Abstract
Background
To investigate the association of FADS gene polymorphisms with age-related changes in polyunsaturated fatty acids (PUFAs) in serum phospholipids and oxidative stress markers.
Methods
We genotyped 122 nonobese men aged 35–59 years without any known diseases at baseline for rs174537 near FADS1 (FEN1 rs174537G > T), FADS2 (rs174575, rs2727270), and FADS3 (rs1000778), and followed them for 3 years.
Results
Among the four single-nucleotide polymorphisms, the minor variants of rs174537 and rs2727270 were significantly associated with lower concentrations of long-chain PUFAs. However, rs174537G > T showed stronger association. At baseline, men with the rs174537T allele had lower arachidonic acid (AA) and AA/linoleic acid (LA), and higher interleukin (IL)-6 levels than rs174537GG counterparts. After 3 years, rs174537GG men had significantly increased AA (P = 0.022), AA/dihomo-γ-linolenic acid (DGLA) (P = 0.007), docosapentaenoic acid (DPA), low-density lipoprotein (LDL) cholesterol, and oxidized LDL (ox-LDL), but decreased eicosatrienoic acid. The rs174537T group showed significantly increased γ-linolenic acid and ox-LDL, and decreased eicosadienoic acid, eicosapentaenoic acid (EPA)/α-linolenic acid (ALA), and IL-6. After 3 years, the rs174537T group had lower AA (P < 0.001), AA/DGLA (P = 0.019), EPA, DPA, EPA/ALA, and urinary 8-epi-prostaglandin F2α (8-epi-PGF2α) (P = 0.011) than rs174537GG. Changes in AA (P = 0.001), AA/DGLA (P = 0.017), EPA, DPA, EPA/ALA, and urinary 8-epi-PGF2α (P < 0.001) were significantly different between the groups after adjusting for baseline values. Overall, changes in AA positively correlated with changes in urinary 8-epi-PGF2α (r = 0.249, P = 0.007), plasma ox-LDL (r = 0.199, P = 0.045), and serum IL-6 (r = 0.289, P = 0.004).
Conclusion
Our data show that FADS polymorphisms can affect age-associated changes in serum phospholipid long-chain PUFAs, Δ5-desaturase activity, and oxidative stress in middle-aged nonobese men. In particular, the rs174537T allele did not show the age-associated increases in AA and Δ5-desaturase activity seen with the rs174537GG genotype.
Introduction
The concentrations and ratios of fat types that people eat have shifted, with marked increases in saturated and ω6 polyunsaturated fatty acids (PUFAs).Citation1 In South Korea, similar profound quantitative and qualitative changes in fat intake have occurred in the last four decades, particularly the rising intake of saturated fatty acids and ω6 PUFAs.Citation2 Dietary PUFAs influence the fatty acid (FA) composition of tissue lipids.Citation3 However, interindividual variability in serum phospholipid FA, as markers of the FA status of an individual, can be attributed to a combination of dietary factors and genetic variation. The key enzymes in PUFA metabolism are Δ5-desaturase and Δ6-desaturase, which are encoded by the FADS1 and FADS2 genes, respectively.Citation4–Citation6 These two genes are located in the desaturase gene cluster on chromosome 11q12–13.1. This cluster also includes FADS3, a gene that shares 52% and 62% sequence identity with the FADS1 and FADS2 genes, respectively, and encodes a desaturase of unknown activity.Citation4 In addition, dietary PUFAs have been shown to suppress the activity of stearoyl-coenzyme A (CoA) desaturase (Δ9-desaturase),Citation7 which is encoded by the stearoyl-CoA desaturase gene family and the rate-limiting enzyme in the cellular synthesis of monounsaturated FA (oleic acid; 18:1 n-9) from saturated FA (stearic acid; 18:0 n-9).Citation8
A genome-wide association study for plasma PUFAs showed strong evidence for association with the region of chromosome 11 that encodes FADS1, FADS2, and FADS3.Citation9 The most significant association was between the single-nucleotide polymorphism (SNP) rs174537 (flap structure-specific endonuclease [FEN1]) near FADS1 and arachidonic acid (AA, 20:4ω6). Recently, Mathias et alCitation1 suggested that variants in the Δ5-desaturase enzymatic step likely regulate the efficiency of conversion of medium-chain PUFAs, such as dietary linoleic acid (LA, 18:ω6), to potentially inflammatory PUFAs, such as AA. In our previous study, we investigated the association of FADS polymorphism, including rs174537, with PUFAs in serum phospholipids and coronary artery disease-related biomarkers in South Koreans through a case-control study. We also determined the effect of these SNPs on lipid peroxides. We found that rs174537T was associated with a lower proportion of AA in serum phospholipids and reduced coronary artery disease risk, in association with reduced total and low-density lipoprotein cholesterol (LDL-C) and lipid peroxides.Citation10 However, there are no reports on the effect of FADS polymorphisms on age-associated changes in serum phospholipid PUFA composition, proinflammatory cytokines, or oxidative stress markers. Therefore, we followed 122 nonobese men aged between 35 and 59 years without a history of known diseases at baseline for 3 years to investigate the association of FADS polymorphisms, including rs174537, with age-associated changes in serum phospholipid PUFA composition. We also examined the effects of these SNPs on lipid peroxides and oxidative stress markers, including oxidized-LDL (ox-LDL) and urinary 8-epi-prostaglandin F2α (8-epi-PGF2α).
Materials and methods
Study population
A total of 160 healthy nonobese (18.5 ≤ body mass index [BMI] < 30 kg/m2) men aged 35–59 years were recruited at a health-promotion center at the National Health Insurance Corporation Ilsan Hospital in South Korea between August and December 2006. Subjects were sedentary, had no history of known disease, and completed a personal health and medical history questionnaire, which served as a screening tool. Exclusion criteria were type 2 diabetes, cardiovascular disease, psychiatric problems, and use of any medication. Written informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board of Yonsei University.
Anthropometric parameters and blood collection
Body weight and height were measured in the morning while participants were unclothed and not wearing shoes. BMI was calculated as body weight in kilograms divided by the square of the height in meters (kg/m2). Systolic and diastolic blood pressures (SBP and DBP, respectively) were obtained from the left arm of seated patients with an automatic blood pressure monitor (TM-2654; A&D, Tokyo, Japan) after 20 minutes of rest. After overnight fasting, venous blood samples were collected in ethylenediaminetetraacetic acid-treated or plain tubes, separated into plasma and serum, and then stored at −70°C until analysis.
Genotyping of FADS gene polymorphisms
Genomic DNA was extracted from 5 mL whole blood using a commercially available DNA isolation kit (Wizard genomic DNA purification kit; Promega, Fitchburg, WI, USA) according to the manufacturer’s protocol. Based on previous reports of genetic studies and public databases on the FADS gene clusterCitation9,Citation11 and the HapMap project (http://www.hapmap.org), eight relevant FADS SNPs were prescreened and four SNPs (FEN1 rs174537G > T, FADS2 rs174575C > G, FADS2 rs2727270C > T, FADS3 1000778C > T) were selected for further analysis ().
Serum lipid profile and fasting glucose
Fasting serum total cholesterol and triglyceride (TG) were measured using a 7150 Autoanalyzer (Hitachi, Tokyo, Japan). After precipitation of serum chylomicrons using dextran sulfate magnesium, HDL-cholesterol concentrations in the supernatants were enzymatically measured. LDL-C was estimated indirectly using the Friedewald formula for subjects with serum TG concentrations <400 mg/dL (4.52 mol/L). In subjects with serum TG concentrations ≥ 4.52 mol/L (400 mg/mL), LDL-C was directly measured by an enzymatic method on the 7150 Autoanalyzer. Fasting glucose was measured by the glucose oxidase method using a glucose analyzer (Beckman Coulter, Brea, CA, USA).
Plasma ox-LDL and serum high-sensitivity C-reactive protein
Plasma ox-LDL was measured using an enzyme immunoassay (Mercodia, Uppsala, Sweden). The resulting color reaction was read at 450 nm on a Wallac Victor2 multilabel counter (PerkinElmer, Waltham, MA, USA). High-sensitivity C-reactive protein (hs-CRP) levels were measured on an Express Plus™ autoanalyzer (Chiron Diagnostics Co., Walpole, MA, USA) using commercially available hs-CRP-Latex (II) X2 kits (Seiken Laboratories Ltd., Tokyo, Japan) that allowed detection of CRP in the range of 0.001–31 mg/dL.
Urinary 8-epi-PGF2α and serum cytokine levels
Urine was collected in polyethylene tubes containing 1% butylated hydroxytoluene after a 12-hour fast. The tubes were immediately covered with aluminum foil and stored at −70°C until analysis. 8-epi-PGF2α was measured using an enzyme immunoassay (Bioxytech Urinary 8-epi-PGF2α Assay Kit, Oxis International, Portland, OR, USA), and the resulting color reaction was read at 650 nm using a Wallac Victor2 multilabel counter. Urinary creatinine was determined by the alkaline picrate (Jaffe) reaction. Urinary 8-epi-PGF2α concentrations were expressed as pg/mg creatinine. Levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-α in serum were measured using the Bio-Plex Reagent Kit on a Bio-Plex instrument (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions.
FA composition in serum phospholipids
Serum phospholipid FA composition was analyzed by gas chromatography (7890A; Hewlett Packard, Palo Alto, CA, USA) using a modification of a previously described method.Citation12,Citation13 Individual FAs were calculated as a relative percentage of the total of 26 FAs (set as 100%) using GC ChemStation software revision B.04.02 (Agilent Technologies, Santa Clara, CA, USA). The activities of Δ5 desaturase and Δ6 desaturase were estimated as the ratios of AA to DGLA and γ-linolenic acid (GLA; 18:3ω6) to LA, respectively.
Assessment of dietary intake and physical activity level
The duration of the study was 3 years. At baseline, usual dietary intake for each subject was assessed using a semi-quantitative food-frequency questionnaire and 24-hour recall method.Citation14 Subjects were encouraged to maintain their body weight within ±2 kg and given general oral and written information about healthy food choices and exercise at baseline and at a subsequent visit (after 3 years). Subjects were instructed by trained dietitians and were also asked to keep food records for 3 days (2 weekdays and 1 weekend day) at each visit. Nutrient intake was determined and calculated as mean values from the 3-day food record using Can-Pro (Korean Nutrition Society, Seoul, South Korea), based on food-composition tables from the National Rural Living Science Institute in South Korea. Total energy expenditure (kcal/d) was calculated from activity patterns, including basal metabolic rate calculated with the Harris–Benedict equation, physical activity for 24 hours, and specific dynamic action of food.
Statistical analysis
Statistical analyses were performed using SPSS version 12.0 for Windows (IBM, Armonk, NY, USA). Hardy–Weinberg equilibrium and linkage disequilibrium tests were examined using Haploview 4.1 (Broad Institute, Cambridge, MA, USA). Frequencies were compared by the Chi-square test. We examined whether each variable was normally distributed before statistical testing, and logarithmic transformation was performed for skewed variables. Paired t-tests were used to test between baseline and follow-up values. Differences in clinical variables between the rs174537GG and T-allele carrier groups were tested by independent t-test, and a general linear model test was applied to adjust for baseline values. Pearson’s correlation coefficients were used to examine the relationships between variables. For descriptive purposes, mean values are presented using untransformed and unadjusted values. Results are expressed as means ± standard error or percentage. A two-tailed value of P < 0.05 was considered statistically significant.
Results
Clinical characteristics, serum phospholipid FA composition, and macronutrient intake at baseline and 3-year follow-up
Among the enrolled men (n = 160), 38 dropped out for personal reasons or poor compliance, leaving 122 men at 3 years. Clinical characteristics, serum phospholipid FA composition, and macronutrient intake at baseline and 3-year follow-up are shown in . After 3 years, subjects showed an increase in LDL-C (P = 0.018), ox-LDL (P < 0.001), serum phospholipid GLA (P = 0.024), and AA (P = 0.043). HDL-C (P = 0.001), IL-6 (P = 0.004), and serum phospholipid eicosadienoic acid (20:2ω6) (P = 0.016) decreased. There was no significant difference in total energy intake or macronutrient intake between baseline and 3-year follow-up ().
Table 1 Clinical characteristics, PUFA composition in serum phospholipids, and macronutrition intake at baseline and at 3 years
Genotype distribution of four selected SNPs
Genotype distributions in Hardy–Weinberg equilibrium with 41. 0% GG, 47. 5% GT, and 11. 5% TT at position rs174537; 88.5% CC, 9.8% CG, and 1.6% GG at position rs174575; 48.4% CC, 44.3% CT, and 7.4% TT at position rs2727270; and 49.2% CC, 38.5% CT, and 12.3% TT at position rs1000778. The major alleles were G at position rs174537 (frequency 0.648, P = 0.647), C at rs174575 (frequency 0.934, P = 0.087), C at rs2727270 (frequency 0.705, P = 0.480), and C at position rs1000778 (frequency 0.684, P = 0.233). Because the FADS3 rs1000778C > T genotype-related PUFA was not significantly different among serum phospholipids, and the FADS2 rs174575 genotype only showed a trend toward an association with serum phospholipid PUFA (data not shown), we did not perform further analysis on FADS3 rs1000778C > T and FADS2 rs174575C > G. The genotype and haplotype distributions of FEN1 rs174537 and FADS2 rs2727270 were both associated with PUFAs in serum phospholipids. However, because haplotype analysis did not provide information beyond that revealed by each SNP (data not shown), we present only the results of FEN1 rs174537 and FADS2 rs2727270.
Serum phospholipid FA composition according to genotypes
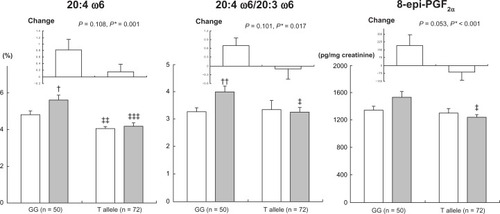
At baseline, men with the FEN1 rs174537T allele showed a lower proportion of AA (P = 0.007) and lower ratio of AA to LA (P = 0.007) in serum phospholipids than those with rs174537GG (). After 3 years, men with rs174537GG showed a significant increase in AA (P = 0.022), ratio of AA to DGLA (P = 0.007, ), and docosapentaenoic acid (DPA; 22:5ω3) (P = 0.044), but a significant decrease in eicosatrienoic acid (20:3ω3) (P = 0.037, ). Carriers of the rs174537T allele showed a significant increase in GLA (P = 0.031) and a significant decrease in EDA (P = 0.030), and the ratio of eicosapentaenoic acid (EPA; 20:5ω3) to α-linolenic acid (ALA; 18:3ω3) (P = 0.024). At 3-year follow-up, men with the rs174537T allele showed lower AA (P > 0.001), AA/DGLA (P = 0.019, ), EPA (P = 0.010), DPA (P = 0.016), and EPA/ALA (P = 0.048) than rs174537GG carriers. Changes in AA (P = 0.001), AA/DGLA (P = 0.017, ), EPA (P = 0.004), DPA (P = 0.011), and EPA/ALA (P = 0.048) were significantly different between rs174537GG men and rs174537T allele carriers after adjusting for baseline values (). Similar but weaker associations of FADS2 rs2727270 with PUFAs were also found ().
Table 2 Associations of FEN1 rs174537 genotypes with PUFA composition in serum phospholipids in men at baseline and 3-year follow-up
Figure 1 Serum phospholipid AA (20:4 ω6), the ratio of 20:4 ω6/20:3 ω6, and urinary levels of 8-epi-PGF2α according to FEN1 rs174537 G > T in men at baseline (□) and 3-year follow-up (▪).
Abbreviation: 8-epi-PGF2α, urinary 8-epi-prostaglandin F2α.
LDL-cholesterol, hs-CRP, cytokines, and oxidative stress markers according to genotypes
At baseline, men with the FEN1 rs174537T allele showed higher serum IL-6 than those with rs174537GG (P = 0.038, ). After 3 years, men with rs174537GG showed a significant increase in serum LDL-C (P = 0.043) and plasma ox-LDL (P < 0.001). Carriers of the rs174537T allele showed a significant decrease in IL-6 (P = 0.001) and a significant increase in ox-LDL (P < 0.001). At 3-year follow-up, men with the rs174537T allele showed lower urinary PGF2α levels (P = 0.011) than rs174537GG carriers. Changes in urinary PGF2α levels (P < 0.001) were significantly different between rs174537GG and rs174537T allele carriers after adjusting for baseline values (). Additionally, changes in serum TNF-α (P = 0.089) tended to be different (). Similar associations were found between FADS2 rs2727270 and LDL-C, hs-CRP, cytokines, and oxidative stress markers ().
Table 3 Associations of FEN1 rs174537 genotypes with LDL-cholesterol, hs-CRP, cytokines, and oxidative stress markers in men at baseline and 3-year follow-up
Relation of serum phospholipid AA with oxidative stress markers and cytokines
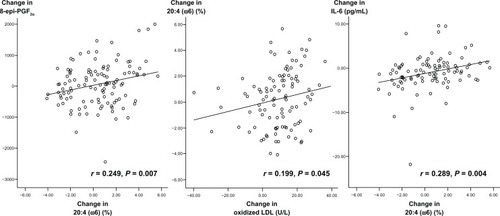
Pearson correlation analysis showed that the changes in the AA proportion in serum phospholipids were positively correlated with changes in urinary 8-epi-PGF2α levels (r = 0.249, P = 0.007), plasma ox-LDL (r = 0.199, P = 0.045), and serum IL-6 (r = 0.289, P = 0.004) in all subjects (). There was a marginal correlation between changes in serum phospholipid AA and changes in serum TNF-α (r = 0.191, P = 0.058). Additionally, changes in urinary 8-epi-PGF2α levels were positively correlated with changes in serum IL-6 (r = 0.242, P = 0.015) and TNF-α (r = 0.246, P = 0.013). Changes in serum IL-6 were positively correlated with changes in serum TNF-α (r = 0.464, P < 0.001) and hs-CRP (r = 0.507, P < 0.001, ).
Table 4 Correlations between 8-epi-PGF2α and cytokine
Figure 2 Relation of changes in serum phospholipid arachidonic acid with changes in oxidized LDL, urinary 8-epi-PGF2α and serum IL-6 in all male subjects.
Abbreviations: 8-epi-PGF2α, urinary 8-epi-prostaglandin F2α; LDL, low-density lipoprotein; IL, interleukin.
Discussion
The major finding of this study is that FADS polymorphisms may affect age-associated changes in serum phospholipid long-chain PUFAs, Δ5-desaturase activity, and oxidative stress in middle-aged nonobese men. At 3-year follow-up, there were significant differences between men with FEN1 rs174537GG and those with the 174537T allele in changes in AA, and Δ5-desaturase activity (determined by the ratio of AA/DGLA), as well as urinary levels of PGF2α, one of the radical peroxides of AA, and an indicator of oxidative stress.Citation15 In particular, the rs174537T allele did not show the age-associated increases in AA and Δ5-desaturase activity seen with the rs174537GG genotype. Therefore, our data show that FADS polymorphisms could affect age-associated changes in serum phospholipid long-chain PUFAs, Δ5-desaturase activity, and oxidative stress in middle-aged nonobese men.
Similar to previous studies,Citation1,Citation16 the minor variants of rs174537G > T and rs2727270 were significantly associated with lower concentrations of long-chain PUFAs. However, rs174537G > T showed stronger association with changes in AA, Δ5-desaturase activity, EPA, DPA, and EPA/ALA than rs2727270. Although numerous SNPs in the FADS gene cluster were reported to be significantly associated with FA alterations in tissues, such as serum and red blood cell membranes,Citation1,Citation9,Citation11,Citation17,Citation18 a recent genome-wide association studyCitation9 found the most significant association to that of rs174537 with AA. However, rs174537 is located in an intron and in linkage disequilibrium with rs174546 (r2 = 0.99) and rs3834458 (r2 = 0.98), which are candidates for a direct influence on gene expression.Citation11,Citation19 Therefore, it is possible that this variant is a marker of other functional polymorphisms or is in linkage with currently unidentified causal variants affecting FA concentrations.
AA, a precursor of eicosanoids including prostaglandins and leukotrienes, is liberated from the hydrolysis of the sn-2 position of glycerophospholipids (phosphatidylcholine).Citation20 Radical peroxidation of AA produces a family of prostaglandin F2-isomers called F2-isoprostanes.Citation21 One such F2-isoprostane, 8-epi-PGF2α, is a sensitive and independent risk marker for coronary artery disease.Citation15,Citation22,Citation23 It is probably released into biological fluids through a phospholipase-mediated pathway and consequently excreted in urine. In this study, the changes in the AA proportion in serum phospholipids were positively correlated with changes in urinary 8-epi-PGF2α levels as well as changes in serum IL-6. Interestingly, changes in urinary 8-epi-PGF2α also correlate with changes in IL-6 and TNF-α. This result is consistent with previous findings of a positive association between urinary 8-epi-PGF2α and circulating proinflammatory cytokines.Citation24
High concentrations of AA may influence the levels of proinflammatory eicosanoids, which in turn appear to be associated with elevated markers of low-level systemic inflammation.Citation25–Citation27 Thus, low synthesis and availability of AA have been suggested to mitigate the inflammatory response by altering, for example, eicosanoid levels.Citation28 At 3-year follow-up, men with the rs174537T allele showed lower AA, Δ5-desaturase activity, and urinary PGF2α levels compared to those with rs174537GG. In addition, men with the rs174537T allele also showed significant reduction in IL-6 at 3-year follow-up compared to baseline, and changes in serum TNF-α tended to be different between rs174537GG and rs174537T allele carriers.
Tekola Ayele et al conducted a genome-wide association study of IL-10, IL-1Ra, and IL-6 level in nondiabetic Africans. They reported that IL-6 levels showed genome-wide significant association with one SNP (RP11-314E23.1; chr6:133397598; P = 8.63 × 10−9), but did not confirm an association of IL-6 with FADS genotypes.Citation29 In addition, Naitza et al investigated a genome-wide association scan on the levels of markers of inflammation including IL-6; however, they did not find an association of IL-6 with FADS genotypes either.Citation30 In our result, at baseline, men with the FEN1 rs174537T allele showed higher serum IL-6 than those with rs174537GG (P = 0.038, ). After adjusting for baseline, changes in IL-6 levels were not significantly different between rs174537GG and rs174537T allele carriers. Thus, we may need to consider further study with an increased number of study subjects in the future to confirm and clarify the result pattern.
Age is known to play an important role in increased LDL oxidation.Citation31 After 3 years, LDL-C increased in men with rs174537GG, and ox-LDL, one of the products of oxidative stress, increased regardless of genotype. Although the change in ox-LDL in subjects with the rs174537T allele was 33% lower than that of men with GG, it failed to reach statistical significance. However, the present findings of direct correlation between serum phospholipid AA and both plasma ox-LDL and urinary 8-epi-PGF2α could suggest a possible role of AA in lipid peroxidation or oxidative stress during aging. More than 95% of the serum phospholipids are phosphatidylcholine, one of the major phospholipids in membranes. Thus, serum phospholipids mirror membrane composition in the body, as markers of the FA status of an individual.Citation32
Several limitations of this study should be mentioned. First, the small sample size was not conducive to identification of weak associations due to low statistical power. Second, PUFA levels were expressed as a percentage of total FAs in serum phospholipids, not as an absolute concentration. Therefore, we were able to detect relative differences in PUFA levels and Δ5-desaturase activity, but unable to decipher the mechanisms, which depend on the absolute values. Third, dietary intake of PUFAs was not investigated in this study, but we confirmed dietary intake of total fat percentage, which did not change for the follow-up period. Finally, we specifically focused on a representative group of South Korean nonobese (18.5 ≤ BMI < 30 kg/m2) men aged 35–59 years. Our subjects were not taking any medications or vitamin/mineral supplementations. Therefore, our data cannot be generalized to other ethnic groups or other populations.
Conclusion
Our data show that FADS polymorphisms could affect age-associated changes in serum phospholipid long-chain PUFAs, Δ5-desaturase activity, and oxidative stress in middle-aged nonobese men. In particular, the rs174537T allele did not show age-associated increase in AA and Δ5-desaturase activity seen in the rs174537GG genotype. Because it has been suggested that appropriate dietary intake of FAs can obviously overcome genetic risk factors,Citation33 these results provide good evidence for tailoring dietary intervention programs to individuals based on their genetic patterns.
Supplementary tables
Table S1 Information for the prescreening of eight single-nucleotide polymorphisms (SNPs) and selection of four SNPs
Table S2 Associations of FADS2 rs 2727270 genotypes with polyunsaturated fatty acid (PUFA) composition in serum phospholipids in men at baseline and 3-year follow-up
Table S3 Associations of FADS2 rs2727270 genotypes with LDL cholesterol, hs-CRP, cytokines, and oxidative stress markers in men at baseline and 3-year follow-up
Acknowledgements
This work was supported by the National Research Foundation, Ministry of Education, Science and Technology (Mid-career Researcher Program: 2012-0005604, M10642120002-06 N4212-00210, C00048, and 2012M3 A9C4048762), South Korea.
Disclosure
None of the authors had any personal or financial conflicts of interest.
References
- MathiasRAVergaraCGaoLFADS genetic variants and omega-6 polyunsaturated fatty acid metabolism in a homogeneous island populationJ Lipid Res2010512766277420562440
- AilhaudGGuesnetPCunnaneSCAn emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development?Br J Nutr200810046147018307824
- KarlssonMMårildSBrandbergJLönnLFribergPStrandvikBSerum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescentsObesity2006141931193917135608
- MarquardtAStöhrHWhiteKWeberBHcDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase familyGenomics20006617518310860662
- ChoHPNakamuraMTClarkeSDCloning, expression, and nutritional regulation of the mammalian delta-6 desaturaseJ Biol Chem19992744714779867867
- ChoHPNakamuraMClarkeSDCloning, expression, and fatty acid regulation of the human delta-5 desaturaseJ Biol Chem1999274373353733910601301
- NtambiJMThe regulation of stearoyl-CoA desaturase (SCD)Prog Lipid Res1995341391507480063
- NtambiJMRegulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterolJ Lipid Res1999401549155810484602
- TanakaTShenJAbecasisGRGenome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI StudyPLoS Genet20095e100033819148276
- KwakJHPaikJKKimOYFADS gene polymorphisms in Koreans: association with ω6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery diseaseAtherosclerosis20112149410021040914
- SchaefferLGohlkeHMüllerMCommon genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipidsHum Mol Genet2006151745175616670158
- FolchJLeesMSloaneStanleyGHA simple method for the isolation and purification of total lipids from animal tissuesJ Biol Chem195722649750913428781
- LepageGRoyCCDirect transesterification of all classes of lipids in a one-step reactionJ Lipid Res1986271141203958609
- ShimJSOhKWSuhIA study on validity of a 299 semiquantitative food frequency questionnaire of Korean adultsKorean J Community Nutr20027484494
- SchwedhelmEBartlingALenzenHUrinary 8-iso prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control studyCirculation200410984384814757688
- MerinoDMJohnstonHClarkeSPolymorphisms in FADS1 and FADS2 alter desaturase activity in young Caucasian and Asian adultsMol Genet Metab201110317117821414826
- MalerbaGSchaefferLXumerleLSNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular diseaseLipids20084328929918320251
- BokorSDumontJSpinnekerASingle nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratiosJ Lipid Res2010512325233320427696
- DixonALLiangLMoffattMFA genome-wide association study of global gene expressionNat Genet2007391202120717873877
- StafforiniDMShellerJRBlackwellTSRelease of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolasesJ Biol Chem20062814616462316371369
- VossPSiemsWClinical oxidation parameters of agingFree Radic Res2006401339134917090423
- WolframROguoghoAPalumboBSinzingerHEnhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8-epi-PGF(2alpha)Eur J Heart Fail2005716717215701462
- KimJYHyunYJJangYLipoprotein-associated phospholipase A2 activity is associated with coronary artery disease and markers of oxidative stress: a case-control studyAm J Clin Nutr20088863063718779277
- KimOYChaeJSPaikJKEffects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese womenAge (Dordr)20123441542521487705
- CesariMPenninxBWNewmanABInflammatory markers and onset of cardiovascular events: results from the Health ABC studyCirculation20031082317232214568895
- ChiltonFHRudelLLParksJSArmJPSeedsMCMechanisms by which botanical lipids affect inflammatory disordersAm J Clin Nutr200887498S503S18258646
- Poudel-TandukarKNanriAMatsushitaYDietary intakes of alpha-linolenic and linoleic acids are inversely associated with serum C-reactive protein levels among Japanese menNutr Res20092936337019628101
- VessbyBUusitupaMHermansenKSubstituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU StudyDiabetologia20014431231911317662
- Tekola AyeleFDoumateyAHuangHGenome-wide associated loci influencing interleukin (IL)-10, IL-1Ra, and IL-6 levels in African AmericansImmunogenetics20126435135922205395
- NaitzaSPorcuESteriMA genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulationPLoS Genet20128e100248022291609
- HolvoetPVanhaeckeJJanssensSVan de WerfFCollenDOxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery diseaseCirculation199898148714949769301
- StubbsCDSmithADThe modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and functionBiochim Biophys Acta1984779891376229284
- LattkaEIlligTHeinrichJKoletzkoBDo FADS genotypes enhance our knowledge about fatty acid related phenotypes?Clin Nutr20102927728719948371