and differences in patients with and without CVD. In both groups, serum PTH levels were positively correlated with age and inversely with serum calcium, eGFR, and cervical type of HF ().
Table 3 Pearson correlation coefficients between serum PTH levels and selected clinical, mineral, and bone metabolism factors in older hip-fracture patients with and without cardiovascular disease
However, only in patients with CVD, PTH correlated positively with BAP, troponin I, and in-hospital death, while only in the non-CVD group higher PTH levels were significantly associated with female sex and dementia. BAP correlated positively with serum magnesium (r = 0.176, P < 0.022) in the CVD group and with calcium (r = 0.349, P < 0.001) and phosphate (r = 0.286, P = 0.049) in patients without CVD. Only in the later group, OC correlated positively with BAP (r = 0.264, P = 0.009), calcium (r = 0.240, P = 0.020), and magnesium (r = 0.279, P = 0.007). In both groups, OC correlated inversely with eGFR (r = −0.456, P < 0.001 for the CVD group and r = −0.202, P = 0.046 for the non-CVD group) and in-hospital death (r = −0.210, P = 0.006 and r = −0.254, P = 0.012, respectively). In both groups, DPD/Cr positively correlated with serum phosphate (r = 0.165, P = 0.048 and r = 0.268, P = 0.014, respectively), and the two urinary bone-resorption markers (DPD/Cr and NTx/Cr) were significantly intercorrelated (r = 0.446, P < 0.001 and r = 0.349, P < 0.001, respectively). 25(OH)D correlated negatively only with NTx/Cr only in the non-CVD patients (r = −0.260, P = 0.021). There was no correlation between PTH and resorption markers (DPD/Cr and NTx/Cr), which is consistent with previous reports.Citation59,Citation60
Factors associated with cardiovascular disease
In univariate analyses of twelve biochemical (25[OH]D, PTH, calcium, phosphate, magnesium, OC, BAP, DPD/Cr, NTx/Cr, hemoglobin, albumin, eGFR) and seven clinical (age, sex, HF type, dementia, smoking, alcohol overuse, use of walking device) parameters, presence of CVD was significantly associated only with age (OR 1.03, 95% confidence interval [CI] 1.00–1.07; P = 0.034), high bone resorption (urinary DPD/Cr > 7.5 nmol/μmol, OR 2.44, 95% CI 1.23–4.87; P = 0.011), and elevated serum PTH levels (>6.8 pmol/L, OR 2.36, 95% CI 1.35–4.11; P = 0.003). PTH as a continuous variable was also associated with CVD (OR 1.06, 95% CI 1.00–1.13; P = 0.033), indicating that the risk of CVD increases by 6% per 1 pmol/L increment in PTH.
To evaluate further whether higher serum PTH levels were associated with CVD, and given the wide range of PTH in our cohort, age-, sex-and 25(OH)D-adjusted ORs were estimated by quartiles of PTH concentration. For the total CVD group as well as for each of the four studied diseases separately, the adjusted ORs increased with quartiles of serum PTH (P for trends < 0.01 in all groups). Compared to the first quartile (PTH < 3.5 pmol/L), in the fourth quartile (PTH > 8.7 pmol/L) the OR for the total CVD group was 2.5 times higher (95% CI 1.12–5.61; P = 0.006), and the highest values were for CAD (OR 3.34, 95% CI 1.35–8.33; P = 0.009) and history of stroke (OR 2.93, 95% CI 1.04–8.21; P = 0.011).
Using serum PTH at a cutoff of 6.8 pmol/L, it was possible to discriminate between presence and absence of CVD with a sensitivity of 42.9%, specificity of 75.8%, positive predictive value of 76.8% and negative predictive value of 41.6%, and using PTH > 8.7 pmol/L with 30.5%, 84.2%, 78.3%, and 39.4%, respectively. A sensitivity of 88.0%, specificity of 25.0%, positive predictive value of 66.7%, and negative predictive value of 55.0% were yielded using DPD/Cr > 7.5 nmol/μmol. These data suggest that SPTH (more specific) and high bone resorption (more sensitive) may be useful indicators of CVD (if previously not diagnosed).
We then examined which factors are independently associated with CVD. Multiple logistic regression analysis with PTH, 25(OH)D, DPD/Cr, albuminemia, eGFR, smoking status, alcohol consumption, HF type, dementia, and sex entered as independent categorical variables and corrected for age showed that elevated serum PTH (>6.8 pmol/L), high urinary DPD/Cr (>7.5 nmol/μmol), and advanced age were the only significant indicators of presence of CVD (), and together explained 32.1% of the variability in presence of CVD. Elevated PTH levels were independently and significantly associated with each of the analyzed CVDs (), and the OR ranged between 1.91 (for AF) and 3.43 (for CAD). Of note, in our models we did not include medication use (see below) as independent variables, as their use was a consequence of presence of CVD.
Table 4 Independent factors associated with the presence of cardiovascular disease in older hip-fracture patients
Table 5 Multivariate-adjusted odds ratio for the presence of cardiovascular disease in older hip-fracture patients with elevated serum parathyroid hormone levels (>6.8 pmol/L)
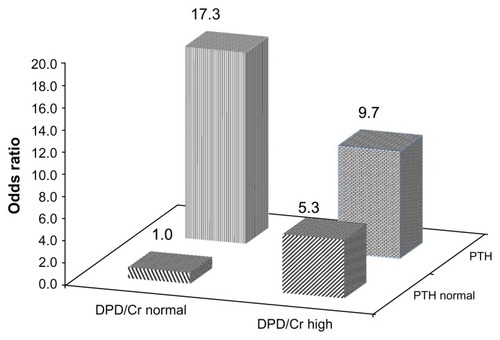
Further, we examined the unique and combined effects of abnormally high PTH and DPD/Cr levels as indicators of presence of CVD (). Compared to subjects with normal serum PTH and urinary DPD/Cr levels (reference group), the age-and sex-adjusted OR for CVD (model 1) was significantly greater in patients with high DPD/Cr and normal PTH levels (OR 4.26, P = 0.005), but the highest risk of CVD was in patients with elevated PTH and both normal (OR 10.27, P = 0.004) and high DPD/Cr (OR 7.61, P < 0.001). After adjustment for seven additional factors was made, the OR for the presence of CVD further increased (model 2, ): 17.32 in patients only with SHPT, 9.68 in patients with SHPT and high DPD/Cr, and 5.33 in subjects only with excess bone resorption. These associations are displayed in . In total, the OR for presence of CVD among HF patients with SHPT or excess bone resorption compared to those with both parameters in the normal range was 7.54 ().
Table 6 Odds ratios for presence of cardiovascular disease according to serum PTH concentrations as urinary deoxypyridinoline excretion in older patients with hip fracture
Figure 1 Odds ratios for presence of cardiovascular disease in older patients with hip fracture according to serum parathyroid hormone and urinary deoxypyridinoline levels.
Abbreviations: PTH, parathyroid hormone; DPD/Cr, deoxypyridinoline corrected by urinary creatinine; 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration rate.
Independent factors associated with serum PTH elevation and high bone resorption
Multivariate logistic regression analyses were performed to determine independent factors associated with elevated PTH (SHPT) and high bone resorption (DPD/Cr > 7.5 nmol/μmol) in older HF patients. These models included age, sex, CVD (yes/no), dementia (yes/no), type 2 diabetes mellitus (yes/no), HF type, smoking status, alcohol consumption, eGFR < 60 mL/minute/1.73 m2 (yes/no), albumin < 33 g/L (yes/no), OC < 14 ng/mL (yes/no), 25(OH)D, calcium, phosphate, magnesium, and DPD/Cr > 7.5 nmol/μmol (in the first model) or PTH > 6.8 pmol/L (in the second model) as independent variables. Independent predictors of SHPT were CVD (any) (OR 2.76, 95% CI 1.30–5.86; P = 0.008), renal impairment (OR 4.70, 95% CI 2.10–10.56; P < 0.001), female sex (OR 2.42, 95% CI 1.03–5.71; P = 0.043), 25(OH) D (OR 0.97, 95% CI 0.95–0.99; P = 0.014), serum calcium corrected for albumin (OR 0.003, 95% CI 0.0002–0.054; P < 0.001), and serum phosphate (OR 4.07, 95% CI 1.26–13.18; P = 0.019). The combined effect of these factors explained 48.7% of the variability in SHPT. These associations remained significant when the analyses were repeated with each CVD separately: OR ranged between 1.7 for AF and 2.9 for CAD.
Independent indicators of high bone resorption were the presence of CVD (OR 2.57, 95% CI 1.11–6.00; P = 0.028), low eGFR (OR 0.19, 95% CI 0.07–0.52; P = 0.001), and low OC (OR 6.20, 95% CI 2.23–17.25; P < 0.001), which explained 38.3% of the variability in high DPD/Cr. The “preventive” effect of renal impairment reflects the fact that DPD is mainly released by degradation of peptide-bound cross-links through renal metabolismCitation61 and is not directly generated from osteoclastic bone resorption.Citation62 Of note, CVD emerged as the second-most significant independent predictor (after eGFR < 60 mL/minute/1.73 m2) of both SHPT and excess bone resorption in this cohort.
Taken together, these data indicate that in older HF patients both SHPT and high bone resorption are significant independent indicators of the presence of CVD and vice versa. The OR for the presence of CVD is 2.68 times higher among subjects with SHPT and 2.58 times higher among patients with high bone resorption compared to patients without such a condition. Furthermore, compared to subjects with both PTH and DPD/Cr in the normal range, the OR for presence of CVD is 17.3 times higher in patients with SHPT and 5.3 times higher in subjects with excess bone resorption. On the other hand, CVD predicts SHPT (OR 2.82) and excess bone resorption (OR 2.53). These findings suggest bidirectional links between CVD and mineral/bone parameters related to osteoporosis. As the 25(OH)D levels in patients with and without CVD were low and did not differ significantly, our results highlight the significance of the independent associations between SHPT, high bone resorption, and CVD, supporting the hypothesis that osteoporosis and CVD share common biological mechanisms.
PTH and use of cardiovascular medications
In order to determine potential effects of different cardiovascular medications on vitamin D and PTH status, the serum mean values of 25(OH)D and PTH in patients receiving and not receiving such drugs were compared. shows that if compared with nonusers, serum PTH levels were significantly higher in users of ACE inhibitors (65.7%) and beta blockers (49.3%), but significantly lower in patients receiving calcium channel blockers (34.6%). PTH levels did not differ in respect to use of ARBs. No significant associations between serum 25(OH)D concentrations and use of any of the five classes of cardiovascular medications have been found.
Table 7 Use of cardiovascular medications and serum parathyroid hormone and vitamin D status
Multiple logistic regression analyses with use of the aforementioned medications as independent variables and controlling for age, sex, CVD, renal impairment, HF type, 25(OH)D, calcium, phosphate, magnesium, and DPD/Cr showed that among these five medication classes only the use of ACE inhibitors was significantly and independently associated with elevated PTH (SHPT) (OR 3.76, 95% CI 1.01–14.16; P = 0.049).
Cardiovascular disease and PTH as predictors of short-term outcomes
In multivariate regression models that considered age, sex, preexisting comorbidities including CAD, hypertension, history of stroke, AF, dementia, CKD ≥ 3 stage, ASA score ≥ 3, and smoking status, having CVD was a strong independent predictor of postoperative myocardial injury (cTnI rise) (OR 6.13, 95% CI 1.57–24.00; P = 0.009) and prolonged hospital stay (LOS > 20 days) (OR 4.27, 95% CI 1.53–11.88; P = 0.005). After further adjusting for SHPT, vitamin D deficiency, and high urinary DPD/Cr excretion, patients with CVD were 2.3 times more likely to develop postoperative myocardial injury (OR 2.25, 95% CI 1.10–4.58; P = 0.006); however, the association between CVD and prolonged length of stay was no longer significant. In fully adjusted models (all clinical and laboratory parameters), CVD did not predict long-term residential care facility need or in-hospital death either. In contrast, SHPT was a strong independent predictor of in-hospital mortality (OR 17.39, 95% CI 3.32–78.63; P < 0.001), LOS > 20 days (OR 2.82, 95% CI 1.47–5.41; P = 0.002). and myocardial injury (OR 1.42, 95% CI 1.25–1.88; P < 0.001). This does not mean that CVD is unrelated to outcomes. It does imply that SHPT is an important pathway contributing to and mediating poorer outcomes. Elevated PTH (>6.8 pmol/L) occurred in 76.9% of in-hospital deaths, 59.2% of postoperative cTnI rises, and in 45.3% of prolonged hospital stays.
Discussion
Main findings
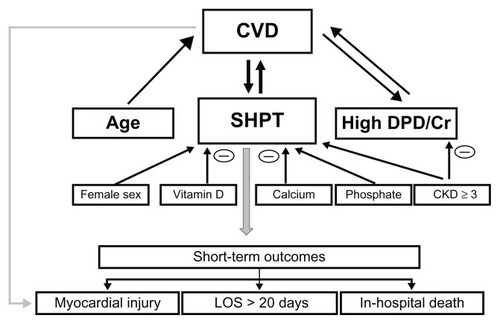
In this study of 746 consecutive older patients with osteoporotic (low-trauma) HF, preoperatively CVD was diagnosed in 63.3% of subjects, among whom 24.9% had two or more cardiovascular diseases. There are three main findings. Firstly, elevated serum PTH levels (SHPT) independently of 25(OH) D and other parameters of mineral and bone metabolism as well as age, sex, smoking status, alcohol consumption, renal impairment, hypoalbuminemia, dementia, and HF type were associated with 2.68-fold greater prevalence of CVD (for hypertension, CAD, history of stroke, and AF 1.97-, 3.43-, 2.57-, and 1.91-fold, respectively) and predictive of poorer short-term outcomes. Secondly, high bone resorption (urinary DPD/Cr > 7.5 nmol/μmol) was also a significant independent indicator of presence of CVD (OR 2.58). With an increasing number of CVDs, mean serum levels of PTH and urinary DPD/Cr levels as well as the proportion of patients with SHPT and excess bone resorption increased significantly. SHPT or excess bone resorption predicted the presence of CVD by factors of 17.32 and 9.68, respectively, compared to subjects with both these parameters in the normal range. Because renal impairment (eGFR < 60 mL/minute/1.73 m2) was prevalent (in 48.3% with CVD and in 37.4% without CVD) and related to SHPT and DPD/Cr in opposite directions, these parameters in combination did not provide additive prognostic information. Thirdly, the presence of CVD was an independent predictor of both SHPT and high DPD/Cr, indicating that links between CVD and osteoporotic HF are bidirectional. Our main findings are shown in .
Figure 2 Diagram showing significant independent relationships (as documented by multiple regression analyses) between cardiovascular disease and parameters of mineral-bone metabolism, age and sex and short-term outcomes.
Abbreviations: CVD, cardiovascular disease; SHPT, secondary hyperparathyroidism (PTH > 6.8 pmol/l); high DPD/Cr, deoxypyridinoline corrected by urinary creatinine excretion > 7.5 nmol/μmol; CKD ≥ 3, chronic kidney disease stage 3 or higher (eGFR < 60 mL/minute/1.73 m2); LOS, length of hospital stay; PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate.
PTH elevation
In agreement with numerous previous reports,Citation63–Citation65 vitamin D insufficiency was highly prevalent in our HF cohort (about 80%). The mean values of 25(OH)D and the prevalence of vitamin D deficiency were similar in patients with and without CVD. However, despite similarity in vitamin D status, in patients with CVD compared to those without CVD, mean serum PTH levels and the prevalence of SHPT were significantly higher, and these increased with increasing number of CVDs. Among patients with SHPT, 76% had CVD.
According to the traditional view, the consequence of vitamin D deficiency is SHPT, leading to bone resorption, osteoporosis, and fractures. However, in a substantial percentage of subjects with hypovitaminosis D, the serum PTH levels were found to be in the reference range. A concept of “functional hypoparathyroidism” with a protective role in bone health has been proposed.Citation64,Citation66 The prevalence of this condition ranges between 16% and 88%.Citation64,Citation67–Citation71 On the other hand, in one study of community-dwelling older women, vitamin D deficiency (25[OH]D < 50 nmol/L) was found in 2%, but elevated PTH (>65 ng/L > 6.9 pmol/L) in 17.4%.Citation72 A recent analysis of a large general health-care database revealed that increased PTH levels are common, even in the absence of 25(OH)D deficiency and renal dysfunction.Citation73
It has been reported that in the presence of hypovitaminosis D, subjects with SHPT compared to those without SHPT demonstrated lower BMD, higher bone loss and bone turnover,Citation64,Citation66,Citation74,Citation75 increased risk of fracture,Citation76 increased severity of heart failureCitation77 and higher mortality rates.Citation29,Citation43,Citation69,Citation77–Citation79
Contrary to these reports, other researchers in postmenopausal women with altered vitamin D status did not observe any difference in hip or spine BMD,Citation68 nor in the incidence of HF or vertebral fracturesCitation58 associated with PTH levels. Increased incidence of vertebral fractures and altered bone turnover (including high urinary DPD) has been reported in postmenopausal women with vitamin D insufficiency without SHPT.Citation56 A recent prospective cohort study of older persons found no evidence of an association between PTH and hip or any nonspine fractures, as well as between 25(OH)D and any nonspine fractures.Citation80 In postmenopausal women with type 2 diabetes and vitamin D insufficiency, functional hypoparathyroidism (not SHPT) was a risk factor for bone fragility.Citation81 In nursing home residents with type 2 diabetes, decreased PTH levels were associated with lower bone turnover and hip-fracture risk.Citation24 A U-shaped association between PTH and mortality has been reported in a hemodialysis population,Citation82 while a recent meta-analysis failed to demonstrate consistent associations between PTH and cardiovascular events and mortality in CKD.Citation40 Thus, it appears that the existing theoretical framework does not fully account for clinical observations, and the pathophysiological role and significance of SHPT remain unclear.
Phylogenetic data demonstrated that although PTH plays a key role in the development of bony skeleton,Citation83 PTH-like peptides and their receptors occurred before the transition from an aquatic to a terrestrial environment and not as adaptation to calcium-depleted milieu.Citation84 The PTH gene family was identified in cartilaginous fish,Citation85,Citation86 indicating that these genes played important metabolic roles other than bone formation and before their recruitment to bone formation. The PTH receptors are present not only in bones but also in a variety of other tissues and organs, including the cardiovascular system (cardiomyocytes, vascular smooth muscle, and endothelial cells), kidneys, adrenal cortex, pancreas, breast, and skin.Citation87–Citation89 Not surprisingly, PTH is involved in numerous physiological functions independent of vitamin D status and beyond calcium regulation, and hyperparathyroidism therefore may have profound effects on morbidity and mortality.
Our findings of the association of elevated PTH levels with CVD are in line with several (many but not all) aforementioned reports and supported by the following lines of evidence. First, in vitro, PTH has direct hypertrophic, inotropic,Citation90 and chronotropicCitation91–Citation93 effects on cardiac muscle. Second, primary hyperparathyroidism is associated with left ventricular hypertrophy,Citation94 aortic valve calcification,Citation95 significant dysfunction in coronary microcirculation,Citation96 increased arterial stiffness,Citation97 CAD, hypertension, and increased cardiovascular and all-cause mortality.Citation98,Citation99 Parathyroidectomy leads to reversal of these effects.Citation27,Citation28,Citation100 Third, SHPT caused by chronic kidney disease is linked to CVD, hypertension, vascular and valvular calcification, left ventricular hypertrophy, fibrosis, and dyslipidemia.Citation101 Fourth, PTH is associated with cardiovascular risk factors in the general population.Citation29,Citation73,Citation102,Citation103 Increased PTH levels are associated with hypertension,Citation31,Citation104–Citation109 CAD,Citation34,Citation73,Citation102 left ventricular hypertrophy,Citation110,Citation111 cardiac arrhythmias,Citation112 heart failure,Citation30,Citation33,Citation110,Citation113–Citation116 and insulin sensitivity,Citation117 as well as cardiovascular and all-cause mortality in patients withCitation87,Citation118,Citation119 and without chronic kidney disease.Citation29,Citation32,Citation43,Citation70,Citation78,Citation79,Citation120–Citation122
Some studies, however, did not find a link between PTH and CAD,Citation8,Citation49,Citation123 left ventricular hypertrophy,Citation124,Citation125 and cardiovascular death.Citation126 PTH levels were not predictive of cardiovascular events in CKD patientsCitation127 and did not emerge as a cardiovascular risk factor in adolescents.Citation128 In primary hyperparathyroidism, parathyroidectomy does not improve renal impairment and may have little effect on hypertension prevalence.Citation28,Citation129 In laboratory animals, PTH infusion was shown to cause vasodilatation, myocardial cell contraction and regeneration attenuating ischemic cardiomyopathy.Citation111,Citation130–Citation132 Although the discrepancy between the studies may reflect, at least in part, differences in patient characteristics (age, comorbidities, etc), experimental conditions, modes of PTH administration, PTH assay methodology, and in the covariates included in the multivariate analyses (in some studies, even 25[OH]D has not been simultaneously measured), they also indicate the complexity of multiple and sometimes contradictory/paradoxical effects of PTH (and PTHrP) on different signaling and metabolic pathways in the cardiovascular system as in the bones.Citation49,Citation111,Citation125,Citation133,Citation134
Our analysis of PTH as a continuous variable demonstrated that increase in serum PTH is associated with CVD, confirming the results of the categorical analysis, in which an arbitrary cutoff point for SHPT was used. Of note in our study, associations of SHPT with CVDs persisted when controlling for multiple covariates, including 25(OH)D, adjusted calcium, and other parameters of mineral and bone metabolism, indicating that pathways linking PTH and CVD are independent from and not mediated by the latter. These data suggest the possibility that PTH acts on the cardiovascular system through other biological mechanisms besides 25(OH)D and bone metabolism.
Experimental and clinical studies revealed that multiple and complex pathways may be involved in the development and progression of CVD in the presence of PTH excess. These include direct effects on cardiomyocytes, endothelial and smooth-muscle cells,Citation111,Citation135 stimulation of endothelial expression of atherosclerotic, inflammatory, and growthCitation136 factors, reduction of endothelial osteoprotegerin (OPG) secretion (a vascular-protective factor that controls vascular calcification),Citation137 increase in sympathetic nerve activity (by facilitating norepinephrine release),Citation138 increase in circulating ionized calcium (because of release from bone and decreased renal excretion), decreased expression of the calcium-sensing receptor,Citation139 stimulation of renin, angiotensin II, and aldosterone synthesis,Citation140–Citation147 indirect effects of PTH-induced hyperphoshatemiaCitation148 and hyperlipidemia,Citation149 and decreased insulin sensitivity.Citation117 Furthermore, the effects of hyperparathyroidism may be amplified by vitamin D insufficiency, as vitamin D is known to contribute directly or indirectly to the (patho) physiological functions mentioned above, such as renin–angiotensin–aldosterone system (RAAS) activation, insulin resistance, and systemic and vascular inflammations.Citation150 Taken together, it may be postulated that SHPT is a significant biomarker of CVD, and in older HF patients predicts postoperative myocardial injury, prolonged hospital stay, and in-hospital death.
Excessive bone resorption
Our finding that in older HF patients excess bone resorption (as defined by high urinary DPD/Cr excretion, the degradation product of the collagen telopeptide cross-linking molecule, which is one of the most specific and sensitive markers of osteoclastic bone resorption)Citation151–Citation156 independently of traditional risk factors is associated with CVD is in accordance with the results reported previously. Prospective studies,Citation58,Citation157,Citation158 although not all,Citation159 showed that high bone-resorption markers were predictive of increased risk for fractures (including hip) in postmenopausal women. Elevated DPD levels predicted a twofold increased risk of cardiovascular events in menCitation8 and mitral annular calcification in patients with renal stone formation.Citation160 Serum bone-resorption markers (which showed good correlation with DPDCitation161) predicted an increased carotid intima-media thickness in the elderly,Citation162 arterial stiffness in predialysis CKD,Citation163 cardiovascular and all-cause mortality in subjects living in residential care,Citation43 and in CKD patients.Citation127
The exact mechanism linking CVD and excess bone resorption is not known, and appears multifactorial. Accumulating evidence supports the hypothesis that CVD and bone resorption share several common cellular, metabolic, and signaling pathways. Both form cells in atheromatous plaques and bone osteoclasts derived from monocyte/macrophage lineage,Citation164,Citation165 and cells resembling osteoclasts have been identified in calcified atherosclerotic plaques.Citation166–Citation168 The receptor activator of nuclear factor-kappaB (RANK), RANK ligand (RANKL), and OPG play an essential role in osteoclastogenesis,Citation169 bone remodeling and resorption, and an imbalance in the RANK/RANKL/OPG system caused by hormones (PTH, 1,25[OH]2 D, estrogens, glucocorticoids), inflammatory cytokines, T-helper cells, oxylipids, oxidative stress, and other interrelated factors mediates both bone loss and vascular/atherosclerotic calcification.Citation170–Citation180 OPG has recently been proposed as a predictor of cardiovascular mortality and morbidity, although its role in CVD is still controversial.Citation180–Citation182 Interestingly, in patients with rheumatoid arthritis, etanercept caused a parallel reduction in serum RANKL and urinary DPD levels, while urinary NTx and serum OPG levels did not change significantly.Citation183
Bidirectional links between CVD and elevated PTH and excess bone resorption
Finally, our data indicate a bidirectional link between CVD and abnormalities in mineral/bone metabolism characteristic for osteoporosis. In older HF patients, both SHPT and excess bone resorption are significant independent indicators of presence of CVD, and CVD is highly predictive of SHPT and/or high bone resorption. The nature of these associations is complex and involves age-and disease-dependent changes, declining renal function, and different altered metabolic and signaling pathways. New information reveals remarkably close interactions between impaired PTH signaling, dysregulation of RAAS, RANK/RANKL/OPG system(axis), FGF23-α-Klotho complex, and Wnt signaling pathway,Citation184–Citation188 all of which have important physiological roles in both cardiovascular and bone health. Recent findings also indicate that oxidative stress, hyperlipidemia, oxidized lipids, and inflammation reciprocally regulate vascular and bone mineralization involving feedback mechanisms interacting with PTH and RANKL.Citation5,Citation171,Citation189
Our findings, when considered together with previous literature data, suggest that the bidirectional PTH–CVD relationships may form a vicious circle in which the interaction between PTH and RAAS plays an important role.Citation146,Citation147,Citation190 It has been hypothesized that in patients with CVD (especially with hypertension and chronic heart failure) inappropriate activation of the RAAS and salt retention causes marked losses of cations (calcium, magnesium) with a consequent increase of PTH production, which in turn stimulates RAAS. PTH elevation leads to paradoxical intracellular calcium overloading, oxidative stress, degeneration of mitochondria followed by cardiomyocyte necrosis and myocardial fibrosis.Citation146,Citation190,Citation191 As PTH increases secretion of aldosterone from the adrenals directly and indirectly (by activating the RAAS),Citation147 this might further contribute to arterial hypertension and cardiovascular injury. In other words, through RAAS-mediated mechanism(s) CVD could contribute to and be the result of SHPT. Furthermore, animal studies (angiotensin II-receptor knockout mice) revealed that the RAAS has a physiologic function in bone metabolism, and that signaling through angiotensin II receptor negatively regulates bone turnover and bone mass.Citation192 Given the lack of data on the status of RAAS in our cohort, we may only speculate regarding its role in CVD and bone pathology. In our study, the group with CVD had a higher proportion of patients with renal impairment (stage CKD > 3), which is known to be associated with higher plasma renin activity.Citation193,Citation194 Moreover, multiple logistic regression analysis showed that the use of ACE inhibitors was significantly and independently associated with elevated PTH. This observation may be explained by the fact that use of ACE inhibitors is associated with increase in plasma renin activity.Citation195,Citation196 Activation of RAAS, both disease-related and drug-related, should be considered in future studies among factors causing and aggravating SHPT. In the setting of CVD and especially with renal impairment, many other factors may also contribute to and be a consequence of SHPT; these include 1,25(OH)2D deficiency, hypocalcemia, hyperphosphatemia, downregulated receptors for vitamin D, calcium (calcium-censoring receptor), and FGF23 (FGFR1c-Klotho complex).Citation197 We observed impaired serum phosphate homeostasis in our cohort: patients with hypertension and CAD as the CVD group in total demonstrated significantly elevated serum phosphate levels compared to the non-CVD group. Furthermore, there was not only a negative correlation between serum calcium and PTH, but serum calcium (inversely) and phosphate (positively) levels were independently associated with SHPT.
Clinical implications
Taken together, ours and previous data on bidirectional bone–cardiovascular regulatory axis suggest the need to revisit conventional patterns of care for older patients: all subjects with osteoporosis and fractures should be screened for CVD, and in patients with CVD the mineral and bone status should be determined, as in both conditions clinical signs may be absent, making their recognition difficult. Moreover, there is increasing evidence that bisphosphonates are effective not only in prevention of osteoporotic fractures but also reduce cardiovascular and total mortality,Citation198–Citation202 while lipid-lowering statinsCitation1,Citation203,Citation204 and beta blockersCitation205 may have a positive effect on osteoporosis.
On the other hand, it should be emphasized that SHPT and excess bone resorption, important pathophysiological characteristics linking CVD and osteoporotic fractures, are present only in a proportion of HF patients with or without CVD, reflecting the disease diversity and significant variations in the related variables exhibited by individual patients. Therefore, identification of the PTH and bone-resorption status may improve the diagnostic and prognostic evaluation of the patient and might offer opportunities for individualized treatment. However, much further work is needed to clarify the hierarchy of genetic, environmental, and modulating factors causing the heterogeneity of both CVD and osteoporotic fractures in regard to PTH and bone-resorption status, and to find if a syndrome-specific approach (eg, interventions targeting SHPT and/or high DPD/Cr excretion) will improve the preventive and therapeutic strategies for these common diseases.
Limitations and strengths
Our study has several limitations as well as strengths. First, because of the cross-sectional design, the described associations do not prove causality. Second, the presence of subclinical CVD was not assessed; therefore, the prevalence of CVD was probably underestimated and the duration and severity of CVD was not analyzed. Third, our results are based on a single measurement of mineral/bone metabolism parameters on hospital admission, and these may change and fluctuate. Fourth, our cohort was of predominantly white Caucasians, so the results may not be applicable to other races or ethnic groups. The relatively large number of consecutive older HF patients that reflects a real-life situation, prospective follow-up for short-term outcomes, simultaneous estimation of 25(OH)D, PTH, calcium, phosphate, magnesium, and four bone-turnover markers levels, and adjustments for multiple confounding factors (sociodemographic, eGFR, albumin, comorbidities) strengthen our results. On the other hand, in multivariate regression analysis, multiple comparisons may potentiate the significance of multicollinearity phenomena. However, the variance inflation factor in all our models (–) was between 1.14 and 1.02, indicating that the amount of multicollinearity was not significant.
Conclusion
In older HF patients, CVD and vitamin D deficiency are highly prevalent (in about 63% and 80%, respectively). Although serum 25(OH)D levels in subjects with and without CVD were similar, serum PTH levels and urinary DPD/Cr excretion as well as the prevalence of SHPT and excess bone resorption were significantly higher in patients with CVD. Both SHPT and high DPD/Cr were strong independent indicators of presence of CVD and vice versa. Presence of CVD was predictive of postoperative myocardial injury, while SHPT was also an independent predictor of prolonged hospital stay and in-hospital death. These findings suggest that: (1) CVD and osteoporotic fractures share common pathogenetic mechanisms, (2) elevated levels of PTH and hypovitaminosis D affect the cardiovascular system and bones through multiple biochemical mechanisms and pathways, (3) SPTH and high urinary DPD/Cr excretion may be used as biomarkers of both osteoporosis and CVD and be helpful for individualized preventive and therapeutic interventions, and (4) patients with one of these diseases should be evaluated and treated for the other.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- McFarlaneSIMuniyappaRShinJJBahtiyarGSowersJROsteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link?Endocrine200423111015034190
- SinnottBSyedISevrukovABarengoltsECoronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with agingCalcif Tissue Int200678419520216604285
- FarhatGNCauleyJAThe link between osteoporosis and cardiovascular diseaseClin Cases Miner Bone Metab200851193422460842
- LampropoulosCEPapaioannouID’CruzDPOsteoporosis – a risk factor for cardiovascular disease?Nat Rev Rheumatol201281058759822890244
- ThompsonBTowlerDAArterial calcification and bone physiology: role of the bone-vascular axisNat Rev Endocrinol20128952954322473330
- CrepaldiGMaggiSEpidemiologic link between osteoporosis and cardiovascular diseaseJ Endocrinol Invest200932Suppl 42519724158
- FarhatGNNewmanABSutton-TyrrellKThe association of bone mineral density measures with incident cardiovascular disease in older adultsOsteoporos Int2007187999100817285350
- SzulcPSamelsonEJKielDPDelmasPDIncreased bone resorption is associated with increased risk of cardiovascular events in men: the MINOS studyJ Bone Miner Res200924122023203119453264
- HyderJAAllisonMAWongNAssociation of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium StudyAm J Epidemiol2009169218619419064643
- ChoiSHAnJHLimSLower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre-and postmenopausal womenClin Endocrinol (Oxf)200971564465119226260
- MussolinoMEMadansJHGillumRFBone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up studyAnn Epidemiol2003131069269714599733
- MussolinoMEMadansJHGillumRFBone mineral density and strokeStroke2003345e20e2212663880
- ChenJSHoganCLyubomirskyGSambrookPNWomen with cardiovascular disease have increased risk of osteoporotic fractureCalcif Tissue Int201188191521046091
- den UylDNurmohamedMTvan TuylLHRatermanHGLemsWF(Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosisArthritis Res Ther2011131R521241491
- LyonsKJMajumdarSREzekowitzJAThe unrecognized burden of osteoporosis-related vertebral fractures in patients with heart failureCirc Heart Fail20114441942421558449
- SennerbyUMelhusHGedeborgRCardiovascular diseases and risk of hip fractureJAMA2009302151666167319843901
- BakhirevaLNBarrett-ConnorELLaughlinGAKritz-SilversteinDDifferences in association of bone mineral density with coronary artery calcification in men and women: the Rancho Bernardo StudyMenopause200512669169816278612
- MussolinoMEArmenianHKLow bone mineral density, coronary heart disease, and stroke mortality in men and women: the Third National Health and Nutrition Examination SurveyAnn Epidemiol2007171184184617728148
- SamelsonEJCupplesLABroeKEHannanMTO’DonnellCJKielDPVascular calcification in middle age and long-term risk of hip fracture: the Framingham StudyJ Bone Miner Res20072291449145417542685
- HolickMFVitamin D: evolutionary, physiological and health perspectivesCurr Drug Targets201112141820795941
- LeeJHGadiRSpertusJATangFO’KeefeJHPrevalence of vitamin D deficiency in patients with acute myocardial infarctionAm J Cardiol2011107111636163821439530
- WangTJPencinaMJBoothSLVitamin D deficiency and risk of cardiovascular diseaseCirculation2008117450351118180395
- de BoerIHKestenbaumBShobenABMichosEDSarnakMJSiscovickDS25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcificationJ Am Soc Nephrol20092081805181219443637
- DobnigHPilzSScharnaglHIndependent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortalityArch Intern Med2008168121340134918574092
- GiovannucciELiuYHollisBWRimmEB25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective studyArch Intern Med2008168111174118018541825
- MelamedMLMichosEDPostWAstorB25-Hydroxyvitamin D levels and the risk of mortality in the general populationArch Intern Med2008168151629163718695076
- AnderssonPRydbergEWillenheimerRPrimary hyperparathyroidism and heart disease – a reviewEur Heart J200425201776178715474692
- Garcia de la TorreNWassJATurnerHEParathyroid adenomas and cardiovascular riskEndocr Relat Cancer200310230932212790792
- HagströmEHellmanPLarssonTEPlasma parathyroid hormone and the risk of cardiovascular mortality in the communityCirculation2009119212765277119451355
- HagströmEIngelssonESundströmJPlasma parathyroid hormone and risk of congestive heart failure in the communityEur J Heart Fail201012111186119220797986
- JordeRSvartbergJSundsfjordJSerum parathyroid hormone as a predictor of increase in systolic blood pressure in menJ Hypertens20052391639164416093907
- SchierbeckLLJensenTSBangUJensenGKøberLJensenJEParathyroid hormone and vitamin D – markers for cardiovascular and all cause mortality in heart failureEur J Heart Fail201213662663221415099
- SugimotoTTanigawaTOnishiKSerum intact parathyroid hormone levels predict hospitalisation for heart failureHeart200995539539819001003
- GrandiNCBreitlingLPHahmannHSerum parathyroid hormone and risk of adverse outcomes in patients with stable coronary heart diseaseHeart201197151215122121586795
- GrandiNCBreitlingLPVossenCYSerum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart diseaseAm Heart J201015961044105120569718
- GrandiNCBrennerHHahmannHCalcium, phosphate and the risk of cardiovascular events and all-cause mortality in a population with stable coronary heart diseaseHeart2012981292693322301505
- DhingraRSullivanLMFoxCSRelations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the communityArch Intern Med2007167987988517502528
- OnufrakSJBellasiACardarelliFInvestigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortalityAm J Epidemiol20091691677718980959
- OnufrakSJBellasiAShawLJPhosphorus levels are associated with subclinical atherosclerosis in the general populationAtherosclerosis2008199242443118093595
- PalmerSCHayenAMacaskillPSerum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysisJAMA2011305111119112721406649
- GoliaschGBlessbergerHAzarDMarkers of bone metabolism in premature myocardial infarction (</= 40 years of age)Bone201148362262621078422
- PennisiPRussoEGaudioAThe association between carotid or femoral atherosclerosis and low bone mass in postmenopausal women referred for osteoporosis screening. Does osteoprotegerin play a role?Maturitas201167435836220727694
- SambrookPNChenCJMarchLHigh bone turnover is an independent predictor of mortality in the frail elderlyJ Bone Miner Res200621454955516598375
- SembAGUelandTAukrustPOsteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand and risk for coronary events: a nested case-control approach in the prospective EPIC-Norfolk population study 1993–2003Arterioscler Thromb Vasc Biol200929697598019325145
- YeapBBChubbSAFlickerLAssociations of total osteocalcin with all-cause and cardiovascular mortality in older men. The Health In Men StudyOsteoporos Int201223259960621359669
- TanAGaoYYangXLow serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population surveyMetabolism20116081186119221353261
- MaderoMWasselCLPeraltaCAMarkers of mineral metabolism are not associated with aortic pulse wave velocity in community-living elderly persons: the Health Aging and Body Composition studyAm J Hypertens201124775576121436791
- Robinson-CohenCKatzRHoofnagleANMineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health studyJ Clin Endocrinol Metab20119672186219321508146
- TaylorENRimmEBStampferMJCurhanGCPlasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart diseaseAm Heart J2011161595696221570529
- ParkerBDBauerDCEnsrudKEIxJHAssociation of osteocalcin and abdominal aortic calcification in older women: the study of osteoporotic fracturesCalcif Tissue Int201086318519120094707
- CameronIDChenJSMarchLMHip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year studyJ Bone Miner Res201025486687219839771
- DabanJLDe Saint MauriceGPBatjomEFalzoneEAussetSPostoperative myocardial damages are a key issue in patients’ outcome after hip fractureAge Ageing2009384488489 author reply 48919411672
- Dawson-BowlingSChettiarKCottamHTroponin T as a predictive marker of morbidity in patients with fractured neck of femurInjury200839777578018407276
- LeveyASStevensLASchmidCHA new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- GarneroPBiomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoringMol Diagn Ther200812315717018510379
- IkegamiSKamimuraMUchiyamaSKatoHWomen with insufficient 25-hydroxyvitamin D without secondary hyperparathyroidism have altered bone turnover and greater incidence of vertebral fracturesJ Orthop Sci201116557358021713425
- GarneroPBauerDCMareauEEffects of PTH and alendronate on type I collagen isomerization in postmenopausal women with osteoporosis: the PaTH studyJ Bone Miner Res20082391442144818442311
- GarneroPHausherrEChapuyMCMarkers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective StudyJ Bone Miner Res19961110153115388889854
- CoenGMantellaDCalabriaSUrinary deoxypyridinoline excretion for the evaluation of bone turnover in chronic renal failureAm J Nephrol200020428329010970981
- LarocheMHeterogeneity of biological bone markers in idiopathic male osteoporosisRheumatol Int20123272101210421499877
- ColwellAEastellRThe renal clearance of free and conjugated pyridinium cross-links of collagenJ Bone Miner Res19961112197619808970901
- AponeSLeeMYEyreDROsteoclasts generate cross-linked collagen N-telopeptides (NTx) but not free pyridinolines when cultured on human boneBone19972121291369267687
- LipsPvan SchoorNMThe effect of vitamin D on bone and osteoporosisBest Pract Res Clin Endocrinol Metab201125458559121872800
- SahotaOGaynorKHarwoodRHHoskingDJHypovitaminosis D and ‘functional hypoparathyroidism’ – the NoNoF (Nottingham Neck of Femur) studyAge Ageing200130646747211742774
- Bischoff-FerrariHAWillettWCOravEJA pooled analysis of vitamin D dose requirements for fracture preventionN Engl J Med20123671404922762317
- SahotaOMundeyMKSanPGodberIMLawsonNHoskingDJThe relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosisBone200435131231915207772
- SakumaMEndoNOinumaTVitamin D and intact PTH status in patients with hip fractureOsteoporos Int200617111608161416874442
- AmouzouganAChopinFLaporteSVicoLThomasTFunctional hypoparathyroidism in postmenopausal women with fragility fractureJoint Bone Spine201279217017521664167
- MadsenCMJorgensenHLLindBSecondary hyperparathyroidism and mortality in hip fracture patients compared to a control group from general practiceInjury20124371052105722261083
- BjorkmanMPSorvaAJRisteliJTilvisRSLow parathyroid hormone levels in bedridden geriatric patients with vitamin D deficiencyJ Am Geriatr Soc20095761045105019473453
- FisherASrikusalanukulWDavisMSmithPHip fracture type: important role of parathyroid hormone (PTH) response to hypovitaminosis DBone201047240040720451678
- von MuhlenDGGreendaleGAGarlandCFWanLBarrett-ConnorEVitamin D, parathyroid hormone levels and bone mineral density in community-dwelling older women: the Rancho Bernardo StudyOsteoporos Int200516121721172615928802
- AndersonJLVanwoerkomRCHorneBDParathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors?Am Heart J20111622331339e221835295
- RejnmarkLVestergaardPBrotCMosekildeLParathyroid response to vitamin D insufficiency: relations to bone, body composition and to lifestyle characteristicsClin Endocrinol (Oxf)2008691293518208576
- CurtisJREwingSKBauerDCAssociation of intact parathyroid hormone levels with subsequent hip BMD loss: the Osteoporotic Fractures in Men (MrOS) StudyJ Clin Endocrinol Metab20129761937194422442276
- RejnmarkLVestergaardPBrotCMosekildeLIncreased fracture risk in normocalcemic postmenopausal women with high parathyroid hormone levels: a 16-year follow-up studyCalcif Tissue Int201188323824521181400
- BozicBLoncarGProdanovicNParathyroid hormone response to vitamin D insufficiency in elderly males with chronic heart failurePhysiol Res201160Suppl 1S155S16321777017
- CawthonPMParimiNBarrett-ConnorESerum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older menJ Clin Endocrinol Metab201095104625463420631024
- FisherAGohSSrikusalanukulWDavisMElevated serum PTH is independently associated with poor outcomes in older patients with hip fracture and vitamin D inadequacyCalcif Tissue Int200985430130919763373
- BarbourKEHoustonDKCummingsSRCalciotropic hormones and the risk of hip and nonspine fractures in older adults: the Health ABC StudyJ Bone Miner Res20122751177118522228250
- YamauchiMKajiHNawataKTakaokaSYamaguchiTSugimotoTRole of parathyroid hormone in bone fragility of postmenopausal women with vitamin D insufficiencyCalcif Tissue Int201188536236921287159
- FloegeJKronenbergFFroissartMMortality in chronic kidney disease and mineral metabolismJAMA20113062159 author reply 159–16021750291
- PottsJTParathyroid hormone: past and presentJ Endocrinol2005187331132516423810
- GuerreiroPMRenfroJLPowerDMCanarioAVThe parathyroid hormone family of peptides: structure, tissue distribution, regulation, and potential functional roles in calcium and phosphate balance in fishAm J Physiol Regul Integr Comp Physiol20072922R679R69617023665
- LiuYIbrahimASTayBHParathyroid hormone gene family in a cartilaginous fish, the elephant shark (Callorhinchus milii)J Bone Miner Res201125122613262320614475
- BhattacharyaPYanYLPostlethwaitJRubinDAEvolution of the vertebrate pth2 (tip39) gene family and the regulation of PTH type 2 receptor (pth2r) and its endogenous ligand pth2 by hedgehog signaling in zebrafish developmentJ Endocrinol2011211218720021880859
- PeirisANYoussefDGrantWBSecondary hyperparathyroidism: benign bystander or culpable contributor to adverse health outcomes?South Med J20121051364222189665
- UrenaPKongXFAbou-SamraABParathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissuesEndocrinology199313326176238393771
- UsdinTBBonnerTIHoareSRThe parathyroid hormone 2 (PTH2) receptorReceptors Channels200283–421121812529938
- OginoKBurkhoffDBilezikianJPThe hemodynamic basis for the cardiac effects of parathyroid hormone (PTH) and PTH-related proteinEndocrinology19951367302430307789328
- HaraMLiuYMZhenLPositive chronotropic actions of parathyroid hormone and parathyroid hormone-related peptide are associated with increases in the current, I(f), and the slope of the pacemaker potentialCirculation19979610370437099396474
- BoginEHararyIThe relationship of calcium and parathyroid hormone in their effect on heart cellsMol Cell Biochem198777129353122019
- ShimoyamaMOginoKFuruseYSignaling pathway and chronotropic action of parathyroid hormone in isolated perfused rat heartJ Cardiovasc Pharmacol200138449149911588519
- PiovesanAMolineriNCasassoFLeft ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomyClin Endocrinol (Oxf)199950332132810435057
- IwataSHyodoEYanagiSParathyroid hormone and systolic blood pressure accelerate the progression of aortic valve stenosis in chronic hemodialysis patientsInt J Cardiol Epub June 24, 2011
- MariniCGiustiMArmoninoRReduced coronary flow reserve in patients with primary hyperparathyroidism: a study by G-SPECT myocardial perfusion imagingEur J Nucl Med Mol Imaging201037122256226320821006
- RubinMRMaurerMSMcMahonDJBilezikianJPSilverbergSJArterial stiffness in mild primary hyperparathyroidismJ Clin Endocrinol Metab20059063326333015769995
- WalkerMDSilverbergSJCardiovascular aspects of primary hyperparathyroidismJ Endocrinol Invest2008311092593119092300
- YuNDonnanPTFlynnRWIncreased mortality and morbidity in mild primary hyperparathyroid patients. The Parathyroid Epidemiology and Audit Research Study (PEARS)Clin Endocrinol (Oxf)2010731303420039887
- HeyligerATangprichaVWeberCSharmaJParathyroidectomy decreases systolic and diastolic blood pressure in hypertensive patients with primary hyperparathyroidismSurgery200914661042104719958931
- CozzolinoMThe calciotropic hormones PTH and vitamin D: from bone to blood vesselsJ Intern Med2012271656656822211583
- KamychevaESundsfjordJJordeRSerum parathyroid hormone levels predict coronary heart disease: the Tromso StudyEur J Cardiovasc Prev Rehabil2004111697415167209
- AhlströmTHagströmELarssonARudbergCLindLHellmanPCorrelation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and womenClin Endocrinol (Oxf)200971567367819250270
- ChanRChanDWooJSerum 25-hydroxyvitamin D and parathyroid hormone levels in relation to blood pressure in a cross-sectional study in older Chinese menJ Hum Hypertens2012261202721248778
- FraserAWilliamsDLawlorDAAssociations of serum 25-hydroxyvitamin D, parathyroid hormone and calcium with cardiovascular risk factors: analysis of 3 NHANES cycles (2001–2006)PLoS One2010511e1388221085485
- MorfisLSmerdelyPHowesLGRelationship between serum parathyroid hormone levels in the elderly and 24 h ambulatory blood pressuresJ Hypertens19971511127112769383176
- SnijderMBLipsPSeidellJCVitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and womenJ Intern Med2007261655856517547711
- TaylorENCurhanGCFormanJPParathyroid hormone and the risk of incident hypertensionJ Hypertens20082671390139418551015
- ZhaoGFordESLiCKris-EthertonPMEthertonTDBalluzLSIndependent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adultsJ Hypertens20102891821182820613627
- LoncarGBozicBDimkovicSAssociation of increased parathyroid hormone with neuroendocrine activation and endothelial dysfunction in elderly men with heart failureJ Endocrinol Invest2011343e78e8520820131
- SchluterKDPiperHMCardiovascular actions of parathyroid hormone and parathyroid hormone-related peptideCardiovasc Res199837134419539855
- McCartyMFBarroso-ArandaJContrerasFCan moderate elevations of parathyroid hormone acutely increase risk for ischemic cardiac arrhythmias?Med Hypotheses200972558158319188028
- AlsafwahSLaguardiaSPNelsonMDHypovitaminosis D in African Americans residing in Memphis, Tennessee with and without heart failureAm J Med Sci2008335429229718414068
- AltayHZorluABiniciSRelation of serum parathyroid hormone level to severity of heart failureAm J Cardiol2012109225225621996143
- KhouzamRNDishmonDAFarahVFlaxSDCarboneLDWeberKTSecondary hyperparathyroidism in patients with untreated and treated congestive heart failureAm J Med Sci20063311303416415661
- ShaneEManciniDAaronsonKBone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failureAm J Med199710331972079316552
- AlvarezJAAshrafAPHunterGRGowerBASerum 25--hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African AmericansAm J Clin Nutr20109261344134920861177
- CovicAKothawalaPBernalMRobbinsSChalianAGoldsmithDSystematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney diseaseNephrol Dial Transplant20092451506152319001560
- GaneshSKStackAGLevinNWHulbert-ShearonTPortFKAssociation of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patientsJ Am Soc Nephrol200112102131213811562412
- CarlstedtFLindLWideLSerum levels of parathyroid hormone are related to the mortality and severity of illness in patients in the emergency departmentEur J Clin Invest199727129779819466123
- PilzSTomaschitzADrechslerCParathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiographyEur Heart J201131131591159820439261
- PremaorMOScalcoRda SilvaMJFurlanettoTWSecondary hyperparathyroidism is associated with increased risk of hospitalization or death in elderly adults living in a geriatric institutionGerontology200955440541019571528
- AradYSpadaroLARothMSerum concentration of calcium, 1,25 vitamin D and parathyroid hormone are not correlated with coronary calcifications. An electron beam computed tomography studyCoron Artery Dis1998985135189847983
- HarnettJDKentGMBarrePETaylorRParfreyPSRisk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patientsJ Am Soc Nephrol199447148614908161730
- WalkerMDFleischerJBDi TullioMRCardiac structure and diastolic function in mild primary hyperparathyroidismJ Clin Endocrinol Metab20109552172217920228165
- KestenbaumBKatzRde BoerIVitamin D, parathyroid hormone, and cardiovascular events among older adultsJ Am Coll Cardiol201158141433144121939825
- Fahrleitner-PammerAHerberthJBrowningSRBone markers predict cardiovascular events in chronic kidney diseaseJ Bone Miner Res200823111850185818597636
- WilliamsDMFraserALawlorDAAssociations of vitamin D, parathyroid hormone and calcium with cardiovascular risk factors in US adolescentsHeart201197431532021193684
- FeldsteinCAAkopianMPietrobelliDOlivieriAGarridoDLong-term effects of parathyroidectomy on hypertension prevalence and circadian blood pressure profile in primary hyperparathyroidismClin Exp Hypertens201032315415820504122
- BrunnerSWeinbergerTHuberBCThe cardioprotective effects of parathyroid hormone are independent of endogenous granulocyte-colony stimulating factor releaseCardiovasc Res201093233033922080594
- NickolsGAActions of parathyroid hormone in the cardiovascular systemBlood Vessels19872431201243036281
- ZarubaMMHuberBCBrunnerSParathyroid hormone treatment after myocardial infarction promotes cardiac repair by enhanced neovascularization and cell survivalCardiovasc Res200877472273118055578
- AslanDAndersenMDGedeLBMechanisms for the bone anabolic effect of parathyroid hormone treatment in humansScand J Clin Lab Invest2012721142222085136
- FeiYHurleyMMRole of fibroblast growth factor 2 and Wnt signaling in anabolic effects of parathyroid hormone on bone formationJ Cell Physiol2012227113539354522378151
- MonegoGArenaVPasquiniSIschemic injury activates PTHrP and PTH1R expression in human ventricular cardiomyocytesBasic Res Cardiol2009104442743419190955
- RashidGBernheimJGreenJBenchetritSParathyroid hormone stimulates the endothelial expression of vascular endothelial growth factorEur J Clin Invest2008381179880319021696
- RashidGPlotkinEKleinOGreenJBernheimJBenchetritSParathyroid hormone decreases endothelial osteoprotegerin secretion: role of protein kinase A and CAm J Physiol Renal Physiol20092961F60F6618945829
- PotthoffSAJanusAHochHPTH-receptors regulate nor-epinephrine release in human heart and kidneyRegul Pept20111711–3354221756942
- UreñaJLópez-BarneoJMetabotropic regulation of RhoA/Rho-associated kinase by L-type Ca(2+) channelsTrends Cardiovasc Med201222615516022902183
- BrunaudLGermainAZarnegarRSerum aldosterone is correlated positively to parathyroid hormone (PTH) levels in patients with primary hyperparathyroidismSurgery200914661035104119958930
- ChhokarVSSunYBhattacharyaSKHyperparathyroidism and the calcium paradox of aldosteronismCirculation2005111787187815710759
- FritschSLindnerVWelschSIntravenous delivery of PTH/PTHrP type 1 receptor cDNA to rats decreases heart rate, blood pressure, renal tone, renin angiotensin system, and stress-induced cardiovascular responsesJ Am Soc Nephrol200415102588260015466263
- LawPHSunYBhattacharyaSKChhokarVSWeberKTDiuretics and bone loss in rats with aldosteronismJ Am Coll Cardiol200546114214615992648
- PilzSTomaschitzAMarzWCavalierERitzEAldosterone and parathyroid hormone: a complex and clinically relevant relationshipCalcif Tissue Int201087437337420721661
- Rodríguez-AyalaEAvila-DíazMFoyo-NiembroEAmatoDRamirez-San-JuanEPaniaguaREffect of parathyroidectomy on cardiac fibrosis and apoptosis: possible role of aldosteroneNephron Physiol20061033112118
- RutledgeMRFarahVAdeboyeAASeawellMRBhattacharyaSKWeberKTParathyroid hormone, a crucial mediator of pathologic cardiac remodeling in aldosteronismCardiovasc Drugs Ther Epub February 29, 2012
- TomaschitzARitzEPieskeBAldosterone and parathyroid hormone: a precarious couple for cardiovascular diseaseCardiovasc Res2012941101922334595
- GoodmanWGThe consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney diseaseSemin Dial200417320921615144547
- AkmalMKasimSESolimanARMassrySGExcess parathyroid hormone adversely affects lipid metabolism in chronic renal failureKidney Int19903738548582313975
- LeeJHO’KeefeJHBellDHensrudDDHolickMFVitamin D deficiency an important, common, and easily treatable cardiovascular risk factor?J Am Coll Cardiol200852241949195619055985
- EastellRHannonRABiomarkers of bone health and osteoporosis riskProc Nutr Soc200867215716218412989
- IbrahimSMojiminiyiSBarronJLPyridinium crosslinks in patients on haemodialysis and continuous ambulatory peritoneal dialysisNephrol Dial Transplant19951012229022948808228
- KnottLBaileyAJCollagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevanceBone19982231811879514209
- NaylorKEastellRBone turnover markers: use in osteoporosisNat Rev Rheumatol20128737938922664836
- PeichlPGriesmacherbAMarteauRSerum crosslaps in comparison to serum osteocalcin and urinary bone resorption markersClin Biochem200134213113911311223
- RobinsSPWoitgeHHesleyRDirect, enzyme-linked immunoassay for urinary deoxypyridinoline as a specific marker for measuring bone resorptionJ Bone Miner Res1994910164316497817812
- ChapurlatRDGarneroPBreartGMeunierPJDelmasPDSerum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS studyBone200027228328610913923
- ChopinFBiverEFunck-BrentanoTPrognostic interest of bone turnover markers in the management of postmenopausal osteoporosisJoint Bone Spine2012791263121723772
- GerdhemPIvaskaKKAlataloSLBiochemical markers of bone metabolism and prediction of fracture in elderly womenJ Bone Miner Res200419338639315040826
- CelikADavutogluVSaricaKRelationship between renal stone formation, mitral annular calcification and bone resorption markersAnn Saudi Med201030430130520622348
- FassbenderWJGoddeMBrandenburgVMUsadelKHStumpfUCUrinary bone resorption markers (deoxypyridinoline and C-terminal telopeptide of type I collagen) in healthy persons, postmenopausal osteoporosis and patients with type I diabetesAdv Med Sci20095411619482729
- LeliCPasqualiniLVaudoGCarotid intima-media thickness and bone turnover: the role of C-terminal telopeptide of type I collagenIntern Emerg Med20105212713420182821
- ManghatPSouleimanovaICheungJAssociation of bone turnover markers and arterial stiffness in pre-dialysis chronic kidney disease (CKD)Bone20114851127113221281749
- Bar-ShavitZThe osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cellJ Cell Biochem200710251130113917955494
- MasseyHMFlanaganAMHuman osteoclasts derive from CD14-positive monocytesBr J Haematol1999106116717010444181
- JeziorskaMMcCollumCWoolleyDECalcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophagesJ Pathol1998185110179713354
- MinHMoronySSarosiIOsteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesisJ Exp Med2000192446347410952716
- MohlerER3rdGannonFReynoldsCZimmermanRKeaneMGKaplanFSBone formation and inflammation in cardiac valvesCirculation2001103111522152811257079
- LaceyDLTimmsETanHLOsteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activationCell19989321651769568710
- D’AmelioPFornelliGRoatoIIsaiaGCInteractions between the immune system and boneWorld J Orthop201123253022474632
- DemerLTintutYThe roles of lipid oxidation products and receptor activator of nuclear factor-kappaB signaling in atherosclerotic calcificationCirc Res2011108121482149321659652
- FengWLiWLiuWWangFLiYYanWIL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failureExp Mol Pathol200987321221819527710
- HofbauerLCSchoppetMClinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseasesJAMA2004292449049515280347
- KadenJJBickelhauptSGrobholzRReceptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcificationJ Mol Cell Cardiol2004361576614734048
- KiechlSWernerPKnoflachMFurtnerMWilleitJSchettGThe osteoprotegerin/RANK/RANKL system: a bone key to vascular diseaseExpert Rev Cardiovasc Ther20064680181117173497
- SchoppetMAl-FakhriNFrankeFELocalization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Mönckeberg’s sclerosis and atherosclerosisJ Clin Endocrinol Metab20048984104411215292354
- TakayanagiHOsteoimmunology and the effects of the immune system on boneNat Rev Rheumatol200951266767619884898
- TintutYDemerLRole of osteoprotegerin and its ligands and competing receptors in atherosclerotic calcificationJ Investig Med2006547395401
- VegaDMaaloufNMSakhaeeKClinical review: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implicationsJ Clin Endocrinol Metab200792124514452117895323
- VenurajuSMYerramasuACorderRLahiriAOsteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidityJ Am Coll Cardiol201055192049206120447527
- HalapasAZacharoulisATheocharisSSerum levels of the osteoprotegerin, receptor activator of nuclear factor kappa-B ligand, metalloproteinase-1 (MMP-1) and tissue inhibitors of MMP-1 levels are increased in men 6 months after acute myocardial infarctionClin Chem Lab Med200846451051618298349
- MalligaDEWagnerDFahrleitner-PammerAThe role of osteoprotegerin (OPG) receptor activator for nuclear factor kappaB ligand (RANKL) in cardiovascular pathology – a reviewWien Med Wochenschr201116123–2456557021870142
- YasunoriKMasaakiTTetsuyukiNHayatoKAkiraNReduction of urinary levels of pyridinoline and deoxypyridinoline and serum levels of soluble receptor activator of NF-kappaB ligand by etanercept in patients with rheumatoid arthritisClin Rheumatol20082791093110118338203
- BodinePVKommBSWnt signaling and osteoblastogenesisRev Endocr Metab Disord200671–2333916960757
- de BorstMHVervloetMGter WeePMNavisGCross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney diseaseJ Am Soc Nephrol20112291603160921852584
- JohnsonRCLeopoldJALoscalzoJVascular calcification: pathobiological mechanisms and clinical implicationsCirc Res200699101044105917095733
- LópezIRodríguez-OrtizMEAlmadénYDirect and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivoKidney Int201180547548221525854
- RheeYBiviNFarrowEParathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivoBone201149463664321726676
- SageAPLuJAttiEHyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipidsJ Bone Miner Res20112661197120621611962
- RobinsonADRamanathanKBMcGeeJENewmanKPWeberKTOxidative stress and cardiomyocyte necrosis with elevated serum troponins: pathophysiologic mechanismsAm J Med Sci2011342212913421747281
- KamalovGBhattacharyaSKWeberKTCongestive heart failure: where homeostasis begets dyshomeostasisJ Cardiovasc Pharmacol201056332032820588190
- KanekoKItoMFumotoTPhysiological function of the angiotensin AT1a receptor in bone remodelingJ Bone Miner Res201126122959296621887703
- PolettiRVergaroGZywLPronteraCPassinoCEmdinMPrognostic value of plasma renin activity in heart failure patients with chronic kidney diseaseInt J Cardiol Epub March 27, 2012
- SimJJShiJCalaraFRasgonSJacobsenSKalantar-ZadehKAssociation of plasma renin activity and aldosterone-renin ratio with prevalence of chronic kidney disease: the Kaiser Permanente Southern California cohortJ Hypertens201129112226223521941205
- DanserAHThe increase in renin during renin inhibition: does it result in harmful effects by the (pro)renin receptor?Hypertens Res201033141019893565
- JonesMRSealeyJELaraghJHEffects of angiotensin receptor blockers on ambulatory plasma renin activity in healthy, normal subjects during unrestricted sodium intakeAm J Hypertens200720890791617679042
- StubbsJRWetmoreJBDoes it matter how parathyroid hormone levels are suppressed in secondary hyperparathyroidism?Semin Dial201124329830621682772
- LylesKWColón-EmericCSMagazinerJSZoledronic acid and clinical fractures and mortality after hip fractureN Engl J Med2007357181799180917878149
- BeaupreLAMorrishDWHanleyDAOral bisphosphonates are associated with reduced mortality after hip fractureOsteoporos Int201122398399121052642
- Colón-EmericCSMesenbrinkPLylesKWPotential mediators of the mortality reduction with zoledronic acid after hip fractureJ Bone Miner Res2010251919719580467
- EriksenEFLylesKWColón-EmericCSAntifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fractureJ Bone Miner Res20092471308131319257818
- SambrookPNCameronIDChenJSOral bisphosphonates are associated with reduced mortality in frail older people: a prospective five-year studyOsteoporos Int20112292551255620959963
- JadhavSBJainGKStatins and osteoporosis: new role for old drugsJ Pharm Pharmacol200658131816393459
- TangQOTranGTGamieZStatins: under investigation for increasing bone mineral density and augmenting fracture healingExpert Opin Investig Drugs2008171014351463
- YangSNguyenNDEismanJANguyenTVAssociation between beta-blockers and fracture risk: a Bayesian meta-analysisBone201251596997422842220