Abstract
As we age, our organ functions gradually decline. Circulating factors in the blood and the integrity of organ barriers can become dysfunctional, resulting in a condition known as leaky syndrome. This condition involves the unregulated exchange or leakage of components between organs. However, the triggers of leaky syndrome, as well as its role in aging-related disorders and illnesses, remain largely unknown. In this editorial, we discuss potential mechanisms that originate from the gut and resident microbes (microbiome) to contribute in leaky syndrome. Furthermore, we explore how the food we consume can impact the development of leaky syndrome, potentially influencing the biology of aging and challenges to diagnose the leaky gut condition accurately and clinically.
Aging is a natural process associated with decreased physiologic function in all organs, ie it not only affects our immune system, but also affects all tissues and cells, resulting in increased risk of several chronic diseases and vulnerability to death. The gut microbiome is now recognized as one of the key elements to maintaining host healthCitation1 and contributing to disease progressions such as high abundance of pathogenic bacteria (such as Escherichia coli, Staphylococcus aureus, and Clostridium difficile) and low abundance of SCFA producing bacteria such as Bifidobacterium, Faecalibacterium, Roseburia.Citation2 Several studies over the past few years revealed that the gut microbiome and its composition changes with age which could have significant implications on overall health during aging,Citation3,Citation4 however, the mechanisms by which it impacts the biology of aging remain largely unknown.
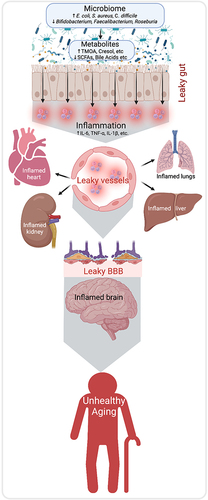
The microbiome is composed of diverse microbes ie, bacteria, archaea, viruses, eukaryotic microbes, and fungi, that have lived in and around our body since birth.Citation2 The gut and skin are the most extensively colonized regions of our body, while other areas including the mouth, eyes, ears, and reproductive organs also harbor dense populations of specific microbes. These microbes establish a symbiotic relationship with the host, playing a crucial role in regulating essential functions such as protection against pathogens, immunomodulation, and maintaining the structural integrity of the gut mucosal barrier, indicating a strong association between abnormalities in gut microbiota and the development of a wide range of diseases including autoimmune disorders,Citation5 depression and neurodegenerative diseases such as Alzheimer’s diseaseCitation6 and metabolic disorders.Citation7,Citation8 However, the mechanisms by which microbiome contributes to the development of these diseases are unclear. There can be several mechanisms but inflammation is a key suspect. Low-grade inflammation is often higher in older adults but the source of inflammation remains largely elusive. Growing evidence indicates that gut dysbiosis, characterized by an imbalance in gut microbial composition, tends to escalate with age. This dysbiosis, in turn, contributes to increased gut permeability, often referred to as “leaky gut”. This heightened permeability facilitates the passage of pro-inflammatory substances such as bacterial toxins and lipopolysaccharide (LPS) from the gut lumen into the bloodstream or mucosal immune system, thereby triggering inflammation. Elevated inflammation is also known to increase the permeability of other epithelial and endothelial barriers such as intestinal epithelia (leaky gut), blood vessel endothelia (leaky vessels), blood–brain barrier (BBB) (leaky brain), and others, and collectively called “leaky syndrome” (). The link between leaky syndrome with chronic inflammation and microbiome dysbiosis in aging biology remains poorly understood.
Leaky Gut
Gut permeability is precisely controlled by various gut barriers, including tight junction proteins and a dense mucin layer. These barriers work in concert to facilitate selective transport, allowing only nutrients and essential molecules to pass from the gut lumen into the blood circulation.Citation9 However, loosening or breaking down of these barriers causes nonselective diffusion of ingredients and the bacterial endotoxin from the gut lumen to mucosal and blood circulation, which instigates inflammation.Citation10,Citation11 Emerging evidence shows that abnormalities in gut microbiome are key to inducing a leaky gut, causing inflammation.Citation12 Increased leaky gut is a common phenomenon in patients with inflammatory bowel diseases like ulcerative colitis, Crohn’s disease, obesity, diabetes, cancer survivors as well as Alzheimer’s disease, which are all age-related disorders;Citation9,Citation13 and result in unhealthy aging. However, the causal and consequential relationship of the leaky gut with these age-related diseases remains elusive. In addition, the mechanisms by which gut microbiome breaks gut barriers also remain elusive, and comprehensive research efforts are needed to understand the etiology of the leaky gut and find therapeutic targets to mitigate it. Presently, there are no medical guidelines for treating and preventing bacterial translocation with leaky gut syndrome;Citation14 however, microbiome modulators such as probiotics and prebiotics can improve gut barrier function and inhibit bacterial invasion.Citation15
Leaky Blood Vessels
Leaky blood vessels refer to a condition known as vascular leakage or increased vascular permeability. Blood vessels have a natural barrier called the endothelium, which prevents substances from leaking out of the blood vessels into the surrounding tissues. However, under certain conditions, the blood vessel walls may become weakened or damaged, resulting in increased permeability and the leakage of fluids, proteins, and cells. Nearly two decades ago, a gut permeability issue was recognized as a potential cause for the decline in mental state unrelated to ammonia levels in advanced liver disease.Citation16 In addition, several studies suggest that the endothelium of blood vessels becomes more permeable with age, increasing the risk of conditions such as sepsis, stroke, traumatic injuries, and ischemic injuries.Citation17 This increased permeability of blood vessels can impact various organs, including the heart, lungs, kidneys, liver, and others. Consequently, the concept of “leaky syndrome” emerges as an interdisciplinary understanding of the pathophysiology underlying many human diseases. As we age, our blood vessels become more fragile and dysfunctional. However, the connection between gut abnormalities, specifically leaky gut, and leaky blood vessels remains poorly understood. Enhancing our understanding of the aging-related leaky syndrome will pave the way for the development of preventive and therapeutic strategies.
Leaky BBB
The BBB is an essential fence that keeps the brain protected from several extrinsic and intrinsic antigens, pathogens, toxins, microbes, and their metabolites that circulate in the blood; however, the disruptions in the BBB cause severe damage to the brain cells and their functions.Citation18 Several neurodegenerative diseases like Alzheimer’s disease, Parkinson's, Huntington’s, Amyotrophic lateral sclerosis (ALS), and others are characterized with leaky BBB.Citation19,Citation20 However, the contribution of leaky BBB in such neurodegenerative diseases is not well known. Emerging evidence indicates that the gut microbiota dysbiosis are linked with increased neurodegenerative pathology in the brain via increasing leaky BBB.Citation21 The gut microbiota generates a variety of metabolites, such as short-chain fatty acids, bile acids, neurotransmitters, and other bioactive molecules within the gut. These metabolites have the ability to enter the circulation and influence the permeability of the blood–brain barrier (BBB). However, the precise mechanisms through which these metabolites affect BBB permeability are still largely unknown.
Leaky Syndrome and Diet
Diet is the most significant modulator of the gut microbiome and thus can impact leaky syndrome, inflammation, and aging biology. A diet with more fruits, vegetables, whole grains, dietary fiber, dairy, and less added sugars, saturated fat, and sodium increases the growth of beneficial microbiota, improving gut health. A high-fat diet, also called Western diet, appears to be a driving factor in developing abnormalities in the gut microbiome. For example, a fiber enriched diet promotes the growth of a beneficial microbiome, which leads to the production of beneficial metabolites like short-chain fatty acids (ie, SCFAs such as acetate, propionate, and butyrate), that exhibit a beneficial impact on host health, including barrier functions of intestine, blood vessels, and BBB. In fact, dietary interventions endowed by substantial content of polyphenols has been tested in a randomized, controlled, crossover study in subjects ≥60 years with proven gut permeability (high serum zonulin).Citation22 Compared to a control diet supplemented, subjects on a polyphenol-rich diet showed a significant decrease of serum zonulin associated with a beneficial enhancement of butyrate-producing and fiber-fermenting bacteria especially in those with higher BMI or impending metabolic syndrome. The gut microbiota modulators such as probiotics, prebiotics, postbiotics, and synbiotics are also acceptable and good tools to beneficially manipulate the microbiome to promote the production of beneficial metabolites, including SCFAs, thus promoting healthy aging.Citation23 However, the research on diet and other microbiome modulators to impact aging biology are understudied. Aging per se besides, frailty is a further aggravating factor of age-related leaky-gut syndrome. Indeed, Rashidah et al,Citation24 by examining extensive literature, have shown that, frail compared to healthy elderly had a significantly different gut microbiota composition with lower diversity and lower abundance of SCFA producers and increased serum zonulin and pro-inflammatory cytokines/factors which are associated to sarcopenia.
Although, the role of leaky syndrome in aging and its related illnesses is not fully understood, emerging evidence indicates the modulation of microbiome using both Pharmacological and nonpharmacological interventions including probiotics, prebiotics, postbiotics and others reduce leaky gut, systemic inflammation and aging-related conditions including metabolic and neuronal functions.Citation8,Citation25 Antibiotics disrupt the microbiome balance, and older adults are often exposed to antibiotics more frequently due to their higher susceptibility to infections. However, role of antibiotics indeveloping leaky syndrome and aging biology is debatable and needs further research.
Challenges and Future Opportunities
Increased leakiness is also a physiological response for many acute responses, including exercise, which helps wound healing, muscle development, and other acute recovery events. However, the chronic appearance of leakiness in the intestine, blood vessels, and the BBB with aging become part of pathology in age-related disorders. Therefore, differentiating leaky syndrome as a physiological event versus a pathological one remains a significant challenge. In recent years, it has been demonstrated that under energetic stress, a specific stress-polarity signaling (SPS) is activated via AMP-kinase.Citation26 This determines an increase of epithelial polarity. However, in the aging process it seems that the SPS-pathway is inhibited but its recharge on an experimental level can bring about a restitutio ad integrum of aging-associated dysbiosis, inflammatory changes and of loss of barrier function. Promising data come also from urolithin A, a main gut microbial metabolite derived from while curbing inflammatory microbiota phenotype.Citation27 Its mechanism leading to tight junction proteins upregulation, albeit still under scrutiny, seems to be related to the activation of aryl hydrocarbon receptor (AhR)- or erythroid 2-related factor 2-dependent pathways. On a pharmacological level, although at experimental level mimicking human metabolic syndrome, metformin was able to decrease leaky gut abnormalities enhancing goblet cell and mucin mass and reducing inflammation by downregulating Wnt signaling.Citation28
As the population is rapidly aging, older adults will supersede young adults, the age-related problems will be the primary health care burden in the coming years. Finding the right time windows and developing preventive and therapeutic strategies to curve leaky syndrome will open new opportunities to delay, prevent and/or treat age-related disorders – debilitating public health problems.
Disclosure
None of the authors report any other conflicts of interest related to this work. Francesco Marotta is affiliated to ReGenera R&D International for Aging Intervention. Dr. Yadav is Co-founder and Chief Scientific Officer of Postbiotics Inc and Dr. Jain is founder of MusB LLC, but their roles have no conflict and influence with the work presented in this manuscript. Dr. Leila Haghshenas is affiliated to the Department of Clinical Bioinformatics, Postdoc Association Member of Harvard Medical School, Boston, MA USA.
References
- Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. 2017;35:36–45. doi:10.1016/j.arr.2017.01.001
- de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi:10.1136/gutjnl-2021-326789
- Badal VD, Vaccariello ED, Murray ER, et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12(12):3759. doi:10.3390/nu12123759
- Nagpal R, Mainali R, Ahmadi S, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4):267–285. doi:10.3233/NHA-170030
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. doi:10.1038/nrgastro.2012.152
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi:10.3389/fncel.2015.00392
- Backhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58(Suppl 2):44–52. doi:10.1159/000328042
- Mishra SP, Wang B, Jain S, et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut. 2023;gutjnl-2022–327365. doi:10.1136/gutjnl-2022-327365
- Aleman RS, Moncada M, Aryana KJ. Leaky gut and the ingredients that help treat it: a review. Molecules. 2023;28(2):619.
- Usuda H, Okamoto T, Wada K. Leaky gut: effect of dietary fiber and fats on microbiome and intestinal barrier. Int J Mol Sci. 2021;22(14):7613. doi:10.3390/ijms22147613
- Hollander D, Kaunitz JD. The “Leaky Gut”: tight junctions but Loose Associations? Dig Dis Sci. 2020;65(5):1277–1287. doi:10.1007/s10620-019-05777-2
- Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80. doi:10.1186/s40168-017-0296-0
- Kohler CA, Maes M, Slyepchenko A, et al. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des. 2016;22(40):6152–6166. doi:10.2174/1381612822666160907093807
- Twardowska A, Makaro A, Binienda A, Fichna J, Salaga M. Preventing bacterial translocation in patients with leaky gut syndrome: nutrition and pharmacological treatment options. Int J Mol Sci. 2022;23(6):3204. doi:10.3390/ijms23063204
- Bagarolli RA, Tobar N, Oliveira AG, et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J Nutr Biochem. 2017;50:16–25. doi:10.1016/j.jnutbio.2017.08.006
- Campion D, Giovo I, Ponzo P, Saracco GM, Balzola F, Alessandria C. Dietary approach and gut microbiota modulation for chronic hepatic encephalopathy in cirrhosis. World J Hepatol. 2019;11(6):489–512. doi:10.4254/wjh.v11.i6.489
- Oakley R, Tharakan B. Vascular hyperpermeability and aging. Aging Dis. 2014;5(2):114–125. doi:10.14336/AD.2014.0500114
- Obrenovich MEM, Gut L. Leaky brain? Microorganisms. 2018;6(4):107. doi:10.3390/microorganisms6040107
- Wu YC, Sonninen TM, Peltonen S, Koistinaho J, Lehtonen S. Blood-brain barrier and neurodegenerative diseases-modeling with iPSC-derived brain cells. Int J Mol Sci. 2021;22(14):7710.
- Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. doi:10.1038/nrneurol.2017.188
- Tang W, Zhu H, Feng Y, Guo R, Wan D. The impact of gut microbiota disorders on the blood-brain barrier. Infect Drug Resist. 2020;13:3351–3363. doi:10.2147/IDR.S254403
- Guglielmetti S, Bernardi S, Del Bo C, et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 2020;20(1):1–10.
- Ghosh TS, Shanahan F, O’Toole PW. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol. 2022;19(9):565–584.
- Rashidah NH, Lim SM, Neoh CF, et al. Differential gut microbiota and intestinal permeability between frail and healthy older adults: a systematic review. Ageing Res Rev. 2022;2022:101744.
- Miller B, Mainali R, Nagpal R, Yadav H. A newly developed synbiotic yogurt prevents diabetes by improving the microbiome–intestine–pancreas axis. Int J Mol Sci. 2021;22(4):1647. doi:10.3390/ijms22041647
- Ghosh P, Swanson L, Sayed IM, et al. The stress polarity signaling (SPS) pathway serves as a marker and a target in the leaky gut barrier: implications in aging and cancer. Life Sci Alliance. 2020;3(3). doi:10.26508/lsa.201900481
- Singh R, Chandrashekharappa S, Bodduluri SR, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10(1):89. doi:10.1038/s41467-018-07859-7
- Ahmadi S, Razazan A, Nagpal R, et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol. 2020;75(7):e9–e21. doi:10.1093/gerona/glaa056