Abstract
Objective
Our objective was to develop and validate a nomogram model aiming at predicting the risk of contrast-induced acute kidney injury (CI-AKI) following percutaneous coronary intervention (PCI) in patients suffering from type 2 diabetes mellitus (T2DM) and also diagnosed with acute coronary syndrome (ACS).
Methods
The study gathered data from 722 T2DM patients with ACS who received PCI treatment at the Affiliated Hospital of Xuzhou Medical University between February 2019 and December 2022, serving as the training set. Considering the validation set, the study included 217 patients who received PCI at the East Affiliated Hospital of Xuzhou Medical University. The patients were classified into CI-AKI and non-CI-AKI groups. The study employed univariate and multivariate logistic analysis for identifying independent risk factors for CI-AKI, followed by developing a predictive nomogram model for CI-AKI risk using R software. The predictive performance and clinical utility of the nomogram were assessed through internal and external validation, utilizing the areas under the receiver operating characteristic curve (AUC-ROC), the Hosmer-Lemeshow test and calibration correction curve, and decision curve analysis (DCA).
Results
The nomogram comprised four variables: age, estimated glomerular filtration rate (eGFR), triglyceride-glucose (TyG) index, and prognostic nutritional index (PNI). The AUC-ROC were 0.785 (95% confidence interval (CI) 0.729–0.841) and 0.802 (95% CI 0.699–0.905) for the training and validation cohorts, respectively, indicating a high discriminative ability of the nomogram. The calibration assessment and decision curve analysis have substantiated the strong concordance and clinical usefulness of the aforementioned.
Conclusion
The nomogram exhibits favorable discrimination and accuracy, enabling it to visually and individually identify pre-procedure high-risk patients, and possesses a predictive capacity regarding CI-AKI incidence after PCI in patients diagnosed with both T2DM and ACS.
Introduction
Contrast-induced acute kidney injury (CI-AKI) is characterized by acute renal impairment subsequent to administering iodinated contrast media throughout medical procedures such as angiography. CI-AKI ranked as the third most prevalent reason for in-hospital AKI, following hypoperfusion and drug-related kidney injury.Citation1,Citation2 CI-AKI is linked to extended hospital stays, thereby heightening the occurrence of cardiovascular disease (CVD), all-cause mortality, and end-stage renal disease.Citation3–5 In light of the restricted availability of effective treatment options for CI-AKI, identifying modifiable risk factors is crucial to implement preventive measures.
The triglyceride-glucose (TyG) index was demonstrated to have a significant relation to insulin resistance (IR) and serves as a dependable surrogate for the same. Moreover, the TyG index is associated with endothelial disorders and cardiometabolic abnormalities, making it an important risk factor for CVD onset.Citation6,Citation7 The TyG index was also found to be correlated with diminished urinary microalbumin, chronic kidney disease, and glomerular filtration rate (GFR).Citation8 Additionally, the preoperative TyG index was reported to serve as an independent risk factor for AKI in patients undergoing coronary angiography.Citation9,Citation10
The prognostic nutritional index (PNI) has garnered heightened interest in recent years owing to its uncomplicated nature and ease of access.Citation11 This index employs albumin and lymphocyte counts to evaluate a patient’s nutritional, inflammatory, and immune status. Originally utilized to anticipate surgical risk and prognosticate outcomes in surgical patients, its application in the cardiovascular domain has been comparatively underexplored.Citation12,Citation13 The impairment of the body’s immune defenses, activation of inflammatory cytokines, autonomic dysfunction, and promotion of a vicious cycle of cachexia resulting from malnutrition can lead to slower disease recovery, heightened surgical complications, and increased mortality.Citation14 As such, it is imperative that preoperative nutritional screening and assessment of patients for percutaneous coronary intervention (PCI) are not disregarded.
The nomogram has gained widespread usage as a precise and uncomplicated visualization instrument for forecasting event incidence across various endpoints in patients.Citation15 Consequently, our study endeavors to construct and authenticate a nomogram that forecasts the probability of CI-AKI following PCI in patients diagnosed with acute coronary syndrome (ACS) and type 2 diabetes mellitus (T2DM), thereby furnishing a foundation for clinical treatment determinations and CI-AKI prevention.
Materials and Methods
Study Subjects
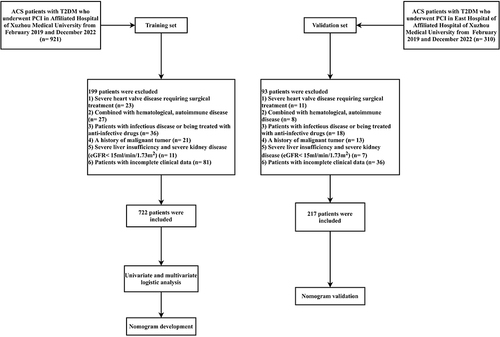
We obtained baseline and clinical characteristics from the electronic medical record system aiming at conducting a retrospective analysis of patients diagnosed with ACS and T2DM who received PCI at the Affiliated Hospital of Xuzhou Medical University and the East Hospital of Xuzhou Medical University between February 2019 and December 2022, with complete clinical data. The study protocol received approval from the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. The study set these inclusion criteria: (1) patients diagnosed with ACS per 2020 ESC guidelinesCitation16 for managing ACS, including ST-segment elevation myocardial infarction (STEMI), non-STEMI, and unstable angina (UA); (2) patients with diabetes mellitus (DM); and (3) who received PCI throughout their hospitalization.
Exclusion criteria were as follows: (1) severe heart valve disease requiring surgical treatment, (2) combined with hematological, autoimmune and neoplastic diseases, (3) patients with infectious disease or being treated with anti-infective drugs, (4) a history of malignant tumor, (4) severe liver insufficiency and severe kidney disease (eGFR <15mL/min/1.73m2), (5) patients with incomplete clinical data.
PCI, Hydration Methods
The present study employed conventional catheters, guidewires, balloon catheters, and stents for PCI procedures conducted via radial artery access. During the procedure, an iso-osmolar contrast medium (Ioversol, Yangtze River Pharmaceutical Group) was administered. Prior to PCI, it was typical to give dual antiplatelet therapy along with 3000 IU of ordinary heparin anticoagulation. Additionally, during PCI, an additional dose of 100 IU/kg of heparin could be administered based on the patient’s body weight. The interventional physicians identified the dosage of medication administered during the procedure depending on the medical condition of the patient. According to the guidelines, it is recommended that all patients be administered normal saline for 12 h at 1.0 mL/kg/h rate pre- and post-procedure. When LVEF is below 40% or the patient has heart failure, it is recommended to decrease the hydration rate to 0.5 mL/kg/h.Citation16,Citation17
Research Methodology
The medical record system was reviewed for baseline and clinical information (including demographics, past medical histories, vital signs at admission, laboratory indicators, and medication at discharge). A venous blood sample was collected from each participant within a 24-h timeframe following admission and also at least 2 days following PCI. Lab indicators were uniformly performed before operation by the testing center’s laboratory.
Definitions
According to the European Society of Urogenital Radiology (ESUR), CI-AKI is defined as the absolute elevation of serum creatinine (sCr) level of ≥ 0.3 mg/dl (26.5 μmol/L) or more than 1.5 times the baseline level from the preoperative level throughout 48–72 h timeframe following the contrast medium exposure, excluding other causes of renal impairment.Citation18 The study employed the simplified MDRD formula for calculating the estimated glomerular filtration rate (eGFR), taking into account the sCr level measured upon admission: eGFR (mL/min/1.73 m2)) = 186 x sCr (mg/dL) − 1.154 x age (years) − 0.203× 0.742 (female). TyG = ln [fasting serum triglycerides (mg/dl) × fasting glucose (mg/dl)/2]. PNI = serum albumin (g/L) + 5 x peripheral blood lymphocyte count (109 /L). The criteria utilized to diagnose DM were fasting blood glucose (FBG) > 7 mmol/L, random blood glucose > 11.1 mmol/L, or a documented DM history involving diet, orally-administrated drugs, or therapeutic insulin.
Statistical Analysis
The study employed SPSS version 26.0 and R version 4.2.3 to conduct the statistical analyses. Shapiro–Wilk and Levene’s tests were utilized to test for normality and homogeneity. The normally distributed data were represented as mean ± standard deviation while representing the non-normally distributed data as median (M) and interquartile range (M P25, P75). Two independent-sample t-tests were employed for comparing the normally distributed data, and analysis of between-group data with a non-normal distribution was conducted using a nonparametric test (Mann–Whitney U-test). The study utilized the chi-square test or Fisher’s exact probability for the comparison of count data between groups. The multivariate regression analysis incorporated variables that exhibited P > 0.05 in the univariate analysis. After performing a multivariate analysis, a nomogram was constructed. This was then verified using receiver operating characteristic (ROC) curves and calibration plots. Furthermore, the Hosmer-Lemeshow test, calibration plot, and decision curve analysis (DCA) were utilized for assessing the model’s clinical validity. In addition, DCA utilization was employed to assess the clinical utility of the nomogram, while a calibration plot was utilized to internally validate the model.
Results
Baseline Patient Features for the Training and Validation Sets
Between February 2019 and December 2022, 722 patients out of 921 diagnosed with ACS in conjunction with T2DM and received PCI at the Affiliated Hospital of Xuzhou Medical University were selected as the training set, following the application of exclusion criteria. Meanwhile, 217 patients out of 310 diagnosed with ACS in conjunction with T2DM and received PCI at the East Hospital of the Affiliated Hospital of Xuzhou Medical University were selected as the validation set. shows the flowchart.
Comparison of General and Laboratory Data for Patients in the Training Set
Of the 722 patients selected in the training set, a total of 69 (9.55%) developed CI-AKI. In comparison to the non-CI-AKI group, CI-AKI group patients were older, exhibiting a significantly lower PNI and eGFR, and significantly higher prevalence of FBG, blood urea nitrogen, sCr, TyG, diuretic use, and hypertension (HTN) (P < 0.05). lists the specific results.
Table 1 Comparison of Baseline Features in the Training Cohort
The Clinical Features of the Two Sets
To prevent overfitting of the clinical prediction model when analyzing influencing factors, we conducted an analysis of the clinical features of the two sets, aiming at identifying any differences between the data in these sets. The features of the two sets did not exhibit any significant differences, suggesting that the datasets were reasonably partitioned. Table S1 lists the basic information, and the two sets are comparable.
Creating a Nomogram
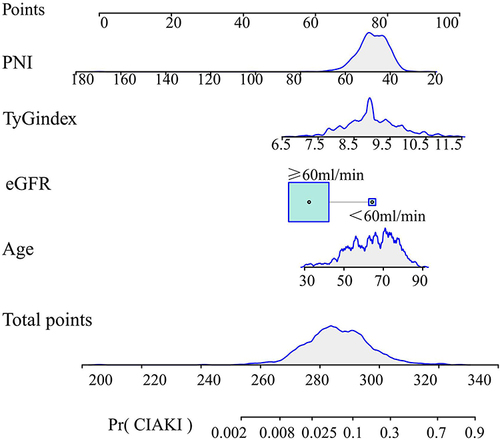
The study employed univariate and multivariate regression analyses for identifying CI-AKI risk factors (). The univariate logistic regression analysis identified several factors, including age, HTN history, fasting blood glycemia, blood urea nitrogen, diuretics, estimated eGFR, TyG index, and PNI (P<0.05). Subsequently, the aforementioned factors were incorporated into a multifactorial analysis, aiming at identifying the CI-AKI independent risk factors such as age (odds ratio (OR) 1.039; 95% confidence interval (CI) 1.008–1.070), eGFR (OR 0.974; 95% CI 0.962–0.985), TyG (OR 2.997; 95% CI 1.911–4.701), and PNI (OR 0.915; 95% CI 0.872–0.961). The results obtained from the multivariate analysis were employed to draw a nomogram that can be utilized for the prediction of CI-AKI occurrence in patients with ACS combined with DM ().
Table 2 Logistic Regression Analysis for the Occurrence of CI-AKI After PCI in T2DM Patients with ACS in Training Cohort
Figure 2 The nomogram for predicting the occurrence of CI-AKI after PCI in T2DM patients with ACS. The cumulative score is determined by adding together the individual scores of each of the four variables incorporated in the nomogram.
Validation of the Nomogram
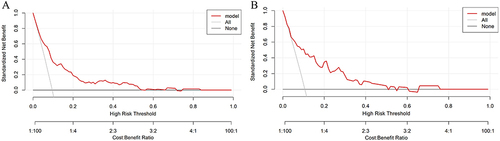
The values of AUCs for predicting CI-AKI in patients with ACS combined with T2DM were 0.785 (95% CI 0.729–0.841) and 0.802 (95% CI 0.699–0.905) in the training () and validation sets (), which indicated that the discriminatory power of this clinical prediction model is very good. The Hosmer-Lemeshow test found a strong fit between the training (χ2=9.987, P=0.266) and validations (χ2= 4.517, P= 0.808) sets. Subsequently, the calibration curves were plotted for the two nomogram sets employing the bootstrap self-sampling method with B=1000 replicates, revealing that the predictions matched well with the actual results (). In addition, the DCA was utilized for assessing the clinical validity of the nomogram (), indicating that the nomogram has a wide range of threshold probabilities, making the model clinically useful.
Figure 3 ROC curve of the nomogram for predicting CI-AKI after PCI in T2DM patients with ACS. (A) ROC curve in the training set; (B) ROC curve in the validation set.
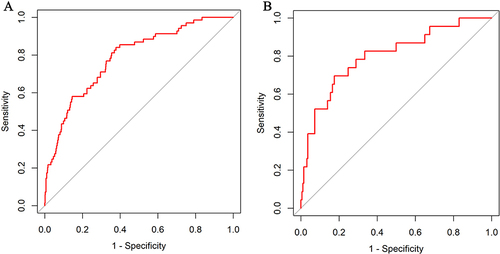
Figure 4 Calibration curve of the nomogram for the training set (A) and the validation set (B). The X-axis denotes the comprehensive predicted probability of contrast-induced acute kidney injury (CI-AKI) subsequent to percutaneous coronary intervention (PCI), while the Y-axis signifies the corresponding actual probability. The calibration of the model is determined by the extent to which the curve aligns with the diagonal.
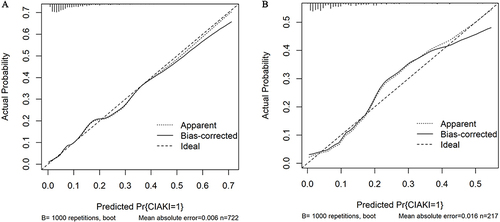
Figure 5 Decision curve analysis for the training set (A) and the validation set (B). A horizontal line signifies the absence of treatment and a negative outcome for all samples, resulting in a net benefit of zero. Conversely, an oblique line denotes the presence of treatment and a positive outcome for all samples, leading to a negative slope in the net benefit.
Discussion
While advanced minimally invasive imaging and interventional techniques are becoming more mature than traditional surgical techniques in the cardiovascular field, CI-AKI remains a prevalent obstacle regarding patients undergoing intravascular imaging, increasing the stay duration and mortality.Citation5,Citation19 Currently, there is a lack of specific clinical medications and interventions for CI-AKI occurrence.Citation20 The primary focus for addressing this condition remains on preventing its pathogenic mechanism and mitigating risk factors.Citation21 The primary objective in preventing CI-AKI is to conduct a risk assessment in high-risk groups, which serves as a crucial component. Consequently, several models have been suggested to address this concern.Citation22,Citation23 However, the TyG index combined with PNI to predict contrast nephropathy has not yet been proposed. This retrospective study enrolled 722 T2DM patients with ACS PCI, of whom 69 (9.55%) developed CI-AKI. Based on the previously reported CI-AKI risk factors, clinical data from patients at Xuzhou Medical University Hospital were retrieved to further analyze CI-AKI risk factors in patients diagnosed with both DM and ACS after PCI. The nomogram was based on the multi-factor regression analysis and integrated the predictors, with the advantage that the values of multiple predictors or variables could be derived using scaled line segments, which can be used to predict the risk of disease development in a more intuitive and individualized way.Citation15 The results of this study showed that age, eGFR <60 mL/min, TyG index, and PNI were independent predictors of CI-AKI. This was used as a basis to develop a nomogram for CI-AKI occurrence following PCI in patients with DM combined with ACS.
Aging is a risk factor for CVD, with decreasing renal function with age, along with increased vascular stiffness and decreasing endothelial function, resulting in reduced vasodilation and the ability of pluripotent stem cells to repair vessels.Citation24–26 Together, these factors diminish the probability of immediate creatinine recuperation in patients after surgery and enhance the susceptibility to CI-AKI in elderly patients.
Baseline renal function is a determining factor in CI-AKI development, and eGFR is a more objective indicator to assess renal function;Citation27,Citation28 patients with lower eGFR levels undergoing PCI are more susceptible to developing CI-AKI, which is strongly related to renal hypoperfusion.Citation29 Renal insufficiency leads to a decreased ability to excrete contrast media, resulting in medullary ischemia and the release of reactive oxygen species, which directly leads to renal tubular apoptosis.Citation30
The current study shows that the TyG index is not only correlated to IR but also has a better performance in evaluating IR compared to the high insulin-normal glucose clamp test.Citation31,Citation32 The mechanism by which IR damages the kidney is thus thought to be related to the following reasons:Citation32,Citation33 (1) IR is closely related to oxidative stress, and increased free radical production can cause kidney damage. (2) has the potential to elevate insulin levels within the body and can coexist with abnormal glucose metabolism and lipid metabolism. Consequently, this combination can participate in the development or progression of metabolic disorders, including obesity, DM, and CVD, which in turn can further aggravate renal impairment. Prior research has established a strong correlation between a high TyG index and a heightened occurrence of CI-AKI, thereby establishing the TyG index as a significant CI-AKI risk factor. This finding lends support to our conclusion.Citation9
Our study revealed that malnutrition is also an independent risk factor for CI-AKI development in T2DM patients receiving PCI. The PNI scoring system, which includes only albumin and lymphocyte count, is an objective nutritional screening tool based on clinical test data and is used in clinical practice because of its simplicity and ease of implementation.Citation34,Citation35 The lymphocyte count is often considered a marker of the body’s inflammatory response and immune status. Multiple studies have suggested that inflammatory cells contributed to the initiation, proliferation, and recovery phases of AKI, infiltrating damaged kidney tissue and producing inflammatory mediators, including cytokines and chemokines, and that lymphocytes play a key role in these processes.Citation36 Protein-calorie malnutrition causes altered renal hemodynamics, reduced renal blood flow, decreased GFR, concentrated urine, and reduced tubular excretion of acids.Citation37,Citation38 In summary, AKI development may be attributed to not only albumin reduction, which alters plasma osmolality and blood flow viscosity and attenuates vasodilatory, antioxidant, and anti-inflammatory mechanisms, but also lymphocyte, which initiates and amplifies immune inflammatory responses. These two factors can work together synergistically, exerting diverse pathophysiological effects and varying degrees of contribution to CI-AKI development.
In summary, we analyzed the risk factors for CI-AKI occurrence and developed a nomogram that can be utilized to predict CI-AKI. Through internal and external validation, the model showed good discrimination and accuracy, which can identify high-risk patients in a more intuitive and individual way. Moreover, the model also possesses predictive value in determining CI-AKI occurrence after PCI in patients with ACS and T2DM. This predictive ability aids clinicians in evaluating CI-AKI risk before contrast exposure and enables them to implement preemptive interventions for patients at a heightened risk of CI-AKI, including appropriate hydration therapy or the use of combined drug prophylaxis.
Limitations
The shortcomings of this study are that it is a retrospective study with data from the hospital medical record system, and although the availability of external validation, the external data are from the branch hospitals, and follow-up is still required for further assessment of the clinical predictive value of the model in a multi-center, expanded sample size. In this paper, patients were not followed up after discharge from the hospital, including medication use and all-cause mortality. A complete follow-up system is expected to enhance the performance of the predictive model in the future.
Conclusions
T2DM has good discrimination and accuracy with our predictive model; CI-AKI can be assessed before PCI through simple calculations, and preventive measures can be taken to reduce the incidence. The concept deserves to be promoted and applied further.
Data Sharing Statement
The Data can be provided by the corresponding author upon a suitable request.
Ethics Approval and Consent Participate
The investigation was conducted in accordance with the Helsinki Declaration and received approval from the Medical Ethics Committee of Xuzhou Medical University’s Affiliated Hospital (ethic number: XYFY2021-KL024-01). Given the retrospective nature of the study, the review committee waived the requirement for written informed consent. Prior to data analysis, all identifiable patient information was removed.
Consent for Publication
All participants and/or their legal guardians given permission for the publishing of their information.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
We extend our gratitude to all the participants and colleagues who actively participated in the study. It is worth noting that Yinghua Zhu and Haiyan He have contributed equally as co-first authors in this research endeavor.
Additional information
Funding
References
- Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. 2016;102(8):638–648. doi:10.1136/heartjnl-2014-306962
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi:10.1053/ajkd.2002.32766
- Griffiths RI, Cavalcante R, McGovern AM, et al. Cost to Medicare of acute kidney injury in percutaneous coronary intervention. Am Heart J. 2023;262:20–28. doi:10.1016/j.ahj.2023.03.013
- Ng AK, Ng PY, Ip A, et al. Impact of contrast-induced acute kidney injury on long-term major adverse cardiovascular events and kidney function after percutaneous coronary intervention: insights from a territory-wide cohort study in Hong Kong. Clin Kidney J. 2022;15(2):338–346. doi:10.1093/ckj/sfab212
- Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35(23):1533–1540. doi:10.1093/eurheartj/ehu063
- Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi:10.1186/s12933-022-01511-x
- Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16(4):203–212. doi:10.1038/s41569-018-0119-4
- Gao YM, Chen WJ, Deng ZL, Shang Z, Wang Y. Association between triglyceride-glucose index and risk of end-stage renal disease in patients with type 2 diabetes mellitus and chronic kidney disease. Front Endocrinol (Lausanne). 2023;14:1150980. doi:10.3389/fendo.2023.1150980
- Qin Y, Tang H, Yan G, et al. A High Triglyceride-Glucose Index Is Associated With Contrast-Induced Acute Kidney Injury in Chinese Patients With Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2020;11:522883. doi:10.3389/fendo.2020.522883
- Ye Z, An S, Gao Y, et al. Association between the triglyceride glucose index and in-hospital and 1-year mortality in patients with chronic kidney disease and coronary artery disease in the intensive care unit. Cardiovasc Diabetol. 2023;22(1):110. doi:10.1186/s12933-023-01843-2
- Zhang S, Wang H, Chen S, et al. Prognostic nutritional index and prognosis of patients with coronary artery disease: a systematic review and meta-analysis. Front Nutrition. 2023;10:1114053. doi:10.3389/fnut.2023.1114053
- Kang N, Gu H, Ni Y, Wei X, Zheng S. Prognostic and clinicopathological significance of the Prognostic Nutritional Index in patients with gastrointestinal stromal tumours undergoing surgery: a meta-analysis. BMJ Open. 2022;12(12):e064577. doi:10.1136/bmjopen-2022-064577
- Yan L, Nakamura T, Casadei-Gardini A, Bruixola G, Huang YL, Hu ZD. Long-term and short-term prognostic value of the prognostic nutritional index in cancer: a narrative review. Ann Translational Med. 2021;9(21):1630. doi:10.21037/atm-21-4528
- Ahmed N, Choe Y, Mustad VA, et al. Impact of malnutrition on survival and healthcare utilization in Medicare beneficiaries with diabetes: a retrospective cohort analysis. BMJ Open Diabetes Research & Care. 2018;6(1):e000471. doi:10.1136/bmjdrc-2017-000471
- Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793. doi:10.1016/j.jtcvs.2017.12.107
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
- Andreucci M, Solomon R, Tasanarong A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed Res Int. 2014;2014:741018. doi:10.1155/2014/741018
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi:10.1007/s00330-011-2225-0
- Connolly M, Kinnin M, McEneaney D, et al. Prediction of contrast induced acute kidney injury using novel biomarkers following contrast coronary angiography. QJM. 2018;111(2):103–110. doi:10.1093/qjmed/hcx201
- Efe SC, Keskin M, Toprak E, et al. A Novel Risk Assessment Model Using Urinary System Contrast Blush Grading to Predict Contrast-Induced Acute Kidney Injury in Low-Risk Profile Patients. Angiology. 2021;72(6):524–532. doi:10.1177/00033197211005206
- Valdenor C, McCullough PA, Paculdo D, et al. Measuring the Variation in the Prevention and Treatment of CI-AKI Among Interventional Cardiologists. Curr Probl Cardiol. 2021;46(9):100851. doi:10.1016/j.cpcardiol.2021.100851
- Ma K, Li J, Shen G, et al. Development and Validation of a Risk Nomogram Model for Predicting Contrast-Induced Acute Kidney Injury in Patients with Non-ST-Elevation Acute Coronary Syndrome Undergoing Primary Percutaneous Coronary Intervention. Clin Interv Aging. 2022;17:65–77. doi:10.2147/cia.S349159
- Qiu H, Zhu Y, Shen G, Wang Z, Li W. A Predictive Model for Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention in Elderly Patients with ST-Segment Elevation Myocardial Infarction. Clin Interv Aging. 2023;18:453–465. doi:10.2147/cia.S402408
- Kaneko H, Yano Y, Okada A, et al. Age-Dependent Association Between Modifiable Risk Factors and Incident Cardiovascular Disease. J Am Heart Assoc. 2023;12(2):e027684. doi:10.1161/jaha.122.027684
- Zhao M, Song L, Sun L, et al. Associations of Type 2 Diabetes Onset Age With Cardiovascular Disease and Mortality: the Kailuan Study. Diabetes Care. 2021;44(6):1426–1432. doi:10.2337/dc20-2375
- Ray N, Reddy PH. Structural and physiological changes of the kidney with age and its impact on chronic conditions and COVID-19. Ageing Res Rev. 2023;88:101932. doi:10.1016/j.arr.2023.101932
- Pan HC, Wu XH, Wan QL, Liu B, Wu XS. Analysis of the risk factors for contrast-induced nephropathy in over-aged patients receiving coronary intervention. Exp biol med. 2018;243(12):970–975. doi:10.1177/1535370218799973
- James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096–2103. doi:10.1016/s0140-6736(10)61271-8
- Patschan D, Kribben A, Müller GA. Postischemic microvasculopathy and endothelial progenitor cell-based therapy in ischemic AKI: update and perspectives. Am J Physiol Renal Physiol. 2016;311(2):F382–94. doi:10.1152/ajprenal.00232.2016
- Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66(2):496–499. doi:10.1111/j.1523-1755.2004.761_5.x
- Dave N, Wu J, Thomas S. Chronic Kidney Disease-Induced Insulin Resistance: current State of the Field. Curr Diab Rep. 2018;18(7):44. doi:10.1007/s11892-018-1010-8
- Sarafidis PA, Grekas DM. Insulin resistance and oxidant stress: an interrelation with deleterious renal consequences? J Cardiometab Syndr. 2007;2(2):139–142. doi:10.1111/j.1559-4564.2007.06666.x
- Huh JH, Yadav D, Kim JS, et al. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism. 2017;67:54–61. doi:10.1016/j.metabol.2016.11.003
- Dai Y, Liu M, Lei L, Lu S. Prognostic significance of preoperative prognostic nutritional index in ovarian cancer: a systematic review and meta-analysis. Medicine. 2020;99(38):e21840. doi:10.1097/md.0000000000021840
- Efe SC, Karagöz A, Doğan C, et al. Prognostic significance of malnutrition scores in elderly patients for the prediction of contrast-induced acute kidney injury. Int J Clin Pract. 2021;75(7):e14274. doi:10.1111/ijcp.14274
- Black LM, Lever JM, Traylor AM, et al. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol. 2018;315(4):F1107–f1118. doi:10.1152/ajprenal.00179.2018
- Kalista-Richards M. The kidney: medical nutrition therapy--yesterday and today. Nutr Clin Pract. 2011;26(2):143–150. doi:10.1177/0884533611399923
- Dilken O, Ince C, Kapucu A, Heeman PM, Ergin B. Furosemide exacerbated the impairment of renal function, oxygenation and medullary damage in a rat model of renal ischemia/reperfusion induced AKI. Intensive Care Med Exp. 2023;11(1):25. doi:10.1186/s40635-023-00509-3