 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and purpose
QT and Tpeak-Tend (Te) intervals are associated with sudden cardiac death in patients with chronic heart failure (CHF). We studied age-dependent influence on short-term temporal dispersion of these two variables in patients with postischemic CHF.
Method
We grouped 75 CHF and 53 healthy control subjects into three age subsets: ≤50 years, >50 years and ≤65 years, and >65 years. We then calculated the following indices: QT and Te variability index (QTVI and TeVI), the ratio between the short-term variability (STV) of QT or Te, and the STV of resting rate (RR) (QT/RR STV and Te/RR STV).
Results
In all different age subgroups, patients with CHF showed a higher level of QTVI than age-matched control subjects (≤50 years: P < 0.0001; >50 years and ≤65 years: P < 0.05; >65 years: P < 0.05). Patients with CHF < 50 years old also had all repolarization variability indices higher than normal age-matched controls (TeVI, P < 0.05; QT/RR STV, P < 0.05; Te/RR STV, P < 0.05), whereas we did not find any difference between the two older classes of subjects. Both QTVI (r2: 0.178, P < 0.05) and TeVI (r2: 0.433, P < 0.001) were positively related to age in normal subjects, even if the first correlation was weaker than the second one.
Conclusion
Our data showed that QTVI could be used in all ages to evaluate repolarization temporal liability, whereas the other indices are deeply influenced by age. Probably, the age-dependent increase in QTVI was more influenced by a reduction of RR variability reported in older normal subjects.
Introduction
Malignant ventricular arrhythmias are common complications of chronic heart failure (CHF) induced by myocardial infarction. SenescenceCitation1,Citation2 and CHFCitation3,Citation4 strongly influence the cardiovascular autonomic control and myocardial repolarization phase.Citation5–Citation9 Indeed, both these conditions are able to increase α-adrenoreceptor-mediated peripheral resistanceCitation1 and to reduce β-adrenergic adrenoreceptorCitation10 and baroreflex function,Citation10,Citation11 with a consistent decrease of heart rate variability.Citation12–Citation16 The increase of temporal dispersion of the repolarization ventricular phaseCitation17–Citation25 and the reduction of heart rate variabilityCitation25–Citation27 are risk factors for ventricular malignant arrhythmias and sudden cardiac death (SCD) and non-SCD. AgingCitation28–Citation30 and CHFCitation31 are clear pathogenetic factors able to induce ventricular arrhythmogenesis. Neurohumoral activation,Citation4,Citation6,Citation32 electroanatomical remodeling,Citation33,Citation34 chronic inflammatory condition,Citation35 and endothelial dysfunctionCitation36–Citation39 are well-recognized causes of ventricular arrhythmia in patients with CHF. Elderly patients with CHF show higher proneness for the malignant ventricular arrhythmias because these subjects have lower levels of functional reserve capable of resisting arrhythmogenesis. Although the influence of CHF and aging on ventricular arrhythmias is well known, the underlying pathogenic mechanisms are not yet completely understood. Particularly, the impact of aging on markers of repolarization’s temporal dispersion during CHF has not been clarified. In a recent large community-based study, an increased risk of SCD has been found related to a prolonged Tpeak-Tend interval (Te).Citation40 In addition, in patients with CHF and SCD, a higher temporal dispersion was reported.Citation5
All in all, we analyzed QT interval (from Q to end T wave electrocardiogram [ECG]) variability index (QTVI) and short-term variability (STV) of QT in CHF subjects with different ages, in order to individuate a possible age-dependent influence on these markers. Finally, we calculated the same indices of variability on the last part of the T wave (considering the peak and end of the T wave) to observe a peculiar age-related effect on this important part of repolarization.
Methods
Study subjects
For this study we selected 75 outpatients (63 men and 12 woman) who had stable CHF secondary to ischemic dilated cardiomyopathy, and 53 healthy control subjects (41 men and 12 women). We defined clinically stable patients as those who had not been hospitalized or had their therapy adjusted or had experienced any other acute coronary artery or noncoronary event during the past 3 months. All participants had undergone revascularization either cutaneously or by aortocoronary artery bypass at least 3 months before the study. None of the patients had malignancy, primary valve disease, atrial fibrillation, premature complexes (one premature complex per minute was permitted), or other arrhythmias likely to interfere with heart rate and QT analysis. None of the patients was New York Heart Association class IV. Before the study, none of the subjects had a documented history of cardiac arrest, ventricular tachycardia, or fibrillation. We suggested that all patients with ejection fraction ≤35% underwent an implant of implantable cardioverter/defibrillator device.
To detect possible statistical differences related to age we divided each of the two study groups into three age subsets: ≤50 years, >50 years and ≤65 years, and >65 years.
Study protocol and offline data analysis
After a 10-minute rest lying down, each subject underwent a 5-minute, single ECG lead recording during controlled breathing (15 breaths per minute, 0.25 Hz). All digitized signal recordings were analyzed by a single physician (GP) blinded to the subjects’ circumstances.
We measured the following intervals from the respective time series of ECG recordings: resting rate (RR), QT (from the Q wave to the T wave end), and Te (from the T peak to T wave end). We therefore calculated mean and variance values of each of these intervals and then used the original formula proposed by Berger et alCitation41 to calculate three different QT variability indices:
Software for data acquisition and storage and for spectral analysis were designed and produced by our research group and are described in detail elsewhere.Citation7–Citation9,Citation42–Citation45
Finally, we used all ECG recordings to measure the STV of the aforementioned intervals. This variable was calculated following the standard method, namely by using the first 60 consecutive beats (STV60) Citation6,Citation46,Citation47 and also by using the total number of beats in the whole 5-minute recording (STVT). The formula used was:
where D was the duration of RR or QTe, or Te interval. Consequently, we were able to obtain the following six STV indices: RR STV60, QT STV60, Te STV60, RR STVT, QTe STVT, and Te STVT.
Finally, we calculated the following ratio between the different STVs:
Moreover, from the same 5-minute ECG segment, the corrected QT and Te intervals were obtained according to the formulas proposed by Bazett (QT/RR0.5; QT; Te/RR0.5), Friedericia (QT/RR0.33; Te/RR0.33), Lilly (QT/RR0.4; Te/RR0.4), and Framingham (QT+ [0.154*{1000-RR}]; Te+ [0.154*{1000–RR}]).
Statistical analysis
Unless otherwise indicated, all data are expressed as means ± standard deviation. Data with skewed distribution are given as median and interquartile range (75th percentile–25th percentile). Categorical variables were analyzed with the χ2 test. One-way analysis of variance and the Bonferroni test were used to compare data for the normally distributed variables. Kruskal–Wallis and Mann–Whitney tests were used to compare non-normally distributed variables (as evaluated by the Kolmogorov–Smirnov test). To detect possible statistical differences related to age, we divided each of the two study groups into three age subsets: ≤50 years, >50 years and ≤65 years, and ≥65 years.
Stepwise multiple regression analysis was used to determine possible relationships between the studied variables. P-values ≤ 0.05 were considered statistically significant. All data were evaluated with the database SPSS-PC+ (SPSS-PC+ Inc, Chicago, IL, USA).
Results
We examined 128 subjects, 75 with CHF and 53 healthy controls. The clinical characteristics of subjects enrolled in the study are shown in .
Table 1 General characteristics of study sample
Age, body mass index, and sex distribution did not differ significantly between the two groups, whereas heart rate, diastolic arterial pressure, left ventricular ejection fraction, and QT interval differed significantly (). Both subgroups had a similar mean intergroup age ().
Table 2 Mean age of subjects in the three age-subgroups
The younger CHF group showed a reduction of RR variance in comparison with the age-matched healthy controls, whereas only the older CHF group reported a longer RRmean than controls (). Only elderly subjects had a significantly larger QTmean (P < 0.05), QT variance (P < 0.05), and Temean (P < 0.05) than age-matched healthy controls. Furthermore, the middle-aged group showed a QTvariance (P < 0.05) significantly higher than age-matched controls (). In all subsets, independently from age, the CHF group showed a significantly higher QTVI (P < 0.001) than age-matched controls, but only the younger CHF group had all other QT variability values significantly higher (TeVI: P < 0.05, QT/RR STV60: P < 0.05, Te/RR STV60: P < 0.05, QT/RR STVT: P < 0.05, and Te/RR STVT: P < 0.05) in comparison with age-matched controls (). In the middle-aged and elderly CHF groups we did not find any differences for the other repolarization variables ().
Table 3 RR interval and QT dynamics data according to age and presence of chronic heart failure
Table 4 QT variability indices according to age and presence of chronic heart failure
We did not find any difference between the three CHF groups in respect of the RR and QT variables ( and ). RRvariance (P < 0.001) was significantly higher in younger and middle-aged control subjects than older subjects (), and this variable was also significantly higher in younger controls compared with healthy middle-aged subjects. Te (P < 0.05) was higher in younger and middle-aged control subjects than older subjects (). On the other hand, Tevariance (P < 0.05) was higher in the two younger groups than the older normal subjects.
In the control group, all temporal dispersion variables (QTVI, TeVI, QT/RR STV60, Te/RR STV60, QT/RR STVT, Te/RR STVT) were significantly lower in the younger group in comparison with the older subjects (P < 0.05) (). QTVI, TeVI, QT/RR STVT, and Te/RR STVT were lower in the middle-aged subjects than in the older ones. Only QTVI results were significantly lower in the younger control group compared with the middle-aged group ().
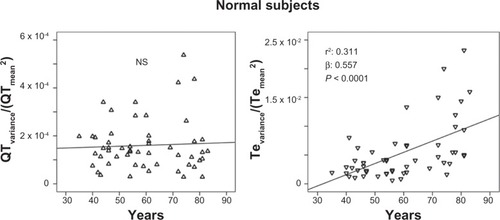
The stepwise multiple regression analysis found a significant relation with QTVI and ejection fraction (R: 0.364; R2: 0.132, β: −0.364, P: 0.001) in CHF subjects. Instead, QTVI (P < 0.05), TeVI (P < 0.001), QT/RR STV60 (P < 0.05), Te/RR STV60 (P < 0.05), QT/RR STVT (P < 0.001), and Te/RR STVT (P < 0.001) showed a significant positive relation with aging in control groups ( and ). We also found a negative significant correlation between all measures of RR variability and age () in the same subjects. Except for Tevariance/(Tmean2) (), we did not find any significant correlation between age and repolarization variables without RR normalization in the control group.
Figure 1 Relationship between QT variability index (QTVI) or Tpeak-Tend variability index (TeVI) and age in healthy control subjects.
Abbreviation: QT, from the Q wave to the T wave end.
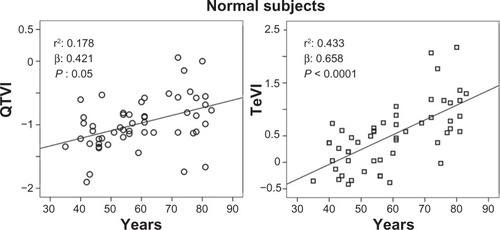
Figure 2 Relationship between age and ratio of short-term QT or Tpeak-Tend (Te) variability (STV) and resting rate (RR) variability indices, calculated on 60 consecutive beats (QT/RR STV60, Te/RRv STV60) or on 5-minute electrocardiogram recordings (QT/RR STVT, Te/RRv STVT) in healthy control subjects.
Abbreviations: QT, from the Q wave to the T wave end; v, variance.
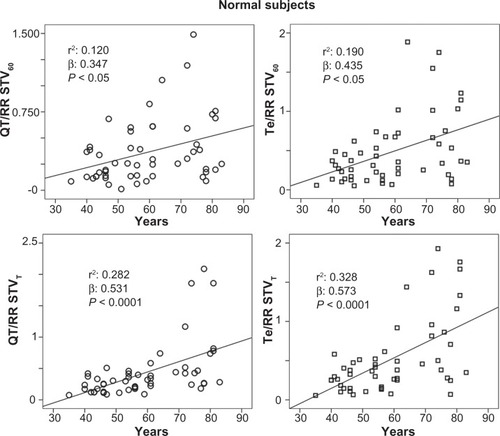
Figure 3 Relationship between age and the resting rate (RR) variability indices (ratio denominator of QTVI, TeVi, and short-term variability [STV], see Methods section), calculated on 5-minute electrocardiogram recordings (RRvariance/RRmean2 and RR STVT) or on 60 consecutive QRSs (RR STV60) in healthy control subjects.
Abbreviations: TeVI, Tend Variability index; Q-T, waves interval.
![Figure 3 Relationship between age and the resting rate (RR) variability indices (ratio denominator of QTVI, TeVi, and short-term variability [STV], see Methods section), calculated on 5-minute electrocardiogram recordings (RRvariance/RRmean2 and RR STVT) or on 60 consecutive QRSs (RR STV60) in healthy control subjects.Abbreviations: TeVI, Tend Variability index; Q-T, waves interval.](/cms/asset/738b5785-a5a6-425c-8a17-2655e85aa4c5/dcia_a_41879_f0003_b.jpg)
Figure 4 Relationship between age and Te variance/Te mean2 (ratio numerator of Tpeak-Tend variability index, see Methods section).
Discussion
Our main and original finding was that only QTVI values remained significantly higher in patients with CHF regardless of their age, whereas all other indices of temporal myocardial repolarization dispersion were significantly higher only in those patients with CHF belonging to the youngest category, namely those characterized with an age ≤50 years. Our second, somewhat confirmatory, finding was the significant positive correlation between QTVI and left ventricular ejection fraction in patients with CHF,Citation10 whereas, in healthy control subjects, all repolarization variability parameters showed a significant relation only with age. Thus, although QTVI suffers a certain age influence, this index seems to be the only useful variable to measure temporal dispersion of myocardial repolarization independently from age of the patients with CHF.
An increase in temporal myocardial repolarization dispersion, as assessed by several QT interval-derived indices, has been proven to be significantly associated with an increased SCD risk in patients with structural heart disease.Citation17–Citation21,Citation41–Citation44 However, it is well known that, particularly in patients with CHF, aging represents an additional feature able to magnify this risk, most likely through a further increase in myocardial repolarization lability. Indeed, aging typically leads to a prolongation of action potential duration due to different mechanisms, such as an increased number and overactivity of cardiac L-type Ca2+ channels with a consequent slow inactivation of calcium influx and a reduction in outward potassium current.Citation48,Citation49 As a result, with aging, the QT length and variability tends to increase. Nonetheless, it should be emphasized that CHF leads to a prolongation and dispersion of action potential duration that is quantitatively more pronounced in respect of pure aging. Specifically, functional downregulation of potassium current and an alteration in depolarizing sodium and calcium currents are responsible for electrical remodeling, with a consequent increase in QT temporal dispersion in CHF.Citation50 Our data, besides confirming that QT variability-derived indices are significantly higher in patients with CHF compared with healthy controls, suggest that aging might represent a confounding factor for a number of them but not for QTVI. Initially, our data also suggested through which mechanisms QTVI remains a solid marker of myocardial repolarization lability despite aging. Indeed, in patients with CHF aged ≤50 years, QTVI suffers because of extremely low RR variance values, whereas, considering those patients with CHF aged >50 years, QTVI was much worse, as indicated by a marked increase in QT variance. Thus, it could be hypothesized that in relatively young patients with CHF, an increased QTVI might mirror a sinus node dysfunction due to a prevalent autonomic nervous system control derangement, whereas, with aging, QTVI mainly reflects an altered myocardial repolarization phase. A possible reason underlying a major role of sinus node dysfunction in worsening QTVI of young patients with CHF could be represented by the well-known age-related decline in heart rate variability.Citation11–Citation16
Evidence indicates that an increased Te intervalCitation40 is related to SCD, and also recent research from our group showed an increased temporal dispersion of the last part of repolarization (TeVI) in patients with CHFCitation5 and SCD, as well as in an animal model with pacing-induced heart failure (TeVI, Te/STVT).Citation6 However, the pathophysiologic meaning of this ECG interval still remains controversial. Some authors believe that Te is a reliable noninvasive marker of transmural repolarization gradient, whereas others affirm that this period reflects the total spatial ventricular repolarization dispersion.Citation41,Citation46,Citation51 Notably, clinical and experimental evidence indicates that Te should be considered as a marker of Iks function, especially during sympathetic activation.Citation52,Citation53 Indeed, an enhancement in IKs was reported after a β-adrenergic stimulation, the latter leading to an elevation of intracellular cyclic adenosine monophosphate (cAMP) and an activation of protein kinase A.Citation54 Supporting this datum, it was reported that subjects with concealed type 1 long QT reported a significant increase of Te interval during an infusion of epinephrine,Citation55 again favoring a close link between adrenergic burst and IKs activity. Our current data seem to identify a possible role of Te-derived indices only when considering those patients with CHF aged <50 years. However, a real comparison is impossible given the different population enrolled and the lack of a follow-up. A possible explanation, albeit merely speculative, could be that aging per se, due to a downregulation of β-adrenoreceptor expression and function, could affect Te-derived indices, leading to an age-related loss of their diagnostic utility in patients with CHF. Accordingly, we can hypothesize that, between all indices of myocardial repolarization dispersion, QTVI might remain the only one that could be used to point out an increased SCD risk regardless of patients’ age, whereas Te indices should be useful just in middle-aged patients.
Conclusion
Our data demonstrate a deep influence of age on the short-term variability of repolarization phases, and tend to suggest that only QTVI is able to characterize temporal myocardial repolarization lability in patients with CHF regardless of aging.
Disclosure
The authors report no conflicts of interest in this work.
References
- HottaHUchidaSAging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulationGeriatr Gerontol Int201010S127S13620590828
- KayeDMEslerMDAutonomic control of the aging heartNeuro Molecular Medicine200810179186
- KishiTHeart failure as an autonomic nervous system dysfunctionJ Cardiol20125911712222341431
- PiccirilloGOgawaMSongJChongVJJoungBHanSPower spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failureHeart Rhythm2009654655219324318
- PiccirilloGRossiPMitraMIndexes of temporal myocardial repolarization dispersion and sudden cardiac death in heart failure: any difference?Ann Noninv Electrocardiol201210.1111/anec.12005
- PiccirilloGMagrìDPappadàMAMaruottiAOgawaMHanSAutonomic nerve activity and the short term variability of Tpeak-Tend interval in dogs with pacing-induced heart failureHeart Rhythm201292044205023063868
- PiccirilloGMagrìDOgawaMSongJChongVJHanSAutonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogsJ Am Coll Cardiol20095484085019695465
- PiccirilloGCacciafestaMLionettiMNoccoMDi GiuseppeVMoisèAThe influence of age, the autonomic nervous system and anxiety on QT interval variabilityClin Sci200110142943811566081
- PiccirilloGMagnantiMMateraSDi CarloSDe LaurentisTTorriniAAge and QT variability index during free breathing, controlled breathing and tilt in patients with chronic heart failure and healthy control subjectsTransl Res2006148727816890147
- JanczewskiAJLakattaEGModulation of sarcoplasmic reticulum Ca2+ cycling in systolic and diastolic heart failure associated with agingHeart Fail Rev20101543144520419345
- PiccirilloGCacciafestaMViolaEInfluence of aging on cardiac baroreflex sensitivity determined noninvasively by power spectral analysisClin Sci200110026727411222112
- PiccirilloGDi GiuseppeVNoccoMLionettiMNasoCTallaricoDInfluence of aging and other cardiovascular risk factors on baroreflex sensitivityJ Am Geriatr Soc2001491059106511555067
- PiccirilloGFimognariFLViolaEMariglianoVAge-adjusted normal confidence intervals for heart rate variability in healthy subjects during head-up tiltInt J Cardiol1995501171247591322
- ColosimoAGiulianiAManciniAMPiccirilloGMariglianoVEstimating a cardiac age by means of heart rate variabilityAm J Physiol1997273H1841H18479362251
- PiccirilloGBuccaCBaucoCCintiAMMicheleDFimognariFLPower spectral analysis of heart rate in subjects over a hundred years oldInt J Cardiol19986353619482145
- PiccirilloGMagrìDNasoCdi CarloSMoisèADe LaurentisTFactors influencing heart rate variability power spectral analysis during controlled breathing in patients with chronic heart failure or hypertension and in healthy normotensive subjectsClin Sci (Lond)200410718319015046616
- TereshchenkoLGCygankiewiczIMcNittSVazquezRBayes-GenisAHanLPredictive value of beat-to-beat QT variability index across the continuum of left ventricular dysfunction: competing risks of noncardiac or cardiovascular death and sudden or nonsudden cardiac deathCirc Arrhythm Electrophysiol2012571972722730411
- DobsonCPLa RovereMTPinnaGDGoldsteinROlsenCBernardinangeliMQT variability index on 24-hour Holter independently predicts mortality in patients with heart failure: analysis of GISSI-HF trial dataHeart Rhythm201181237124221457791
- DobsonCPLa RovereMTOlsenCBerardinangeliMVenianiMMidiP24-hour QT variability in heart failureJ Electrocardiol20094250050419647268
- HaigneyMCZarebaWNasirJMMcNittSMcAdamsDGentleskPJMADIT II investigatorsGender differences and risk of ventricular tachycardia or ventricular fibrillationHeart Rhythm2009618018619187907
- PiccirilloGMagrìDMateraSMagnantiMTorriniAPasquazziEQT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective studyEur Heart J2007281344135017101636
- HaigneyMCZarebaWGentleskPJGoldsteinREIllovskyMMcNittSMulticenter Automatic Defibrillator Implantation Trial II investigatorsQT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial [MADIT] IIJ Am Coll Cardiol2004441481148715464332
- AtigaWLCalkinsHLawrenceJHTomaselliGFSmithJMBergerRDBeat-to-beat repolarization lability identifies patients at risk for sudden cardiac deathJ Cardiovasc Electrophysiol199898999089786070
- PiccirilloGMagrìDMateraSMagnantiMPasquazziESchifanoEEffects of pink grapefruit juice on QT variability in patients with dilated or hypertensive cardiomyopathy and in healthy subjectsTransl Res200815126727218433709
- PiccirilloGMagrìDdi CarloSDe LaurentisTTorriniAMateraSInfluence of cardiac-resynchronization therapy on heart rate and blood pressure variability: 1-year follow-upEur J Heart Fail2006871672216513420
- HuikuriHVSteinPKClinical application of heart rate variability after acute myocardial infarctionFront Physiol201234122375128
- ThayerJFYamamotoSSBrosschotJFThe relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factorsInt J Cardiol201014112213119910061
- CooperLLOdeningKEHwangMSChavesLSchofieldLTaylorCAElectromechanical and structural alterations in the aging rabbit heart and aortaAm J Physiol Heart Circ Physiol2012302H1625H163522307668
- BapatANguyenTPLeeJHSovariAAFishbeinMCWeissJNKaragueuzianHSEnhanced sensitivity of aged fibrotic hearts to angiotensin II- and hypokalemia-induced early after depolarization-mediated ventricular arrhythmiasAm J Physiol Heart Circ Physiol2012302H2331H234022467308
- MoritaNSovariAAXieYFishbeinMCMandelWJGarfinkelAIncreased susceptibility of aged hearts to ventricular fibrillation during oxidative stressAm J Physiol Heart Circ Physiol2009297H1594H160519767530
- AibaTTomaselliGFElectrical remodelling of the failing heartCurr Opin Cardiol201025293619907317
- ZuckerIHPatelKPSchultzHDNeurohumoral stimulationHeart Fail Clin20128879922108729
- HanSKobayashiKJoungBPiccirilloGMaruyamaMVintersHVElectroanatomic remodeling of the left stellate ganglion after myocardial infarctionJ Am Coll Cardiol20125995496122381432
- AjijolaOAWiscoJJLambertHWMahajanAStarkEFishbeinMCShivkumarKExtracardiac neural remodeling in humans with cardiomyopathyCirc Arrhythm Electrophysiol201251010111622923270
- RossiPRicciADe PaulisRPapiEPavaciHPorcelliDEpicardial ganglionated plexus stimulation decreases post-operative inflammatory response in humansHeart Rhythm2012994395022306617
- BrackKECooteJHNgGAVagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activationCardiovasc Res20119143744621576131
- HassanabadZFFurmanBLParrattJRAugheyECoronary endothelial dysfunction increases the severity of ischaemia-induced ventricular arrhythmias in rat isolated perfused heartsBasic Res Cardiol1998932412499782365
- PiccirilloGQuaglioneRFimognariFMoisèAMarioMLionettiMInfluence of L-arginine and vitamin C on the autonomic nervous system in chronic heart failure secondary to ischemic cardiomyopathyAm J Cardiol20049365065414996603
- PiccirilloGNoccoMMoisèAInfluence of vitamin C on baroreflex sensitivity in chronic heart failureHypertension2003411240124512743013
- PanikkathRReinierKUy-EvanadoATeodorescuCHattenhauerJMarianiRProlonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac deathCirc Arrhythm Electrophysiol2011444144721593198
- BergerRDKasperEKBaughmanKLMarbanECalkinsHTomaselliGFBeat-to-beat QT interval variability. Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathyCirculation199796155715659315547
- MagrìDPiccirilloGBucciEPignatelliGCautiFMMorinoSIncreased temporal dispersion of myocardial repolarization in myotonic dystrophy type 1: beyond the cardiac conduction systemInt J Cardiol 3201215625926421112106
- PiccirilloGMagrìDMitraMRufaAZicariEStromilloMLIncreased QT variability in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathyEur J Neurol2008151216122118803652
- MagrìDSciomerSFedeleFGualdiGCascianiEPugliesePIncreased QT variability in young asymptomatic patients with beta-thalassemia majorEur J Haematol20077932232917655692
- MagrìDPiccirilloGQuaglioneRDell’armiAMitraMVelittiSEffect of acute mental stress on heart rate and QT variability in postmyocardial infarction patientsISRN Cardiol201291267222844616
- ThomsenMBOrosASchoenmakersMProarrhythmic electrical remodelling is associated with increased beat-to-beat variability of repolarizationCardiovasc Res20077352153017196569
- OosterhoffPTereshchenkoLGvan der HeydenMAShort-term variability of repolarization predicts ventricular tachycardia and sudden cardiac death in patients with structural heart disease: a comparison with QT variability indexHeart Rhythm201181584159021699842
- JanczewskiAMLakattaEGModulation of sarcoplasmic reticulum Ca2+ in systolic and diastolic heart failureHeart Rev201015431445
- OcorrKReevesNLWessellsRJFinkMChenHSAkasakaTKCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of agingPNAS20071043943394817360457
- AibaTTomaselliGFElectrical remodeling in the failing heartCurr Opin Cardiol2010252636
- PatelCBurkeJFPatelHIs there a significant transmural gradient in repolarization time in the intact heart? Cellular basis of the T wave: a century of controversyCirc Arrhythm Electrophysiol20092808819808446
- XiaYLiangYKongstadOIn vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swineHeart Rhythm2005216216915851290
- IzumiDChinushiMIijimaKThe peak-to-end of the T wave in the limb ECG leads reflects total spatial rather than transmural dispersion of ventricular repolarization in an anthopleurin-A model of prolonged QT intervalHeart Rhythm2012979680322123313
- ChengJHKodamaITwo components of delayed rectifier K+ current in heart: molecular basis, functional diversity, and contribution to repolarizationActa Pharmacol Sin20042513714514769199
- ShimizuWNodaTTakakiHKuritaTNagayaNSatomiKEpinephrine unmasks latent mutation carriers with LQT1 form of congenital long-QT syndromeJ Am Coll Cardiol20034163364212598076