Abstract
Fungi are major causes of infections among immunocompromised or hospitalized patients with serious underlying diseases and comorbidities. Candida species remain the most important cause of opportunistic infections worldwide, affecting predominantly patients over 65 years old, while they are considered to be the fourth most common cause of nosocomial bloodstream infections. The rapidly growing elderly population has specific physiological characteristics, which makes it susceptible to colonization and subsequent infection due to Candida species. Comorbidities and multidrug use should be taken into account any time the therapeutic regimen is under consideration. Different classes of antifungal drugs are available for the treatment of invasive fungal infections but echinocandins, apart from their activity against resistant strains (Candida glabrata and Candida krusei), seem to be safe, with limited adverse events and minimal drug–drug interactions in comparison to the other regimens. Therefore, these agents are strongly recommended when dealing with elderly patients suffering from an invasive form of Candida infection.
Introduction
Candidemia and invasive candidiasis (C/IC) show an increasing incidence in the nosocomial setting. The crude mortality of those infections ranges between 36%–63% depending on patient population.Citation1–Citation4 Comorbidities, aging and age-associated physiological changes, higher rates of oropharyngeal colonization with Candida species, and concomitant drug use make elderly patients (>65years old) more vulnerable to infections. For this reason fungal infections have become a major problem in older adults, since age is a well-documented predisposing factor with increased impact on mortality.Citation5–Citation7
The cutoff age for the elderly population cannot be clearly defined because aging is a continuous process. It is also clear that aging is a multifactorial process influenced by both genetic and environmental parameters. Many studies dealing with Candida infection in the elderly have used several different arbitrary cutoff points such as over 60, 65, or 70 years old. However, most prospective epidemiological studies define “elderly population” as the age group >65 years.Citation5–Citation9
The elderly population is large and is growing in proportion to the general hospitalized population, but available data on epidemiology, clinical impact, and outcome of nosocomial fungal infections are limited.Citation7–Citation10 The indications for antifungal therapy are the same for older as well as younger individuals, and the initial antifungal therapy should be selected on the basis of the infecting organism and the local epidemiology.Citation9,Citation11 Fluconazole is generally effective against C/IC but its use may be limited by the increasing prevalence of Candida species (spp.) with acquired or intrinsic resistance. Echinocandins are recommended as first-line treatment for C/IC in all patients, and more specifically in hemodynamically unstable patients or in those with prior azole exposure, or for invasive infections caused by C. krusei or C. glabrata because of their activity against azole-resistant strains, while Amphotericin B remains the cornerstone of antifungal treatment.Citation1,Citation9,Citation11–Citation15
In this review, we aim to discuss the management and treatment options of fungal infections in the elderly population, considering additionally the specific conditions and the impact of potential comorbidities and drug interactions.
Epidemiology
Invasive candidiasis (IC) (also called systemic), is the invasion of Candida spp. in a human organ (invasion via the bloodstream is called candidemia). If multiple organs such as the brain, heart, kidneys, lungs, and liver are affected, the condition is called disseminated candidiasis. Candida spp. are ubiquitous (more than 200 species have been described); most consist partly of the human microbiological flora, although only 10% of these species are known to be responsible for infections in humans.Citation16 Indeed, at least 17 different Candida spp. cause IC in humans; C. albicans is the most common worldwide, presenting a global average of 66% of all Candida spp., with large geographical discrepancies according to the ARTEMIS DISK Global Antifungal Surveillance Study.Citation17,Citation18 Candida spp. account for 8%–10% of all nosocomial infections, next to coagulase-negative Staphylococci (31%), Staphylococcus aureus (20%), and Enterococci (9%) and have become the fourth most common cause of hospital-acquired bloodstream infections (BSIs) in the USA.Citation19 Other studies in Europe and Canada, with the exception of Denmark, reported a lower incidence of candidemia than that reported in the USA (1.9% in Finland, 4.9% in Iceland and Spain), reflecting probable differences in patient demographics and comorbidities, as well as in medical practices and/or diagnostic methods.Citation6,Citation20 In all of these studies the highest incidence occurred at the extremities of the age spectrum (infants of less than one year old and in adults >65 years old).Citation6,Citation21–Citation24
Candida albicans is the most common pathogen causing IC worldwide, though a shift towards non-albicans species has been noted over the years. C. glabrata represents an important fungal pathogen, ranking second to C. albicans as a cause of bloodstream infection.Citation6 In the USA, C. glabrata accounts for 20%–24% of all Candida BSIs but in other geographical regions, including Europe, lower rates have been reported.Citation6 The origins of these discrepancies are unclear but previous exposure to azoles, increasing patient age, presence of underlying diseases such as malignancies, and different geographic locations or technical methodologies regarding blood cultures could be considered as possible explanations. Older patients (>65 years) have increased risk of candidemia due to C. glabrata and increased risk of dying (29%) as well.Citation25 Systemic broad spectrum antibiotic use, central venous catheters, long stay in the intensive care unit (ICU), renal insufficiency, and total parenteral nutrition were identified as the most important risk factors. It is unclear whether the higher identified rate of oropharyngeal colonization with C. glabrata in older, compared to younger, adults, is related to candidemia.Citation26,Citation27 Blot et al have studied the outcome of critically ill patients with candidemia and found that fungemia from C. glabrata was significantly associated with older age. Older age, polymicrobial bloodstream infection and acute renal failure were independent predictors of mortality.Citation11 A significant epidemiological shift towards C. glabrata as a cause of candidemia has been reported in oncology centers, compared to individual hospitals.Citation6,Citation25,Citation28–Citation30
C. paraspilosis is an exogenous pathogen, found mostly on skin rather than mucosal surfaces, and is known for its ability to form biofilm on catheters and other implanted devices. It is spread through hand contamination in hospitals and nursing homes. About 38% of C. paraspilosis BSIs are acquired outside the hospital, a finding consistent with the fact that older patients often receive home health care with indwelling catheter use due to various chronic diseases.Citation26,Citation31–Citation33 BSIs due to this species are associated with a lower mortality rate than other Candida spp.Citation2,Citation6 C. tropicalis is an important pathogen in neutropenic patients with hematologic malignancies and mucositis. This pathogen is very common in Latin America, responsible for 22% of BSI isolates but, according to the ARTEMIS DISK Global Antifungal Surveillance Study, its incidence worldwide has reached 4%–7% with an increasing trend.Citation18 C. krusei is another important pathogen among patients with hematologic malignancies, and among blood marrow transplant recipients, characterized by intrinsic resistance to fluconazole.Citation1 C. krusei accounts for 2%–5% of all Candida infections worldwide, having emerged in oncology patients under prophylaxis with fluconazole.Citation28,Citation34 Interestingly, it has been reported that exposure to piperacillin-tazobactam and vancomycin leads more often to C. krusei infections than does exposure to fluconazole, because the former drugs promote skin and gastrointestinal colonization, rendering the human host more vulnerable to C. krusei BSIs.Citation35
C. guillermondii and C. rugosa are common in Latin America and responsible for clusters of hospital infection, exhibiting low susceptibility to fluconazole, while they are considered as rare causes of catheter-related candidemias in other countries.Citation36–Citation39 C. incospicua and C. norvegensis are both phenotypically similar to C. krusei, exhibiting intrinsic resistance to fluconazole, causing candidemia in human immunodeficiency virus-infected patients and in patients with hematologic malignancies. C. norvegensis has been found mostly in respiratory specimens.Citation40–Citation42
Physiological alterations in the elderly
The aging process leads to variable changes in physiological and morphological functions, rendering older patients potentially more vulnerable to infections, particularly from fungal species ().Citation7 Aging leads to hyposalivation, which in turn alters the normal microflora of the oral cavity, so that it has fewer anaerobic bacteria, such as enterococci.Citation43 Less saliva production limits peptide and protein presence in the oral cavity, and the lack of substances with broad antimicrobial activity, such as lysozyme, contributes to oral candidiasis.Citation43 In the supragingival plaque, which is responsible for caries formation, Candida species are the predominant pathogens, especially in adults >70 years old.Citation44 Moreover, in dental prostheses various Candida species can be found, with C. albicans being the most prevalent, followed by C. glabrata and C. tropicalis.Citation44 This colonization is further influenced by (a) poor oral hygiene, (b) drugs that irritate or damage the oral mucosa, such as cytostatics, (c) drugs that alter the oral flora synthesis, such as antibiotics, or (d) by concurrent diseases, such as iron deficiency anemia. Once the oral cavity is colonized, it is easier for the yeasts to reach the respiratory system, and since Candida is a commensal of the gut lumen and the cutaneous surfaces, the colonization index is increasing.Citation43,Citation45 Candida spp. are common in the urine of the elderly, especially after treatment with broad spectrum antibiotics. Differentiating asymptomatic candiduria, even in high concentrations (>105/mL urine), from a true infection which triggers a systematic inflammatory response is difficult, and treatment is influenced by the biofilm formation in the urinary catheter.Citation43
Table 1 Normal physiological alterations in the elderly
The biofilm formation is an aggregate of microorganisms where cells adhere to each other on a surface. It may become a problem in patients with indwelling catheters, such as older people in hospitals or nursing homes. Candida colonization is one of the main reasons why older people are so prone to bloodstream infections, but not the only reason. Older age is always accompanied by normal physiological alterations and/or various metabolic disorders or neoplastic diseases, which disrupt the mucosal and cutaneous barrier and make the organism more vulnerable to Candida infection ().Citation6 Thus, selection of a suitable drug is based not only on the specific microorganism, and on the clinical condition and its severity, but also on all the underlying pathophysiological characteristics of the patient’s advanced age.
Table 2 Physiological effects of aging and their impact on drug metabolism
Aging is characterized by diminished immunological response to infection, especially due to functional insufficiency of monocytes and macrophages, which leads to inadequate phagocytosis.Citation43 Other antigen presenting cells, such as dendritic cells, are lacking, and so are naive T-cells due to thymus gland involution.Citation46 Mature T-cells lose their memory capacity and exhibit poor and/or altered cytokine production.Citation46 Moreover, the number of circulating B-cells is diminished and their response to antigenic challenges through immunoglobulin production is weaker.Citation47 Animal studies on the aging liver have shown modifications of the hepatic physiology which affect drug metabolism. Possible mechanisms, occurring normally with aging, involve reduction in total liver mass, hepatic blood flow, and protein synthesis. These factors compromise drug metabolism, such as hydroxylation, dealkylation, and reduction; reactions occurring in Phase I drug metabolism, performed by microsomal cytochrome p450.Citation48,Citation49 Phase I is necessary to prepare the drug or toxin to undergo Phase II metabolism (conjugation, acetylation, and methylation), altering its form and promoting its effective excretion.Citation49
Renal function is also impaired with advanced age. Glomerular filtration rate (GFR) decreases by 1% per year of life. This is not reflected in serum creatinine because a 25% rise in serum creatinine level actually represents a substantial fall in GFR, probably as much as 50%, due to the exponential rise in creatinine level with declining renal function.Citation50 This decline of renal function is often underestimated, since serum creatinine is dependent on muscle mass, which also attenuates with age and remains almost normal. Therefore, older and very sick patients, with a normal creatinine value, have a GFR of only 30% of that of a young, healthy adult. This is associated with serious clinical problems with drugs dependent on renal excretion.Citation50
Polypharmacy in the elderly is another important issue, relevant to both adverse effects and drugs interactions. A recent Dutch study demonstrated that almost 75% of the elderly population was being treated with at least 4 drugs, suggesting that elderly patients are not only prescribed a greater number of medications than younger patients, but they also receive drugs in a more inappropriate manner.Citation51–Citation53 Analgesics, including nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, acetylsalicylic acid, ibuprofen, indomethacin, naproxen), narcotics (eg, hydrocodone), non-narcotic pain medications (eg, acetaminophen), or drugs with other mechanisms of action that act synergistically on pain relief (benzodiazepines, tricyclic antidepressants), comprise the most popular drug categories.Citation53 Furthermore, polypharmacy involves, apart from pain relief, treatment of other diseases, such as hypertension, diabetes, chronic obstructive pulmonary disease, heart failure, or cancer. Moreover, alterations in body composition such as decrease in total body water or increase in body fat may result in unexpected toxic effect or duration of action of various drugs. The prevalence of the effect of drug–drug interactions on the liver is >74% in older women of which 63% involve NSAID use.Citation54 Apart from multiple drug use, the mechanisms of drug-induced liver injury in the elderly include gender, dosage and treatment duration, drug formulations, nutritional status, genetic susceptibility, environmental factors (eg, alcohol abuse), and underlying comorbidities.Citation53
Diagnosis
Invasive Candida infections include clinical syndromes of different severity where the diagnosis is a challenge, especially in critically ill, immunocompromised, or elderly patients. The signs and symptoms vary from silent or atypical, to that of a bacterial infection. The diagnosis relies on clinical, microbiological, and biochemical evidence. Newer culture methods have raised the sensitivity of Candida detection to almost 70%, but it takes a minimum of 24 to 48 hours to become positive and this may come late in the course of the infection.Citation55 Moreover, patients are often under fluconazole prophylaxis which may render the cultures negative at time of testing.Citation56 Two antigen based tests are currently available for the early diagnosis of candidemia, relying on detecting components of the fungal cell wall. The first method detects mannan levels, which is a major component of the Candida cell wall. In high risk patients, it is recommended to be performed two to three times per week, since its circulation in the bloodstream is intermittent. Sensitivity and specificity of this test, when combined with anti-mannan antibodies detection in critically ill but not immunocompromised patients, are 83% and 86% respectively.Citation56–Citation59 The second diagnostic tool is based on the detection of 1,3-β-D-glucan. This test has been evaluated mostly in critically ill patients and has demonstrated an overall sensitivity of 77% and specificity of 85% for subjects with proven or probable IC.Citation58–Citation60 One single positive test is indicative of the infection, but it must be interpreted with caution due to false positive results.Citation55 A negative 1,3-β-D-glucan test is associated with high negative predictive value (>90%) and can be used to rule out IC, especially in patients with neutropenia.Citation60–Citation62 Finally, nucleic acid-based detection methods (real-time polymerase chain reaction) have been developed for five different Candida spp. of major clinical importance. Although these techniques have shown significant advances in the early and specific diagnosis of IC, further evaluation must be conducted in specific populations such as the elderly, considering the relatively weak immune systems and variable immunological responses among this age group, which render the diagnostic accuracy of the above methods less precise.Citation55
Therapeutic targets and drug selection
The management of Candida infection includes prophylactic, preemptive, empiric, and targeted treatment (). Prophylaxis is used in high risk patients with no symptoms and signs of infection. Preemptive therapy is justified in the presence of positive inflammatory markers – biomarkers in conjunction with certain predisposing risk factors. Empiric therapy is warranted in patients with a currently unknown infection, for whom treatment is justified based on clinical judgment, while targeted treatment is administered when the diagnosis of a certain pathogen is documented. Currently available drugs against IC include amphotericin B (AmB) and its derived lipid formulations (LFAmB), azoles (triazoles) including fluconazole (FLU), voriconazole (VOR), posaconazole (POS), and itraconazole, and echinocandins including. caspofungin (CFG), micafungin (MIC), and anidulafungin. 5-fluorocytocine (5FC), a fluorinated pyrimidine analog has also antimycotic properties. Most Candida spp. are susceptible to these agents, except for those with intrinsic or acquired resistance after exposure to other drugs.
Figure 1 Algorithm for the management of candidiasis in the elderly patient.
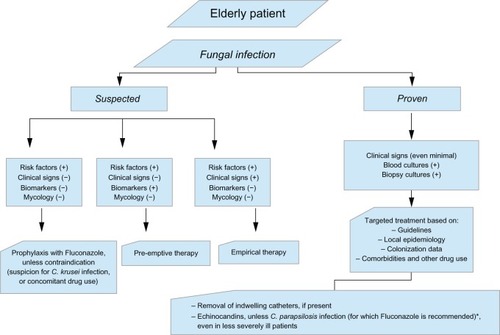
AmB is a polyene macrolide antifungal regimen with fungicidal action, which was considered in the past to be the “gold standard” for the treatment of invasive fungal infections.Citation62 Derived from Streptomyces spp., it has a high affinity for the sterols of fungal and bacterial membranes, forming small transmembrane channels which lead to monovalent ion leakage and cause fungal cell death. Derivatives of AmB were developed in order to limit toxicity, especially renal failure, which has been rated between 49%–65%.Citation50,Citation63–Citation65 Three lipid formulations of AmB (LFAmB) are commercially available, all with a good fungicidal activity and no differences in efficacy. Indeed, all Candida spp. are susceptible to AmB, along with Aspergillus spp., Cryptococcus spp., and Zugomecetes spp. The formulations include a true liposome structure LFAmB (AmBisome®, Gilead, Cambridge, UK); a ribbon-like structure AmB lipid complex (Abelcet®, Sigma-Tau Pharmaceuticals, Inc, Gaithersburg, MD, USA); and a colloid dispersion, amphotericin B colloidal dispersion (ABCD) (Amphocil/Amphotec®, EvaluatePharma, London, UK) with a disc-like structure. All lipid formulations have less nephrotoxicity than conventional AmB-deoxycholate.Citation64–Citation66 Among these, the liposome structure of AmB seems to have lower nephrotoxicity rates (15%) than the ribbon-like structure (>40%).Citation67 LFAmB is used in a dose of 3 to 5 mg/kg daily in life-threatening mycoses, as well as for empirical treatment of suspected IC, or in situations where an antifungal agent with rapid time-kill rate and high post-antifungal effect is needed.Citation11,Citation68 However, in older patients it should be used with caution, especially if parameters such as dehydration, large cumulative dosage, abnormal baseline renal function, and concomitant nephrotoxic drug use exist.
The azoles FLU, itraconazole, VOR, and POS inhibit the fungal cytochrome P450 enzyme 14α-demethylase and prevent the conversion of lanosterol to ergosterol, which is essential for the fungal cell membrane integrity. All the above azoles demonstrate activity against Candida spp. but reduce activity against C. glabrata and C. krusei.
FLU, with primarily fungistatic effect (800 mg or 12 mg/kg loading dose, followed by 400 mg or 6 mg/kg daily), has comparable efficacy to AmB for the treatment of candidemia, while it is indicated as empirical and curative treatment in non-neutropenic patients.Citation69–Citation70 FLU is recommended for the treatment of C. parapsilosis BSI, while among all the triazoles, it possesses the greatest penetration to the cerebrospinal fluid and it is therefore indicated for the treatment of central nervous system and intraocular infections. Prophylactic use of FLU led to a shift to resistant Candida species, and its use alters (and may even increase) the frequency of infection due to molds. Prophylaxis (6 mg/kg daily) is recommended in solid organ transplant recipients (liver, pancreas, and small bowel), during induction chemotherapy and in stem cell transplant recipients during the period of neutropenia.Citation11 FLU is an inhibitor of the human cytochrome P450 system and therefore it decreases the metabolism or increases the concentration of any drug metabolized by these enzymes. This should be kept in mind, considering the high number of concomitant drugs that older people take, which also undergo hepatic metabolism, in order to avoid serious and life-threatening drug–drug interactions. Serum levels of warfarin, phenyntoin, or oral hypoglycemic agents are increased by the azoles, whereas serum digoxin levels may increase. Another rare adverse event is the potential effect on electrocardiographic QT interval, whose elongation increases the risk of ventricular arrhythmias, especially if drugs are used concurrently, which also prolong its duration (eg, macrolides, fluoroquinolones, anticholinergic, antihistamines, diuretics, and the gastroprokinetic agent cisapride). Itraconazole has a broader spectrum of activity than FLU (in vitro activity against Candida spp., Aspergillus spp., and dimorphic fungi, but not as broad as VOR or POS), but is not able to penetrate cerebrospinal fluid. VOR is active against Candida spp., Mucor spp., and Aspergillus spp. in severely immunocompromised patients, while it is active against C. krusei, C. guillermondii, and C. lusitaniae.Citation71 Intravenous VOR is complexed to a cyclodextrin molecule and after two loading doses of 6 mg/kg every 12 hours, a lower maintenance dosage of 3–4 mg/kg twice daily is recommended. Due to cyclodextrin accumulation, VOR is not indicated in patients with renal dysfunction and creatinine clearance of <50 mL/min.Citation72 VOR is effective in candidemia, but it offers little advantage over FLU and is therefore recommended as a step-down oral therapy for C. krusei infection and for FLU-resistant, VOR-sensitive C. glabrata infection. Oral VOR does not require dosage adjustment in renal insufficiency, but is the only triazole that requires dosage reduction in patients with mild-to-moderate hepatic insufficiency. In a randomized, international, multicenter trial comparing VOR with LFAmB as empirical antifungal treatment, the authors suggested that VOR could be a suitable alternative to LFAmB in patients with neutropenia and persistent fever.Citation73 POS is available only as an oral suspension with high oral availability and seems to be more active than the other triazoles. POS exhibits a broad spectrum activity against yeasts, molds, or rare fungal strains.
Echinocandins (ECs) are a new class of antifungal agents that target the fungal cell wall by inhibiting 1,3-β-D-glucan synthetase, leading to osmotic instability and cell death. ECs are considered to be safe drugs, with few reported side effects. The three members of the group, CFG (loading dose of 70 mg, then 50 mg daily), MIC (100 mg daily), and anidulafungin (loading dose of 200 mg, then 100 mg daily), are all available only for parenteral use. Each of these agents has been studied for the treatment of IC in comparative and noncomparative clinical trials.Citation74–Citation77 The MICs of the echinocandins are low for a broad spectrum of Candida spp., including C. krusei and C. glabrata. C. parapsilosis demonstrates less in vitro susceptibility (higher MICs) than most other Candida spp., and that has raised the concern of its being less responsive.Citation71,Citation78,Citation79 Similarly, there have been reports of increased clinical failure and persistence of infection with this species, claiming that C. parapsilosis infection may indeed require higher echinocandin dosage.Citation1,Citation80 Therefore, in the recent clinical practice guidelines for the management of candidiasis, FLU is the treatment recommendation for C. parapsilosis infection, unless the patient has already received an echinocandin, is clinically improved, and has negative follow-up cultures.Citation11 Until now, this matter was still under debate, since a recent meta-analysis showed that ECs are effective for the treatment of candidemia or invasive candidiasis due to C. parapsilosis.Citation81
Another important issue, unique for the ECs, is the “eagle effect”, a term used to describe the paradoxical in vitro and in vivo growth of Candida and Aspergillus isolates when the dose of the drug gets over the MIC level.Citation82 This phenomenon has similarities to the “eagle effect” observed in other cell wall active antimicrobial agents, such as penicillins. Although the clinical impact of this phenomenon has not been elucidated, it might be of some importance in biofilm tretment.Citation62 None of the ECs require dosage adjustment for renal insufficiency or dialysis. Both CFG and MIC undergo minimal hepatic metabolism, but neither is a major substrate for cytochrome P450 and therefore they have minimal drug-drug interactions. Anidulafungin has not hepatic metabolism; it undergoes slow chemical degradation to a ring opened peptide with no antifungal activity. Though concerns have been raised about the potential hepatotoxicity of MIC due to tumor formation in rodents, CFG is the only EC for which dosage adjustment is recommended for patients with moderate to severe hepatic dysfunction. The clinical practice guidelines favor the use of an EC as initial therapy for candidemia in non-neutropenic as well as neutropenic adult patients, with moderate to severe illness.Citation11 Alternatively, FLU and LFAmB may be used, but for infection due to C. glabrata an EC is preferred, since the triazoles have diminished activity against this species.
Moreover, very little is known about the pharmacological/pharmacokinetic properties of antifungal drugs in the elderly. The diminished drug clearance that occurs naturally with aging, along with the presence of other comorbidities and drug use, make the pharmacodynamic and pharmacokinetic issues very intriguing. Drug interactions and comorbidities are the main reasons why we would not recommend azoles or amphotericin B as our first therapeutic choice in this specific population. Dose modification is not warranted unless indicated for other reasons (eg, hepatic or renal dysfunction).Citation83 Monitoring of plasma levels could be an option, but it is time and resource consuming, even in health care facilities where the method is available. Monitoring drug levels in plasma cannot be indicated for routine use unless future studies provide us with more data.
Flucytocine (or 5-fluorocytosine, 5FC) is an antimetabolite that acts as an antifungal against Candida spp., Cryptococcus spp., and other fungi. 5FC enters the fungal cell via cytosine permease, and is metabolized to 5-fluorouracil, which is incorporated extremely closely into the fungal RNA, inhibiting both DNA and RNA synthesis. Most of the drug is excreted unchanged in the urine, so that dose adjustment is necessary for patients with renal dysfunction. Considering the fact that 5FC is rarely administered as a single agent but in combination with other antifungal drugs (mainly LFAmB) for patients with IC, it is not suggested as a combination in elderly patients due to the accumulative nephrotoxicity risk.
Nosocomial candidemia is associated with increased mortality and this seems to be further aggravated in case of delay in antifungal drug initiation.Citation84–Citation86 Morrell et al have shown that initiating empiric antifungal treatment more than 12 hours after the first blood culture sample is associated with a greater risk of hospital mortality than when patients are started on antifungal therapy within the first 12 hours.Citation84 In a 5-year study, Parkins et al studied 207 patients with IC; 64 patients (32%) received empirical therapy, in 51 (26%) of which was deemed appropriate.Citation86 Similarly, Kumar et al demonstrated a 12% decreased survival probability for every hour’s delay in patients with fungal septic shock.Citation87 Therefore, prompt initiation of early empiric therapy is warranted in high risk patients.
Knowledge of risk factors for IC may help to identify those patients who could benefit from early antifungal therapy (). The Candida score was first introduced by León et al and the EPCAN study group, from data available from the surveillance study of fungal infection and colonization in critically ill patients.Citation88 Clinical sepsis (2 points), multifocal colonization (1 point), surgery (1 point), and total parenteral nutrition (1 point) are the risk factors that must be evaluated by the physician in order to identify patients who are candidates for empirical treatment. A score of >2.5 showed 81% sensitivity and 74% specificity for the early administration of empirical treatment in ICU patients.Citation88,Citation89
Table 3 Risk factors for fungal infections in the elderly
Conclusion
In summary, the increase in invasive candidiasis in older adults has become an important clinical problem, since the older population is growing and is nowadays more likely to take aggressive chemotherapeutic regimens for cancer, or immunosuppressive drugs for nonmalignant diseases. Moreover, aging leads to variable physiological changes, rendering older patients potentially more vulnerable to fungal infections. Elderly patients are more easily colonized by pathogenic fungi and have an increased incidence of C. glabrata fungemia, which has higher mortality rates as well as higher rates of resistance to fluconazole, especially after exposure to the drug. Therefore, although clinical manifestations in older and younger adults may be similar, for the treatment of the former the use of an echinocandin is safer, since treatment with amphotericin B is associated with increased nephrotoxicity risk. Azoles are less toxic but they must be used with caution, since older adults are usually under a number of medications and the risk of serious drug–drug interactions is more likely to appear.
Disclosure
The authors have no conflicts of interest to declare.
References
- KullbergBJVerweijPEAkovaMEuropean expert opinion on the management of invasive candidiasis in adultsClin Microbiol Infect201117Suppl 5S1S1222069786
- PappasPGRexJHLeeJA prospective observational study of candidemia: epidemiology, therapy and influences on mortality in hospitalized adult and pediatric patientsClin Infect Dis20033763464312942393
- TortoranoAMPemanJBernhardtHECMM Working Group on CandidaemiaEpidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) in hospital based surveillance studyEur J Clin Microbiol Infect Dis20042331732215029512
- GueryBPArendrupMCAuzingerGManagement of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: Part I. Epidemiology and diagnosisIntensive Care Med200935556218972101
- KauffmanCFungal infections in older adultsClin Infect Dis20013355055511462194
- PfallerMADiekemaDJEpidemiology of invasive candidiasis: a persistent public health problemClin Microbiol Rev20072013316317223626
- DimopoulosGKoulentiDBlotSElderly critically ill patients with infection: Analysis of the Extended Prevalence of Infection in Intensive Care Unit (EPIC II) StudyJ Amer Geriatr Soc2013 (In press)
- BlotSCankurtaranMPetrovicMEpidemiology and outcome of nosocomial bloodstream infection in elderly critically patients: a comparison between middle-aged, old, and very old patientsCrit Care Med2009371634164119325489
- DimopoulosGPaivaJAMeerssemanWEfficacy and safety of anidulafungin in elderly, critically ill patients with invasive Candida infections: a post hoc analysisInt J Antimicrob Agents20124052152622998997
- NicolasFLe GallJRAlperovitchALoiratPVillersDInfluence of patients’ age on survival, level of therapy and length of stay in intensive care unitsIntensive Care Med1987139133558942
- BlotSVandewoudeKHosteEPoelaertJColardynFOutcome in critically ill patients with candida fungaemia: Candida albicans versus Candida glabrataJ Hosp Infect20014730831311289775
- PappasPGKauffmanCAAndesDClinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of AmericaClin Infect Dis20094850353519191635
- UllmannAJGuidelines for the treatment of invasive fungal infections: ESCMID Candida guidelines 2011/2012Mycoses201154Suppl 241
- DiekemaDJMesserSABoykenLBIn vitro activity of seven systemically active antifungal agents against a large global collection of rare Candida species as determined by CLSI broth microdilution methodsJ Clin Microbiol2009473170317719710283
- PfallerMABoykenLHollisRJIn vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillanceJ Clin Microbiol20084615015618032613
- EggimannPGarbinoJPittetDEpidemiology of Candida species infections in critically ill non-immunosuppressed patientsThe Lancet20033685702
- LimCS-YRosliRSeowHFChongPPCandida and invasive candidiasis: back to the basicsEur J Clin Microbiol Infect Dis201231213121544694
- PfallerMADiekemaDJRinaldiMGResults from the ARTEMIS DISK Global Antifungal Surveillance Study: a 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testingJ Clin Microbiol2005435848585916333066
- WisplinghoffHBischoffTTallentSMSeifertHWenzelRPEdmondMBNosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance studyClin Infect Dis20043930931715306996
- ArendrupMCFuurstedKGahrn-HansenBSeminational surveillance of fungemia in Denmark: notably high rates of fungemia and number of isolates with reduced azole susceptibilityJ Clin Microbiol2005434434444016145088
- LauplandKBGregsonDBChurchDLRossTElsayedSInvasive Candida species infections: a 5 year population-based assessmentJ Antimicrob Chemother20055653253716040623
- SandvenPBevanagerLDigranesAHauklandHHMannsakerTGaustedPNorwegian Yeast Study GroupCandidemia in Norway, 1991 to 2003: results from a nationwide studyJ Clin Microbiol2006441977198116757587
- AsmundsdottirLRErlendsdottirHGottfredssonMIncreasing incidence of candidemia: results from a 20-year nationwide study in IcelandJ Clin Microbiol2002403489349212202600
- AlmiranteBRodriguezDParkBJEpidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003J Clin Microbiol2005431829183515815004
- MalaniAHmoudJChiuLCarverPLBielaczycAKauffmanCACandida glabrata fungemia: experience in a tertiary care centerClin Infect Dis20054197598116142662
- HedderwickSAWanJYBradleySFSangeorzanJATerpeningMSKauffmanCARisk factors for colonization with yeast species in a Veterans Affairs long-term care facilityJ Am Geriatr Soc1998468498539670871
- LockhartSRJolySVargasKSwails-WengerJEngerLSollDRNatural defences against Candida colonization break down in the oral cavities of the elderlyJ Dent Res19997885786810326730
- Abi-SaidDAnaissieEUzunORaadIPinzcowskiHVartivarianSThe epidemiology of hematogenous candidiasis caused by different Candida speciesClin Infect Dis199724112211289195068
- MarrKASeidelKWhiteTCBowdenRACandidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazoleJ Infect Dis200018130931610608780
- SafdarAChaturvediVCrossEWProspective study of Candida species in patients at a comprehensive cancer centerAntimicrob Agents Chemother2001452129213311408236
- ClarkTASlavinskiSAMorganJEdemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospitalJ Clin Microbiol2004424468447215472295
- DiekemaDJMesserSAHollisRJWenzelRPPfallerMAAn outbreak of Candida parapsilosis prosthetic valve endocarditisDiagn Microbiol Infect Dis1997291471539401807
- FridkinSKThe changing face of fungal infections in health care settingsClin Infect Dis2005411455146016231257
- ViscoliCGirmeniaCMarinusACandidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organizaiton for Research and Treatment of Cancer (EORTC)Clin Infect Dis1999281071107910452637
- LinMYCarmeliYZumstegJPrior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: a case-case-control studyAntimicrob Agents Chemother2005494555456016251295
- ColomboALNucciMSalomãoRHigh rate of non-albicans candidemia in Brazilian tertiary care hospitalsDiagn Microbiol Infect Dis19993428128610459478
- ColomboALMeloASARosasRFCOutbreak of Candida rugosa candidemia: an emerging pathogen that may be refractory to Amphotericin B therapyDiagn Microbiol Infect Dis20034625325712944016
- DubeMPHeseltinePNRinaldiMGEvansEZawackiBFungemia and colonization with nystatin-resistant Candida rugosa in a burn unitClin Infect Dis19941877828054438
- ReinhardtJFRuanePJWalkerLJGeorgeWLIntravenous catheter-associated fungemia due to Candida rugosaJ Clin Microbiol198522105610574066918
- SugitaTTakeoKOhkusuMFluconazole-resistant pathogens Candida inconspicua and Candida norvegensis: DNA sequence diversity of the rRNA intergenic spacer region, antifungal drug susceptibility, and extracellular enzyme productionMicrobiol Immunol20044876176615502409
- D’AntonioDViolanteBMazzoniAA nosocomial cluster of Candida inconspicua infections in patients with hematological malignanciesJ Clin Microbiol1998367927959508314
- BailyGGMooreCBEssayagSMde WitSBurnieJPDenningDWCandida inconspicua, a fluconazole-resistant pathogen in patients infected with human immunodeficiency virusClin Infect Dis1997251611629243058
- HofHMikusGCandida infections in the elderlyZ Gerontol Geriatr2013466470 German [with English abstract]22297919
- ZarembaMLDanilukTRozkiewiczDIncidence rate of Candida species in the oral cavity of middle-aged and elderly subjectsAdv Med Sci200651Suppl 123323617458099
- FanelloSBoucharaJPSauteronMPredictive value of oral colonization by Candida yeasts for the onset of a nosocomial infection in elderly hospitalized patientsJ Med Microbiol20065522322816434716
- AwDSilvaABPalmerDBIs the thymocyte development functional in the aged?Aging2009114615320157506
- Colonna-RomanoGBuffaSBulatiMB cells compartment in centenarian offspring and old peopleCurr Pharm Des20101660460820388070
- StineJGSateeshPLewisJHDrug-induced liver injury in the elderlyCurr Gastroenterol Rep20131529923250699
- WoodhouseKWynneHAAge-related changes in hepatic function. Implications in drug therapyDrugs Aging199222432551606355
- DerayGAmphotericin B nephrotoxicityJ Antimicrob Chemother200249Suppl 1S37S41
- TulnerLRKuperIMFrankfortSVDiscrepancies in reported drug use in geriatric outpatients: relevance to adverse events and drug-drug interactionsAm J Geriatr Pharmacother200979310419447362
- FickDMCooperJWWadeWEWallerJLMacleanJRBeersMHUpdating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of expertsArch Intern Med20031632716272414662625
- GallagherPBarryPO’ MahonyDInappropriate prescribing in the elderlyJ Clin Pharm Ther20073211312117381661
- YoonSLSchafferSDHerbal, prescribed and over-the-counter drug use in older women: prevalence of drug interactionsGeriatr Nurs20062711812916638483
- AhmadSKhanZInvasive candidiasis: A review of nonculture-base laboratory diagnosting methodsIndian J Microbiol201230264269
- FalagasMEApostolouKEPappasVDAttributable mortality of candidemia: a systematic review of matched cohort and case-control studiesEur J Clin Microbiol Infect Dis20062541942516773391
- SendidBTabouretMPoirotJLMathieuDFruitJPoulainDNew enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasisJ Clin Microbiol1999371510151710203514
- MokaddasEKhanZUAhmadSNampooryMRBurhamahMValue of (1–3)-β-d-glucan, Candida mannan and Candida DNA detection in the diagnosis of candidemiaClin Microbiol Infect2011171549155321883664
- SendidBPoirotJLTabouretMCombined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida speciesJ Med Microbiol20025143344211990496
- KooSBryarJMPageJHBadenLRMartyFMDiagnostic performance of the (1–>3) -β-D-glucan assay for invasive fungal diseaseClin Infect Dis2009491650165919863452
- Ostrosky-ZeichnerLAlexanderBDKettDHMulticenter clinical evaluation of the (1–>3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humansClin Infect Dis20054165465916080087
- DimopoulosGAntonopoulouAArmaganidisAVincentJLHow to select an antifungal agent in critically-ill patientsJ Crit Care2013 (In press)
- NucciMLoureiroMSilveiraFComparison of the toxicity of amphotericin B in 5% dextrose with that of amphotericin B in fat emulsion in a randomized trial with cancer patientsAntimicrob Agents Chemother1999431445144810348768
- WhiteMHBowdenRASandlerESRandomized, double-blind clinical trial of amphotericin B colloidal dispersion vs amphotericin B in the empirical treatment of fever and neutropeniaClin Infect Dis1998272963029709879
- WalshTJFinbergRWArndtCLiposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study GroupN Engl J Med199934076477110072411
- SharkeyPKGraybillJRJohnsonESAmphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal menignitis in patients with AIDSClin Infect Dis1996223153218838189
- WingardJRWhiteMHAnaissieERaffalliJGoodmanJArrietaALAmph/ABLC Collaborative Study GroupA randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropeniaClin Infect Dis2000311155116311073745
- PovoaPGoncalves-PereiraJTreatment of candidemia in adult patients without neutropenia – an inconvenient truthCrit Care20111511421345263
- RexJHBennettJESugarAMA randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National InstituteN Engl J Med1994331132513307935701
- RexJHPappasPGKarchmerAWA randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in non-neutropenic subjectsClin Infect Dis2003361221122812746765
- LoefflerJStevensDAntifungal drug resistanceClin Infect Dis200336Suppl 1S31S4112516028
- von MachMABurhenneJWeilemannLSAccumulation of the solvent vehicle sulphobutylether beta cyclodextrin sodium in critically ill patients treated with intravenous voriconazole under renal replacement therapyBMC Clin Pharmacol2006661216981986
- WalshTJPappasPWinstonDJNational Institute of Allergy and Infectious Diseases Mycoses Study GroupVoriconazole compared with Liposomal Amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent feverN Engl J Med200234622523411807146
- Mora-DuarteJBettsRRotsteinCComparison of caspofungin and amphotericin B for invasive candidiasisN Engl J Med20023472020202912490683
- KuseERChetchotisakdPda CunhaCAMicafungin versus liposomal amphotericin B for candidemia and invasive candidiasis: a phase III randomised double-blind trialLancet20073691519152717482982
- ReboliACRotsteinCPappasPGAnidulafungin versus fluconazole for invasive candidiasisN Engl J Med20073562472248217568028
- PappasPGRotsteinCMBettsRFMicafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasisClin Infect Dis20074588389317806055
- ArendrupMCGarcia-EffronGLass-FlorlCEchinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI m27-a3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest mediaAntimicrob Agents Chemother20105442643919884370
- Garcia-EffronGKatiyarSKParkSEdlindTDPerlinDSA naturally occurring proline-to-alanine amino acid change in fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibilityAntimicrob Agents Chemother2008522305231218443110
- PfeifferCDGarcia-EffronGZaasAKPerfectJRPerlinDSAlexanderBDBreakthrough invasive candidiasis on micafunginJ Clin Microbiol2010482373238020421445
- Kale-PradhanPBMorganGWilhelmSMJohnsonLBComparative efficacy of echinocandins and nonechinocandins for the treatment of Candida parapsilosis infections: a meta-analysisPharmacotherapy2010301207121321114387
- ChamilosGLewisREAlbertNKontoyiannisDPParadoxical effect of Echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differencesAntimicrob Agents Chemother2007512257225917438060
- EschenauerGDePestelDDCarverPLComparison of echinocandin antifungalsTher Clin Risk Manag20073719718360617
- MorrellMFraserVJKollefMHDelaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortalityAntimicrob Agents Chemother2005493640364516127033
- KumarAEllisPArabiYInitiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shockChest20091361237124819696123
- ParkinsMDSabudaDMElsayedSLauplandKBAdequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infectionsJ Antimicrob Chemother20076061361817576697
- KumarARobertsDWoodKEDuration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shockCrit Care Med2006341589159616625125
- LeónCRuiz-SantanaSSaavedraPEPCAN Study GroupA bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonizationCrit Care Med20063473073716505659
- LeónCRuiz-SantanaSSaavedraPCava Study GroupUsefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter studyCrit Care Med2009371624163319325481
- ColomboALGuimarãesTSilvaLRBFProspective observational study of candidemia in São Paulo, Brazil: incidence rate, epidemiology, and predictors of mortalityInfect Control Hosp Epidemiol20072857057617464917
- BassettiMTrecarichiEMRighiEIncidence, risk factors, and predictors of outcome of candidemia. Survey in 2 Italian university hospitalsDiagn Microbiol Infect Dis20075832533117350205