Abstract
Background
With the development of population aging worldwide, sarcopenia and knee osteoarthritis (KOA), two age-related diseases, will continue to impose increasing medical and economic burdens on the society. Previous studies have discovered an association between the two, but the causality remains controversial, and it is difficult to eliminate confounding factors. Therefore, a Mendelian randomization (MR) study was conducted to overcome these confounding factors and investigate the causal relationship between sarcopenia and KOA.
Objective
The present work focused on assessing the causality between KOA and sarcopenia, so as to provide new strategies to prevent and treat these two conditions in clinic.
Methods
We registered the title with PROSPERO (ID: CRD42023421096). The two-sample bidirectional MR analysis was conducted in two steps, with sarcopenia being the exposure whereas KOA being the outcome in the first step, and vice versa in the second step. Genome-wide association studies (GWAS) data on low hand-grip strength (n=256,523), walking pace (n=459,915), appendicular lean mass (ALM, n=450,243), and KOA (n=403,124) were obtained from the UK Biobank. Methods such as the inverse variance weighted (IVW) and weighted median were utilized for assessing the causality of KOA with sarcopenia, and sensitivity analyses were also conducted.
Results
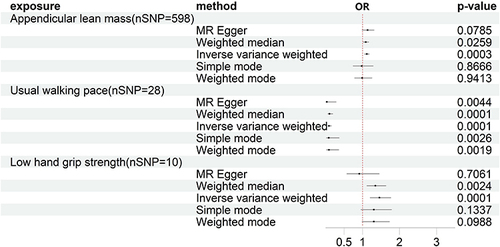
In the main MR analysis using the IVW method, evidence suggested that low hand-grip strength, walking pace, and ALM had adverse effects on KOA (p-value 0.0001, odds ratio (OR) 1.4569, 95% confidence interval (CI) 1.2007–1.7677 for low hand-grip strength; p-value 0.0003, OR 1.1500, 95% CI 1.050–1.183 for ALM; p-value 5.29E-19, OR 0.0932, 95% CI 0.0553–0.1572 for walking pace). However, there was no causality of KOA with sarcopenia in the opposite direction.
Conclusion
Our study suggests an obvious unidirectional causality of KOA with sarcopenia, and supports the notion that patients with sarcopenia are more susceptible to the development of KOA.
Introduction
Knee Osteoarthritis represents the chronic knee joint degenerative disorder caused by various factors, including mechanical and chemical factors.Citation1 Obesity, history of joint injuries, age, gender, anatomical abnormalities, and genetic predisposition are key risk factors for knee osteoarthritis.Citation2–4 Its primary pathological mechanism is related to the changes in joint tissue structure, such as degenerative changes in cartilage, subchondral bone sclerosis or cystic changes, joint space narrowing and proliferation, synovial inflammation, meniscal injury, and periarticular ligament contracture.Citation1 Simultaneously, inflammation within the infrapatellar fat pad beneath the patella may potentially exert a latent influence on these pathological processes, consequently exacerbating disease progression.Citation5 KOA is typically characterized by recurrent knee joint pain, swelling, stiffness, and impaired mobility.Citation6
As reported in the systematic analysis of Global Burden of Disease Study, over 300 million people worldwide suffer from impaired mobility caused by osteoarthritis, and 75% of them are due to KOA.Citation7 Additionally, an increasing body of research has suggested that KOA is among the significant diseases causing pain and disability in elderly patients.Citation8
On the other hand, sarcopenia is a prevalent geriatric syndrome and a leading cause of mortality among the elderly population.Citation9 The primary physiological mechanism responsible for this condition is the progressive decline in muscle strength together with the degenerative skeletal muscle mass and functional losses with aging.Citation10 Sarcopenia is associated with multiple negative health outcomes, including impaired physical function and mobility, an increased risk of falls and fractures, loss of independence, difficulties in the daily living activities, and frailty.Citation11 Ultimately, sarcopenia can contribute to mortality.
Due to the biomechanical effects between bones and muscles, as well as the shared lifestyle and risk factors, KOA and sarcopenia often co-occur and exacerbate the mutual effects.Citation12 It is common that patients with KOA experience lower limb muscle atrophy to varying degrees. Accurate assessment of the muscle mass in KOA patients can effectively predict their prognosis.Citation13 Furthermore, as indicated in a meta-analysis, the risk of sarcopenia is high in patients with KOA,Citation14 but the causality between KOA and sarcopenia remains unclear due to a lack of foundational research.Citation15
Although the association between KOA and sarcopenia has been explored, evidence supporting this relationship remains controversial, and conflicting results are reported in literature.Citation14 Meanwhile, there is no direct evidence supporting the notion that sarcopenia directly affects the development of KOA or the opposite conclusion, in this regard, more research is warranted to understand the relationship between these two conditions. Therefore, a new research method is needed to demonstrate the causality of KOA with sarcopenia.
Mendelian randomization has been developed as the research approach, in which genetic variants from the non-experimental data are utilized for the sake of inferring the causal relation between an exposure and an outcome variable, typically a disease.Citation16 The exposure can encompass any factor that may affect the outcome, including biomarkers, anthropometric measurements, dietary and lifestyle factors, and other risk factors. MR has been recognized as the creditable approach to overcome drawbacks observed in observational studies and to assess causality.Citation17 Due to the random assignment of genetic variants during conception, the relation with outcome may not be affected by external confounding factors.
As far as we know, there is no bidirectional MR study investigating the relationship between sarcopenia and KOA, or randomized controlled trial (RCT) for the specific evaluation of bidirectional relation. Consequently, the bidirectional two-sample MR study was conducted for exploring associations of sarcopenia with KOA. As sarcopenia symptoms often present as a cluster of clinical symptoms, the three most commonly used indicators for sarcopenia (hand-grip strength, ALM, walking pace) were selected as the exposure factors for our MR studyCitation18 according to the 2019 revised consensus.Citation19
Materials and Methods
Study Design
We registered the title with the International Prospective Register of Systematic Reviews (PROSPERO): CRD42023421096. The present work employed the two-sample MR analysis approach and extracted instrumental variables (IVs) for sarcopenia-associated traits from the publicly available summary statistics. This helps to effectively overcome the confounding bias of traditional epidemiological studies and investigate the causality of sarcopenia with KOA.Citation18 Three assumptions need to be satisfied for a genetic variant to be a valid IV in MR analysis, as shown below:
The variant is related to the exposure.
The variant is not related to the outcome through a confounding pathway.
The variant has no direct impact on the outcome, but only possibly indirect impact via the exposure.Citation20
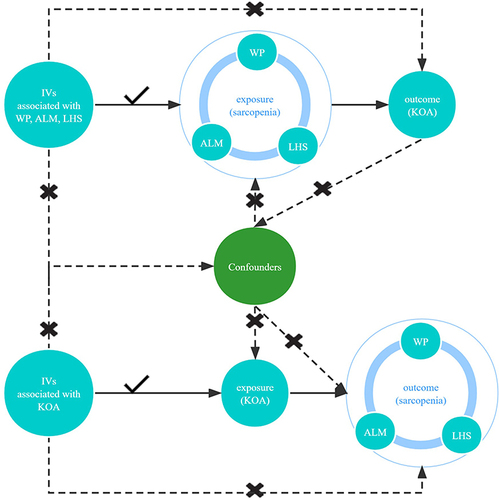
displays our study design.
Figure 1 The design of bidirectional Mendelian randomization (MR) study. The “×” means that genetic variants are not associated with confounders or cannot be directly involved in outcome but via the exposure pathway. The “√” means that genetic variants are highly correlated with exposure. Solid paths are significant; dashed paths should not exist in the MR study.
Sources of Data
Summary-level data utilized in the present workCitation21 were mostly obtained from the MRC Integrated Epidemiology Unit (IEU) through its open GWAS database (https://gwas.mrcieu.ac.uk/).Citation22 Phenotypic and consortium details are presented in .
Table 1 Source of Data for This MR Study
The GWAS summary data regarding hand-grip strength were obtained in one meta-analysis including 256,523 Europeans aged ≥60 years in 22 cohorts.Citation23 According to the definition in the European Working Group on Sarcopenia in Older People, 46,596 participants (18.9%) had muscular weakness. Additionally, GWAS summary data concerning ALM and walking pace were also extracted from the UK Biobank, which included a total of 450,243 and 459,915 individuals, respectively.Citation24
Data on KOA in this study were provided by the European Bioinformatics Institute, including 77,052 cases as well as 29,999,696 SNPs.Citation25 This dataset has a large sample size and has been widely used in MR analyses related to KOA, with acceptable imputation quality.Citation26
Genetic Instrumental Variable Selection
Selecting genome-wide significant SNPs that are both independent and highly correlated with the exposure factor and outcome variable as initial IVs. Subsequently, harmonies data function in Two-Sample MR package was employed to “harmonize” each of these three exposures with the outcome, matching the effect allele in every SNP in the exposure with specific allele in the outcome.Citation27 Thereafter, SNPs with no linkage disequilibrium (thresholds: r2<0.001 and clump distance>10,000 kb) were adopted to be IVs. At last, SNPs with minor allele frequency (MAF) <0.01 and palindromic SNPs were eliminated. We then removed palindromic SNPs showing intermediate allele frequencies. To adhere to the assumptions of MR, SNPs with P-values greater than 5.0e-8 in the exposure and those with P-values less than 5.0e-8 in the outcome were also removed. The final obtained SNP information can be found in Supplementary Tables 1-6. SNPs related to additional phenotypes, such as possible confounders and mediators (body mass index), were assessed and removed based on PhenoScanner database V2Citation28(The specific SNP information can be found in Supplementary Table 7). As a result, IVs were obtained that are strongly associated with the exposure, weakly associated with the outcome, and unrelated to confounding factors. Please refer to for detailed information on the SNP selection process.
Table 2 Selection Process of Snps
MR Analysis
In the MR analysis, the causal relationship between sarcopenia and KOA was evaluated by an inverse variance weighted (IVW) approach. The IVM method has been shown to be the most efficient and unbiased method for estimating the causal effects in MR study when these assumptions hold.Citation29 In addition, the weighted median method was also applied in this MR analysis. It represents a robust method that can generate valid causal estimates, even when up to 50% of the genetic instruments (ie, instrumental variables) are invalid or subjected to pleiotropic effects.Citation30 In the weighted median method, the causal effect estimate is calculated by taking the median of individual instrumental variable estimates weighted by the inverse of their variance.Citation31 Furthermore, the Simple mode and Weight mode methods were also employed as the supplementary reference standards.
Sensitivity Analysis
For ensuring that our MR analysis results were reliable, a multi-step verification process was needed. First, heterogeneity among IVs was tested using the Cochran’s Q statistics. If heterogeneity is detected, then MR-Presso is adopted to detect and remove SNPs with high outlier values, and later MR analysis is performed again.Citation32 Next, a test for pleiotropy was conducted, which might occur when the IVs affected the outcome not only through the exposure pathway, but also via other pathways. Once pleiotropy is detected, the credibility of the causal relationship between exposure and outcome will be greatly reduced.Citation33 Finally, a leave-one-out analysis was conducted by sequentially removing each SNP and calculating the meta-effect of the remaining SNPs, so as to observe whether the results changed significantly after each SNP was removed. If the results change significantly after a particular SNP is removed, it indicates that this SNP has a significant impact on the outcome, which is not desirable. On the contrary, the ideal situation is that the results do not change significantly after a SNP is eliminated.
The results of the sensitivity analysis are presented in .
Table 3 The Results of Sensitivity Analysis
Ethical Approval
No ethical approval was needed because the present work did not use primary data.
Reported Results and Software
The results of MR study were reported as estimates of odd ratios (OR) or β values with a 95% confidence interval (CI), depending on whether the variable was binary or continuous. The P-value <0.05 (two-sided) stood for significant difference. Both types of estimates were consistently reported as the point estimate. MR and MR-PRESSO based on version 4.0.0 were utilized for statistical analysis.Citation34
Results
After calculating the F-statistics for all SNPs based on the outcome and exposure data, it was determined that all SNPs met the criterion of F > 10, suggesting the absence of weak instrument bias. The intercept of MR-Egger regression revealed the absence of horizontal pleiotropy in SNPs related to the exposure factor (As shown in Supplementary Figure 1).
Influence of Sarcopenia-Related Traits on KOA
In the MR analysis with ALM, LHS, and WP as exposures and KOA as the outcome, a total of 598, 10, and 28 SNPs were included after filtering. The results of the MR analysis showed a causal relationship between features related to sarcopenia and the occurrence of KOA. Specifically, the IVW method revealed a p-value of 0.0003 and an odds ratio (95% CI) of 1.1500 (1.050–1.183) for ALM, a p-value of 0.0001 and an odds ratio (95% CI) of 1.4569 (1.2007–1.7677) for LHS, and a p-value of 5.29E-19 and an odds ratio (95% CI) of 0.0932 (0.0553–0.1572) for WP (). The results indicated that features associated with decreased muscle mass were causally related to the occurrence of KOA. Furthermore, improving walking speed and enhancing skeletal muscle mass may potentially reduce the risk of KOA to some extent. Additionally, the intercept results of MR-Egger regression indicated the absence of pleiotropy (p > 0.05). All visualized results of MR analysis, heterogeneity, and “leave-one-out” analysis are presented in Supplementary Figures 1-3.
Influence of KOA on Sarcopenia-Related Traits
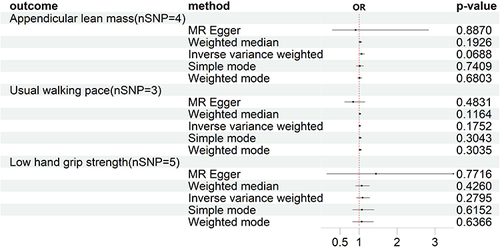
In the reverse MR analysis, after selection, 4, 3, and 5 SNPs were included for the analysis of ALM, WP, and LHS, respectively. The results of the MR analysis showed no causal relationship between KOA and sarcopenia-related traits. Specifically, the IVW method yielded a p-value of 0.06 and an odds ratio (95% CI) of 1.066 (0.995–1.142) for ALM; a p-value of 0.175 and an odds ratio (95% CI) of 1.017 (0.992–1.043) for WP; and a p-value of 0.279 and an odds ratio (95% CI) of 1.093 (0.930–1.284) for LHS. Please refer to for detailed information.
Discussion
In this study, a bidirectional two-sample MR study was conducted using GWAS data to explore the potential causal association of sarcopenia-related traits (grip strength, ALM, walking speed) with KOA. The main findings of our study are as follows:
The genetically predicted sarcopenia-related traits may act as the causal factors for KOA, with no evidence of reverse causation.
The results suggest a possible strong causal relationship between walking speed and KOA.
Sarcopenia and KOA often coexist in elderly patients. The relationship between these two diseases is complex, as reported in some observational studies, muscle mass in KOA cases is markedly lower than that of patients without KOA.Citation35,Citation36 At the same time, research has indicated a higher incidence rate of sarcopenia in KOA patients.Citation37 It seems that the two conditions are causally related, but so far, the relation of sarcopenia with osteoarthritis has not been clearly established, and it remains unknown whether there is a causal relationship between the two.Citation15 This may be due to the differences in the diagnostic criteria and classification of sarcopenia, because the muscle wasting observed in KOA patients is mainly related to lower limb muscle weakness and has not been evaluated for specific hormone levels like testosterone, estrogen, insulin-like growth factor-1 (IGF-1) and growth hormone.Citation38 Our MR analysis results indicated that, different from previous controversial studies, there was a unidirectional causal relationship between sarcopenia and KOA, indicating that sarcopenia is the cause of KOA. This unidirectional causal relationship may be attributed to the self-regulation within the musculoskeletal system and the mutual regulation between the musculoskeletal system and the endocrine system.Citation39,Citation40 In terms of the self-regulation within the musculoskeletal system, osteokines and myokines can regulate each other via the secretion of cytokines, such as the muscle factor irisin, which has a positive effect on bone tissue. Irisin, an independent predictor for sarcopenia, is related to sarcopenia independent of BMI and age.Citation41 Additionally, irisin can up-regulate autophagy, 8-OHdG, and apoptosis levels in the injured cartilage, thus slowing down the process of cartilage injury.Citation42 Recent studies have reported that irisin levels significantly decrease in patients with sarcopenia.Citation43 This indicates that the lower limb strength will decrease in patients with sarcopenia, accompanied by the decreasing load-bearing capacity of the knee joint and more easy wearing of articular cartilage due to a lack of irisin, leading to an accelerated progression of KOA. This is consistent with our MR results. In contrast, among KOA patients, local inflammatory cells can produce bone morphogenetic proteins (BMPs),Citation44 which thereby activate Pax7-positive satellite cells and promote muscle fiber formation.Citation45 Through detecting muscle tissue in patients with osteoarthritis, a significant number of Pax7-positive satellite cells were found, which help to maintain the population of stem cells by means of self-renewal and provide a large number of muscle-forming cells. The proliferation and differentiation of these cells contribute to the formation of new muscle fibers, resulting in the reconstruction of the muscle system.Citation46 On the other hand, the muscle growth inhibitor myostatin can inhibit muscle regeneration by affecting the expression of BMPs via the specific Smad-4 pathway.Citation47 Meanwhile, the high expression of BMP also inhibits myostatin as well as its ability to suppress muscle regeneration. Based on further morphological analysis, a low number of atrophic fibers and a high number of activated satellite cells can be detected in the muscles of OA patients. These molecular mechanisms suggest that excessive expression of BMP-4 inhibits the age-related muscle atrophy and promotes muscle recovery.Citation48 It may be one of the reasons why KOA does not cause muscle atrophy, as shown in our MR results. In the context of mutual regulation between the musculoskeletal and endocrine systems, research has unveiled that insulin resistance, mediated by the PI3K/Akt pathway, can activate caspase-3.Citation49 This activation subsequently diminishes protein synthesis and augments protein degradation within muscles, leading to muscle atrophy. Additionally, impeding the binding of insulin to its receptor weakens its capacity to degrade intra-articular inflammatory factors, intensifying synovial inflammation and propelling the progression of osteoarthritis.Citation50 Lastly, insulin inhibition results in an increased production of free fatty acids, which, via the TLR-4 pathway, triggers apoptosis in synovial cells, ultimately initiating osteoarthritis.Citation51 These mechanisms potentially offer an initial explanation for the association between sarcopenia and KOA.
Overall, our MR analysis suggests that muscle loss may accelerate the occurrence and progression of KOA via pathways such as reducing muscle quality and accelerating chondrocyte apoptosis. Conversely, a moderate balance between BMP and myostatin in the body of KOA patients may account for the lower probability of muscle atrophy occurrence.
The strengths of this study lie in its utilization of bidirectional Mendelian randomization analysis to investigate the causal relationship between sarcopenia and KOA, thus providing genetic evidence to address controversies in the field. Additionally, multiple MR analysis methods were applied in ensuring result accuracy and validity, and the consistent findings enhanced the reliability of the study. Furthermore, compared with previous MR studies in this field, the use of MR-PRESSO to detect and remove outliers led to the more precise and credible findings.
However, certain limitations should also be noted in this work. First, GWAS data just recruited populations of European ancestry, which possibly restricts our result generalizability to other populations. More investigations are warranted to validate the applicability of these findings in additional ethnicities. Secondly, because of the limited resources, the latest individual-level statistical data could not be accessed. Lastly, as the diagnosis of sarcopenia is still evolving, three widely accepted features were chosen in this study to reduce controversies, but there may be other relevant features not included, potentially leading to bias.
Data Sharing Statement
Summary statistics for the genetic associations with KOA GWAS were obtained from IEU, and the sarcopenia-related GWAS obtained from UKB.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to sincerely thank the original GWASs and the related consortiums for sharing and managing the summary statistics.
Additional information
Funding
References
- Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi:10.1001/jama.2020.22171
- Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. doi:10.1016/j.rehab.2016.01.006
- He Y, Li Z, Alexander PG, et al. Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. Biology. 2020;9(8):194. doi:10.3390/biology9080194
- Belluzzi E, El Hadi H, Granzotto M, et al. Systemic and local adipose tissue in knee osteoarthritis. J Cell Physiol. 2017;232(8):1971–1978. doi:10.1002/jcp.25716
- Belluzzi E, Macchi V, Fontanella CG, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21(17):6016. doi:10.3390/ijms21176016
- Yue L, Berman J. What is osteoarthritis? JAMA. 2022;327(13):1300. doi:10.1001/jama.2022.1980
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
- Lv Z, Yang YX, Li J, et al. Molecular classification of knee osteoarthritis. Front Cell Dev Biol. 2021;9:725568. doi:10.3389/fcell.2021.725568
- Xia L, Zhao R, Wan Q, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. 2020;9(21):7964–7978. doi:10.1002/cam4.3428
- Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. 2022;51(10):afac220. doi:10.1093/ageing/afac220
- Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical Independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–250. doi:10.1002/jcsm.12160
- Isaacson J, Brotto M. Physiology of Mechanotransduction: how Do Muscle and Bone “Talk” to One Another? Clin Rev Bone Miner Metab. 2014;12(2):77–85. doi:10.1007/s12018-013-9152-3
- Lunt E, Ong T, Gordon AL, Greenhaff PL, Gladman JRF. The clinical usefulness of muscle mass and strength measures in older people: a systematic review. Age Ageing. 2021;50(1):88–95. doi:10.1093/ageing/afaa123
- Pegreffi F, Balestra A, De Lucia O, Smith L, Barbagallo M, Veronese N. Prevalence of Sarcopenia in knee osteoarthritis: a systematic review and meta-analysis. J Clin Med. 2023;12(4):1532. doi:10.3390/jcm12041532
- Papalia R, Zampogna B, Torre G, et al. Sarcopenia and its relationship with osteoarthritis: risk factor or direct consequence? Musculoskelet Surg. 2014;98(1):9–14. doi:10.1007/s12306-014-0311-6
- Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nature Reviews Methods Primers. 2022;2(1):6. doi:10.1038/s43586-021-00092-5
- Budu-Aggrey A, Paternoster L. Research Techniques Made Simple: using Genetic Variants for Randomization. J Invest Dermatol. 2019;139(7):1416–1421.e1. doi:10.1016/j.jid.2019.03.1138
- Chen S, Yan S, Aiheti N, et al. A bi-directional Mendelian randomization study of sarcopenia-related traits and type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1109800. doi:10.3389/fendo.2023.1109800
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi:10.1007/s10654-017-0255-x
- Cho Y, Haycock PC, Sanderson E, et al. Exploiting horizontal pleiotropy to search for causal pathways within a Mendelian randomization framework. Nat Commun. 2020;11(1):1010. doi:10.1038/s41467-020-14452-4
- Conroy M, Sellors J, Effingham M, et al. The advantages of UK Biobank’s open-access strategy for health research. J Intern Med. 2019;286(4):389–397. doi:10.1111/joim.12955
- Jones G, Trajanoska K, Santanasto AJ, et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun. 2021;12(1):654. doi:10.1038/s41467-021-20918-w
- Pei YF, Liu YZ, Yang XL, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the UK Biobank study. Commun Biol. 2020;3(1):608. doi:10.1038/s42003-020-01334-0
- Tachmazidou I, Hatzikotoulas K, Southam L, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;51(2):230–236. doi:10.1038/s41588-018-0327-1
- Ruan G, Ying Y, Lu S, et al. The effect of systemic iron status on osteoarthritis: a mendelian randomization study. Front Genet. 2023;14:1122955. doi:10.3389/fgene.2023.1122955
- Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. doi:10.1002/gepi.21998
- Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–4853. doi:10.1093/bioinformatics/btz469
- Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inform. 2016;14(4):173–180. doi:10.5808/GI.2016.14.4.173
- Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi:10.1093/ije/dyx102
- Walker VM, Davies NM, Hemani G, et al. Using the MR-Base platform to investigate risk factors and drug targets for thousands of phenotypes. Wellcome Open Res. 2019;4:113. doi:10.12688/wellcomeopenres.15334.2
- Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi:10.1038/s41588-018-0099-7
- Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. doi:10.1093/hmg/ddy163
- Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. doi:10.7554/eLife.34408
- Zhang X, Pan X, Deng L, Fu W. Relationship between Knee Muscle Strength and Fat/Muscle Mass in Elderly Women with Knee Osteoarthritis Based on Dual-Energy X-Ray Absorptiometry. Int J Environ Res Public Health. 2020;17(2):573. doi:10.3390/ijerph17020573
- Kim HT, Kim HJ, Ahn HY, Hong YH. An analysis of age-related loss of skeletal muscle mass and its significance on osteoarthritis in a Korean population. Korean J Intern Med. 2016;31(3):585–593. doi:10.3904/kjim.2015.156
- Shorter E, Sannicandro AJ, Poulet B, Goljanek-Whysall K. Skeletal Muscle Wasting and Its Relationship With Osteoarthritis: a Mini-Review of Mechanisms and Current Interventions. Curr Rheumatol Rep. 2019;21(8):40. doi:10.1007/s11926-019-0839-4
- van der SE, van V. Viewpoint on the role of tissue maintenance in ageing: focus on biomarkers of bone, cartilage, muscle, and brain tissue maintenance. Ageing Res Rev. 2019;56:100964. doi:10.1016/j.arr.2019.100964
- Herrmann M, Engelke K, Ebert R, et al. Interactions between Muscle and Bone-Where Physics Meets Biology. Biomolecules. 2020;10(3):432. doi:10.3390/biom10030432
- Chen S, Han H, Jin J, Zhou G, Li Z. Osteoarthritis and sarcopenia-related traits: the cross-sectional study from NHANES 2011–2014 and Mendelian randomization study. J Orthop Surg Res. 2023;18:502. doi:10.1186/s13018-023-03960-w
- Wang Y, Gu Y, Huang J, et al. Serum vitamin D status and circulating irisin levels in older adults with sarcopenia. Front Nutr. 2022;9:1051870. doi:10.3389/fnut.2022.1051870
- Wang FS, Kuo CW, Ko JY, et al. Irisin Mitigates Oxidative Stress, Chondrocyte Dysfunction and Osteoarthritis Development through Regulating Mitochondrial Integrity and Autophagy. Antioxidants. 2020;9(9):810. doi:10.3390/antiox9090810
- Guo M, Yao J, Li J, et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J Cachexia Sarcopenia Muscle. 2023;14(1):391–405. doi:10.1002/jcsm.13141
- Katagiri T, Watabe T. Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol. 2016;8(6):a021899. doi:10.1101/cshperspect.a021899
- Sondermann P, Szymkowski DE. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr Opin Immunol. 2016;40:78–87. doi:10.1016/j.coi.2016.03.005
- Waseem R, Shamsi A, Mohammad T, et al. FNDC5/Irisin: physiology and Pathophysiology. Molecules. 2022;27(3):1118. doi:10.3390/molecules27031118
- Kim H, Wrann CD, Jedrychowski M, et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell. 2018;175(7):1756–1768.e17. doi:10.1016/j.cell.2018.10.025
- Scimeca M, Piccirilli E, Mastrangeli F, et al. Bone Morphogenetic Proteins and myostatin pathways: key mediator of human sarcopenia. J Transl Med. 2017;15(1):34. doi:10.1186/s12967-017-1143-6
- Umegaki H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr Gerontol Int. 2016;16(3):293–299. doi:10.1111/ggi.12688
- Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: what are the links? Diabetes Res Clin Pract. 2016;122:198–206. doi:10.1016/j.diabres.2016.10.021
- Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1955–1965. doi:10.1016/j.joca.2015.05.016