Abstract
Bile acids play a crucial role in promoting intestinal nutrient absorption and biliary cholesterol excretion, thereby protecting the liver from cholesterol accumulation and bile acid toxicity. Additionally, bile acids can bind to specific nuclear and membrane receptors to regulate energy expenditure and specific functions of particular tissues. Vascular calcification refers to the pathological process of calcium-phosphate deposition in blood vessel walls, which serves as an independent predictor for cardiovascular adverse events. In addition to aging, this pathological change is associated with aging-related diseases such as atherosclerosis, hypertension, chronic kidney disease, diabetes mellitus, and osteoporosis. Emerging evidence suggests a close association between the bile acid network and these aforementioned vascular calcification-associated conditions. Several bile acids have been proven to participate in calcium-phosphate metabolism, affecting the transdifferentiation of vascular smooth muscle cells and thus influencing vascular calcification. Targeting the bile acid network shows potential for ameliorating these diseases and their concomitant vascular calcification by regulating pathways such as energy metabolism, inflammatory response, oxidative stress, and cell differentiation. Here, we present a summary of the metabolism and functions of the bile acid network and aim to provide insights into the current research on the profound connections between the bile acid network and these vascular calcification-associated diseases, as well as the therapeutic potential.
Introduction
Bile acids (BAs) are synthesized in the liver, stored in the gallbladder, and circulated within the enterohepatic circulation. Apart from their role as emulsifiers for lipid absorption, BAs also function as metabolic regulators, influencing overall metabolism and organ functions. Specific receptors, such as the nuclear farnesoid X receptor (FXR), the G protein-coupled membrane receptor (TGR5), vitamin D receptor (VDR) and pregnane X receptor (PXR) respond to BAs and further affect the expression of an abundance of target genes to regulate glucose and lipid metabolism or adjust the physiological function of specific organs.Citation1 Disruption of BA homeostasis contributes to the pathogenesis of a range of metabolic disorders, as well as digestive, cardiovascular, nervous, and systemic diseases.Citation2,Citation3 Given the significant regulatory effects of BAs in different situations, a host of natural or synthesized BA receptor agonists and certain BAs have been applied to treat BA network-associated diseases. Therefore, understanding the metabolism and function of the BA network in different pathological situations is crucial for developing effective targeted treatment strategies.
Calcification refers to the process of crystalline hydroxyapatite deposition and could be categorized into physiological and pathological calcification. Physiological calcification usually occurs in the bone to maintain its strength and structure.Citation4 In contrast, pathological calcification is recognized as an active process that primarily occurs in blood vessels. Dysfunction of calcium (Ca) or phosphate (Pi) homeostasis, imbalance of calcification inhibitors and promoters, osteogenic/chondrogenic differentiation of vascular smooth muscle cells (VSMCs), extracellular matrix remodeling, oxidative stress, inflammation, mitochondria function, iron homeostasis and miRNAs from exosomes are important factors involved in this process.Citation5 Multiple conditions, including aging and aging-related diseases, such as atherosclerosis (AS), hypertension, chronic kidney disease (CKD), diabetes mellitus (DM) and osteoporosis (OP), are considered contributors to the development of vascular calcification (VC) and undoubtedly, treatment for these diseases and accompanied VC will be beneficial for maintaining cardiovascular health.Citation6
Several studies have investigated the influence of the BA network on vascular function. Notably, the synthesis and excretion of BAs serve as physiological mechanisms to eliminate cholesterol from the body thus insufficient BA excretion could promote lipid deposition, thereby contributing to the development of AS.Citation7 Deoxycholic acid (DCA), a classic secondary BA, has been shown to promote the proliferation and migration of VSMCs, thereby accelerating the development of AS.Citation8 In cirrhotic portal hypertension models, activation of FXR can inhibit BA synthesis while alleviating pathological angiogenesis and sinusoidal remodeling.Citation9 For years, many BA metabolites have been shown to act as vasodilators by activating TGR or FXR. However, a recent study has indicated that chronic activation of FXR reduces nitric oxide (NO) sensitivity of smooth muscle, leading to impaired endothelium-dependent vasodilation. This may partially explain the relationship between higher BA levels and vascular complications in patients with liver disease.Citation10 Recent studies indicated that the BA network is tightly connected with VC and above-mentioned VC-associated diseases. In these diseases, there are changes in the levels of BA metabolites, and targeting BA receptors has been proven to be significant in the development of the diseases. In this review, we provide a detailed overview of the BA metabolism and the functions of its nuclear and membrane receptors under physiological conditions and then we place emphasis on the latest advances in intricate interaction between the BA network and VC-associated diseases, as well as the therapeutic therapy.
Bile Acid Synthesis, Transformation, and Excretion
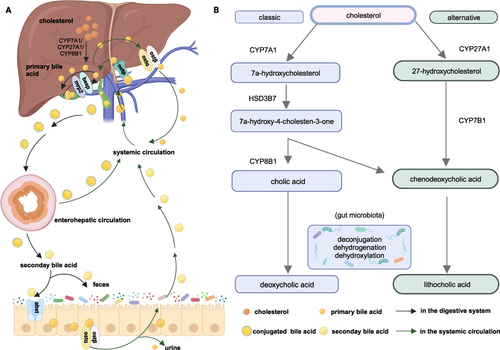
BAs, containing primary and secondary BAs, are derivatives of cholesterol metabolism. In the human liver, there are two main synthesis pathways for the production of primary BAs cholic acid (CA) and chenodeoxycholic acid (CDCA): the classical pathway and the alternative pathway, as shown in .Citation11–13 In neonates, the alternative pathway plays a dominant role due to the loss of cholesterol 7α-hydroxylase (CYP7A1) expression. However, after weaning, the classical pathway dominates, and approximately 80% of BAs are synthesized in this way.Citation14 Apart from CA and CDCA, mice also produce muricholic acid (MCA) and ursodeoxycholic acid (UDCA) as primary BAs. Primary BAs are stored in the gallbladder in the form of conjugating with glycine or taurine. In humans, BAs are mainly conjugated with glycine, while conjugation with taurine is relatively low. However, in mice and rats, BAs are almost completely conjugated with taurine.Citation15 Upon ingestion of food, conjugated BAs are released into the intestine and undergo a series of reactions, including deconjugation, dehydrogenation, and dehydroxylation, to form secondary BAs and achieve chemical diversity. These reactions are highly dependent on the activity of bacteria equipped with specific enzymes.Citation16,Citation17 The most common secondary BAs in the human body are lithocholic acid (LCA) derived from CDCA and DCA derived from CA. In mice, MCA undergoes the same reaction to form murideoxycholic acid, hyodeoxycholic acid and hydrochloric acid.Citation15 For normal adults, approximately 0.5 grams of primary BAs are synthesized daily to maintain the volume of BA. With the help of a bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2), primary BAs are secreted into the bile duct. A small portion of primary BAs will be resorbed subsequently by cholangiocytes (the cholangiohepatic shunt) subsequently. For those BAs secreted to the intestine, there are two ways to re-enter the liver through the blood circulation system: passive absorption in the upper intestine and active transportation in the ileum. The efflux of BAs into blood circulation occurs with the upregulation of organic solute transporter alpha (OSTα) and OSTβ.Citation18 BAs in the blood are ingested into the hepatocytes via sodium-dependent taurocholate co-transporting peptide (NTCP) to restart the loop.Citation19 Almost equal amounts of newly synthesized BAs are excreted in the form of feces, and almost 0.5 mg of BA is lost in the urine of humans. This whole process is called enterohepatic circulation, which ensures the stability of the BA pool.Citation14
Figure 1 The Bile Acid Metabolism figure for human (created with BioRender.com.) (A) illustrates the metabolic pathways of BAs in the hepatic-intestinal and circulatory systems and (B) offers a succinct summary of the key steps in BA metabolism. Cholesterol is converted to primary acids by the classic and alternative pathways. In the classic pathway, with the existence of CYP7A1 and HSD3B7, cholesterol is metabolized to 7α-hydroxycholesterol and C4 successively. C4 is the precursor of CDCA and CA. In the alternative pathway, cholesterol is biotransformed into 27-hydroxycholesterol and CA with the presence of CYP27A1 and CYP7B1. Primary BAs were stored in the gallbladder and secreted into the bile duct after food intake via BESP and MRP2 in conjugated forms. BAs in bile duct could be reabsorbed by the cholangiohepatic shunt directly. Conjugated BAs experienced deconjugation, dehydrogenation, and dihydroxylation reactions by variable bacteria to form LCA and DCA. BAs could enter systemic circulation via OSTα and OSTβ to be reabsorbed by enterohepatic circulation. A small amount of BAs will be excreted from the body in the form of faeces and urine.
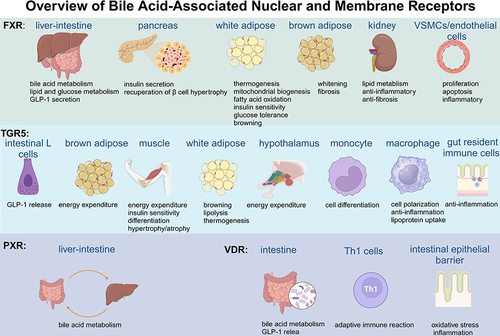
BA Receptors
BAs represent the ultimate metabolite of cholesterol catabolism, and as physiological detergents, BAs play pivotal roles in the absorption, digestion and solubilization process of dietary lipids, fat-soluble vitamins, nutrients, and drugs. Furthermore, as endogenous ligands for several nuclear and plasma membrane receptors, BAs exhibit a diverse range of hydrophilic properties that enable them to bind with FXR, TGR5, VDR, or PXR, thereby contributing to the regulation of metabolism and function in various cells and organs,Citation20 as is shown in .
FXR
FXR, an essential receptor for BAs, is widely expressed in all kinds of organs, especially in the liver and intestine. CDCA is recognized as the most efficient activator of FXR, followed by DCA and LCA, whilst CA is inactive and tauro-muricholic acid is the antagonist for FXR in the intestine.Citation21,Citation22 Activation of FXR could regulate targeted genes via induction of negative regulators and competition for other nuclear receptors or transcriptional coregulators. Importantly, FXR occupies a principal place in BA homeostasis. BAs can be transported to the liver directly via the portal vein system, where they regulate BA synthesis in an FXR-dependent manner by activating FXR/SHP/LRH-1 (FXR-small heterodimer partner, SHP; liver receptor homolog-1, LRH-1) cascade and this pathway has a more significant inhibitory effect on sterol 12-alpha-hydroxylase (CYP8B1) than CYP7A1.Citation23 Furthermore, the activation of FXR in the ileum triggers the release of fibroblast growth factor 15/19 (FGF15/19), which can bind with the FGF receptor 4/β-klotho heterodimer complex and inhibit CYP7A1 by initiating a Jun N-terminal kinase 1/2 (JNK1/2) and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade to restrain the synthesis rate of BA in the liver.Citation24 Emerging evidence has shown that inflammatory cytokines, such as Interleukin-1β and tumor necrosis factor-α (TNFα) can suppress CYP7A1 and oxysterol 7alpha-hydroxylase (CYP7B1) gene transcription through the protein kinase C/JNK pathway. This “FXR/SHP-independent” mechanism ensures a rapid reaction to acute liver injury without the participation of transcription factors.Citation25 In addition to its role in regulating BA synthesis, FXR can increase BAs’ secretion into the gallbladder and efflux into circulation to abate the accumulated toxicity of BAs in the liver. Moreover, FXR can also inhibit apical sodium-dependent bile acid transporter (ASBT), thereby restraining BA reabsorption.
The expression of FXR in the liver plays a crucial role in regulating glucose and lipid metabolism. FXR-/- mice exhibited elevated serum glucose and impaired glucose tolerance.Citation26 However, this function is effective only within a certain dose range, as FXR inhibition of glucagon-like peptide-1 (GLP-1) becomes more significant at higher doses. Notably, although (HCA) and its derivative constitute only approximately 3% of all BAs in humans and mice, a study aimed at supplying HCA to diabetic mice suggested that HCA could improve insulin tolerance by inhibiting FXR and activating TGR5 in a dose-dependent manner.Citation27 However, acetylation of FXR leads to opposite results, as it could activate proinflammatory genes and macrophage infiltration to ruin the function of insulin signaling and plasma glucose homeostasis owing to the imbalance between acetylation and SUMOylation of FXR.Citation28 FXR plays a prominent role in the synthesis, oxidation, transfer, absorption and clearance of lipids. There are three main ways to achieve this effect: (1) Targeting the FXR-SHP-SREBP-1c regulatory cascade (sterol-regulatory element binding proteins, SREBPs). (2) stimulating PPARα and FGF21 (peroxisome proliferator-activated receptor α, PPARα). (3) controlling genes in lipoprotein metabolism.Citation18 In FXR-/- mice, higher levels of triglycerides and cholesterol were observed and supplementation with a selective agonist for FXR reversed hyperlipemia.Citation29 FXR agonists might decrease lipid synthesis in the liver and lipid absorption in the intestine, which could be a latent therapy for nonalcoholic fatty liver disease.Citation30 The process of autophagy was proven to be an essential component in lipid metabolism, especially under conditions of nutrient deprivation and/or starvation. Previous work has indicated FXR could suppress this process by damaging the functional CREB-CRTC2 complex (cyclic-AMP response binding protein, CREB; CREB regulated transcription coactivator 2, CRTC2).Citation31,Citation32 Moreover, FXR in the pancreas would promote insulin secretion and recuperation of β cell hypertrophy to benefit glucose and lipid metabolism.Citation33
FXR is also expressed in adipose tissue and its function in this tissue has garnered increasing interest. In 3t3-l1 cell lines, the activation of FXR enhances preadipocyte differentiation by PPARγ-dependent and PPARγ-independent pathways.Citation34 Fexaramine, a gut-restricted FXR agonist, was proven to promote thermogenesis, mitochondrial biogenesis and fatty acid oxidation in white adipose tissue, thereby alleviating diet-induced metabolic disorders such as obesity, inflammation and hyperglycemia.Citation35 Brown adipose tissue is abundant with mitochondria and is known for dispersing stored energy. When FXR is activated, unforeseen browning of white adipose tissue occurs by downregulating inflammatory cytokine levels while enhancing β-adrenergic signaling.Citation35,Citation36 However, Dehondt deemed that the loss of FXR in white adipose tissue induced the upregulation of anti-oxidant enzymes to benefit systemic glucose tolerance and insulin sensitivity.Citation37 Slightly detected in brown adipose tissue, overexpression of FXR could hinder the development and function of this tissue, leading to whitening and fibrosis.Citation38 FXR is widely expressed in renal tubular epithelial cells, protecting the kidney from lipid toxicity, and pro-inflammatory and pro-fibrotic environment.Citation33 Moreover, FXR also exists in VSMCs and endothelial cells (ECs) to regulate their proliferation, apoptosis and inflammatory activities.Citation39
TGR5
Existing in almost the whole body, TGR5 is primarily activated by LCA and DCA. Dysfunction of TGR5 triggers a series of disorders such as obesity, inflammation, liver steatosis, AS and metabolic syndrome. Activation of TGR5 could stimulate the secretion of GLP-1 in intestinal L cells and promote energy expenditure in brown adipose tissue and muscle through the stimulation of mitochondrial oxidative phosphorylation.Citation40 These changes maintain glucose homeostasis and insulin sensitivity to protect the functions of the liver and pancreas and prevent or treat metabolic disorders. Notoginsenoside Ft1, as an agonist for TGR5 but antagonist for FXR, showed the potential for reversing fat mass and weight gain in diet-induced obese mice via stimulating lipolysis and thermogenesis in adipose tissue.Citation41 HCA could also induce the secretion of GLP-1 through the TGR5-cyclic adenosine monophosphate pathway and this effect on glucose metabolism is more significant than the impact of inhibiting FXR.Citation27 Further study indicated that higher levels of GLP-1 induced by TGR5 could also promote white adipose tissue browning to metabolic conditions.Citation36 The BA–TGR5–cAMP–type 2 iodothyronine deiodinase (D2) signaling pathway enhances energy expenditure in brown adipose tissue, preventing obesity and insulin resistance.Citation42 Since the downregulation of TGR5 in the hypothalamus accelerated weight gain and deteriorated established obesity, it can be concluded that the pathway by which BAs regulate energy metabolism is not restricted to peripheral tissues.Citation43
As reported previously, TGR5 mitigates insulin resistance and enhances differentiation and hypertrophy in skeletal muscle.Citation44 However, the surplus of DCA and CA could stimulate TGR5 to facilitate atrophic conditions in skeletal muscle fibers by means of oxidative stress and protein catabolic catabolism.Citation45 TGR5 is also expressed in immune cells, and its expression in monocytes is downregulated during differentiation into dendritic cells.Citation46 The activation of TGR5 promotes macrophage polarization from the M1 to the M2 phenotype and inhibits nuclear factor kappa-B (NF-κB) inflammation to alleviate inflammatory reactions.Citation47,Citation48 Furthermore, it is documented that through TGR5, BAs could facilitate intestinal healing with the capacity of anti-inflammation on gut resident immune cells and pro-regenerative response in epithelial cells.Citation49 Considering the role of TGR5 in multiple physiological processes, it could be regarded as a therapeutic target for various diseases.
PXR and VDR
PXR is a ligand-activated transcription factor located abundantly in the intestine, liver and bladder. It is highly associated with gene expression related to biotransformation, transport, inflammation and oxidative stress. The activation of PXR is pertinent to the detoxification of BAs to a certain extent. For instance, PXR is considered a physiological sensor to protect the liver from LCA toxification, indicating that PXR agonists could serve as a new therapeutic target for cholestatic liver diseases.Citation50 However, the opposite conclusion has been drawn from studies indicating that Pxr knockout mice are resistant to LCA-mediated hepatotoxicity due to higher urinary bile acid excretion and overexpression of drug metabolism enzymes and hepatic sulfate donor synthesis enzyme Papss2.Citation34 The reason for the discrepancy may be attributed to the different environment, dosage regimen and the proportion of other detoxification pathways.
The vitamin D receptor has a higher binding affinity for LCA in the lower intestine. This combination could induce the expression of enterohepatic cytochrome P450 to detoxify LCA and inhibit the expression of CYP7A1 gene transcription by activating the MEK1/2/ERK1/2 pathway (mitogen-activated protein kinase 1, MEK1).Citation51 Considering that VDR is widely expressed throughout the body thus combination of VDR and LCA could affect the body in multiple aspects. Following sleeve gastrectomy, a higher concentration of LCA in the portal vein stimulates VDR to drive cholic acid-7-sulfate (CA7S) production. CA7S is an efficient agonist for TGR5, which could further modulate GLP-1 secretion and glucose metabolism.Citation52 The immune effects of combining VDR and LCA have also been widely studied. Th1 cells are highly involved in cellular immunity and delayed hypersensitivity inflammatory reactions. Activation of VDR by LCA has a tremendous effect on Th1 cells by diminishing the expression of Th1-connected cytokines (Interferon-γ and TNFα), genes (T-box protein, Stat-1 and Stat4) and signal transducers and activators of transcription 1α/β (STAT 1α/β) phosphorylation to control adaptive immune reactions.Citation53 The injury of intestinal barrier function induced by TNF-α could be protected by the combination of LCA and VDR by upregulating the Sirtuin 1/ nuclear factor erythroid-2 related factor (Nrf2) pathway and downregulating the NF-κB pathway.Citation54
Bile Acid Network and Vascular Calcification-Associated Diseases
VC refers to the Ca-Pi deposition process in blood vessels due to the dysfunction of the vascular microenvironment. Multiple pathophysiological mechanisms, such as Ca–Pi abnormality, imbalanced calcification promoters and inhibitors, VSMCs transdifferentiation, matrix vesicle production and phosphate hydroxyapatite deposition are highly involved in the occurrence of this lesion. More specific cellular and molecular mechanisms include chronic inflammation, endoplasmic reticulum stress (ERs), mitochondria dysfunction, reactive oxygen species, iron homeostasis, and Ca–Pi metabolic imbalance.Citation5,Citation55 The VC in aging results from the interplay of genetic, environmental, and modifiable factors, which create an environment of cellular senescence, oxidative stress, and inflammation to lead to the loss of smooth muscle cell contraction ability in blood vessels, as well as the increase in osteogenic differentiation and calcification. Furthermore, VC in aging can be significantly accelerated in several clinical conditions, especially in metabolic abnormalities.Citation56
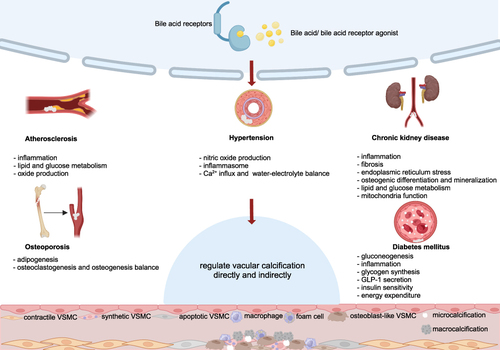
Apart from aging, VC is quite common in various diseases, including but not limited to AS, hypertension, CKD, DM and OP. Many Studies have exhibited significant changes in BA metabolism in these VC-related diseases. However, only a few have explored the direct correlation and most of the research is based on CKD models. Jovanovich et al have hypothesized that there is a positive relationship between the levels of serum CDCA, DCA and glycolithocholic acid and the severity of coronary artery calcification. LCA and DCA have been shown to be involved in regulating Ca-Pi metabolism and regulating ERs to aggravate the osteogenic differentiation of VSMCs, while tauroursodeoxycholic acid (TUDCA) showed the potential for reducing VC by alleviating ERs.Citation57,Citation58 More studies focused on the relationship between BA receptors and calcification. FXR has been shown to regulate the transcription factors msh homeobox 2 (MSX2), bone morphogenetic protein (BMP2) and osterix, which are important for VSMCs osteogenic-like differentiation. This suggests that FXR may be a promising therapeutic target for treating VC.Citation59 Although there is no direct research evidence, given the promoting effects of osteogenic differentiation markers such as runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP) and osteocalcin (OCN) on VC, as well as the regulatory role of FXR and TGR5 on these factors in bone, we believe that future exploration in this area will provide more evidence to elucidate the inherent connection between the BA network and VC. Moreover, non-alcoholic fatty liver disease (NAFLD) is one of the important diseases leading to BA imbalances. It has been found that the low expression of osteoprotegerin (OPG) in NAFLD is associated with an increased risk of coronary artery calcification. Whether BAs play an important role in this process is worth further investigation. Overall, the underlying mechanisms regarding BA metabolism and VC remain largely unknown.Citation60 In this section, we discussed the mechanism of VC and the changes in BA metabolism in aforementioned VC-related diseases, with a particular emphasis on BA network signaling and its therapeutic potential, with a summary in .
Figure 3 The potential effects of BA network on vascular calcification-associated diseases (created with BioRender.com). For AS, attenuating inflammation and increasing lipid and glucose metabolism and NO production are the main approaches for these substances to ameliorate artery calcification. For hypertension, increasing NO, decreasing inflammasome and regulation of Ca2+ and water-electrolyte balance are the main mechanisms of the BA network for modulating blood pressure. For CKD, the action of BA receptors mitigates inflammation, fibrosis, endoplasmic reticulum stress, osteogenic differentiation and mineralization. At the same time, lipid and glucose metabolism and mitochondria function were improved to protect the kidney and vessels. For DM, gluconeogenesis and inflammation are decreased and glycogen synthesis, GLP-1 secretion, insulin sensitivity and energy expenditure were enhanced to regulate the systemic state. For OP, activating nuclear and membrane receptors could regulate adipogenesis and the balance between osteogenesis and osteoclastogenesis to benefit bone mass and the bone microenvironment. The BA network improves VC-related diseases through the above pathways, and may indirectly or directly benefit VC.
Atherosclerosis
Lipid deposition, macrophage infiltration, and foam cell development initiate the formation of atherosclerotic plaques. With intimal calcification being the most common form, VC is greater than 80% of most arterial beds in men and over 60% of all arterial beds in women and the common sites include the carotid artery, coronary artery, proximal and distal aorta, and iliac artery.Citation61 Under the stimulation of inflammation, cell apoptosis, and oxidative stress, VSMCs differentiate into osteo/chondrogenic phenotype and upregulate the expression of osteochondrogenic markers, such as Runx2, osterix, osteopontin, OCN, and ALP, Sox9, Type II, and X collagen, which contribute to the formation of microcalcification to weaken the stability of the plaque.Citation55,Citation62 Actually, the question of whether calcification is beneficial for plaque stability remains a topic that has not been fully settled. The progression of calcification in AS is a sigmoid-shaped curve and high coronary calcification did not necessarily mean a higher chance of myocardial infarction in that some results have shown that the formation of VC, followed by unstable and ruptured lesions, can stabilize plaques to some extent.Citation63 This phenomenon can be attributed to the pro-inflammatory effect of microcalcification by M1 macrophages and the stable ability of subsequent macrocalcification by M2 macrophages.Citation64,Citation65 Another opposing view holds that matrix macrocalcification is prone to form rare but lethal nodular calcification that can cause rupture of the medial wall and rarely extend to the adventitia.Citation66 The degree, location, and type of plaque in VC may be related to these contradictory results and further detailed research will provide answers to this confusion.
Hypercholesterolemia, acting as an independent risk factor for AS, promotes lipid accumulation and plaque formation. The primary pathway for cholesterol elimination is BA metabolism and impaired BA excretion is associated with the occurrence of atherosclerotic coronary artery disease.Citation67 Therefore, the modulation of the BA network has been extensively studied as a potential therapeutic strategy for treating AS. Taurine has been shown to mitigate Trimethylamine N-oxide (TMAO)-induced AS by modulating BA metabolism, and similar pharmacological effects and BA alteration were also observed in usnea and resveratrol trials.Citation68–70 Since FXR is an essential regulatory for BA metabolism, its activation holds great promise as a therapeutic approach. The hepatic FGF19-Src-FXR phosphorylation signaling cascade reduced cholesterol levels to avoid AS.Citation71 Alisol B 23-acetate was proven to enhance cholesterol and BA excretion in an FXR-BSEP-dependent manner, which slowed down the progression of AS.Citation72 Loss of FXR in the ApoE−/− mouse model resulted in worse hyperlipidemia and AS, while FXR activation benefited AS by regulating proinflammatory mediators, decreasing triglyceride and cholesterol levels, and lowering the inflammation and lipid uptake of macrophage.Citation73 Li et al believed that besides the functions of anti-inflammation, FXR activation also inhibited VSMCs proliferation and migration to stabilize plaques in a FXR-SHP dependent manner.Citation39 A contradictory result implied that loss of FXR decreased the size of atherosclerotic lesions, but this phenomenon was restricted in male Ldlr−/− mice.Citation74 Lower levels of ceramide were able to ameliorate AS, and a study suggested that the inhibition of the intestinal FXR/ Sphingomyelin Phosphodiesterase 3 (SMPD3) axis alleviated AS by reducing the levels of circulating ceramide.Citation75 Overall, the effects of FXR on AS are complex and the final effects depend on the disease progression, individual status, and the dominant type of FXR.Citation76
Macrophage inflammation is the core trigger for the development and progression of AS. The activation of TGR5 could regulate this inflammation response through the TGR5-cAMP-NF-κB pathway. Moreover, TGR5 activation could alleviate the accumulation of modified lipoproteins, slowing the progression of AS.Citation63 Dual activation of FXR and TGR5 showed great potential in reducing lipid levels and inhibiting systemic inflammation to treat AS.Citation77 However, another study illustrated that the effect of FXR and TGR5 on AS was attributed to the anti-inflammatory effect rather than the lipid-lowering effect.Citation78 NO was regarded as a key antiatherogenic molecule and evidence showed TGR5 activation induced NO production to suppress the process of adhesion molecule expression and subsequent cell adhesion.Citation79 There was a positive correlation between lower VDR quantities and the occurrence of AS, even when 25- (OH)2-VitD3concentrations are high.Citation80 The expression of VDR in plaques is highly correlated with pro-inflammatory M1 macrophages, and deletion of macrophage VDR accelerated the progression of AS sufficiently.Citation81 A deficiency of PXR showed a beneficial effect in ameliorating AS, which was associated with a decrease in macrophage lipid uptake and accumulation.Citation82 However, another study indicated that chronic PXR activation could promote AS by increasing lipid accumulation and disturbing the balance between atherogenic and antiatherogenic lipoproteins in ApoE−/− mice.Citation83 Furthermore, the coadministration of VDR and retinoid X receptor agonists (represented by calcitriol and bexarotene, respectively) had a synergistic effect in mitigating the development of AS, primarily attributed to the antioxidant stress response of VDR.Citation84 BA sequestrations are alkaline anion exchange resins which could promote continuous biotransformation of cholesterol by inhibiting the enterohepatic circulation of BAs and colesevelam hydrochloride, as a classic type of BA sequestration, was proven to largely decrease atherosclerotic lesion size.Citation85 Moreover, the powerful ability of the BA network to regulate GLP-1 secretion also implied its significant impact on AS. Thus, we can speculate that targeting the BA network will be beneficial for the development of AS, but further research is needed to determine whether it is beneficial for concomitant VC.
Hypertension
Hypertension could promote both intimal calcification indirectly and medial calcification directly and the aorta and coronary arteries are the most susceptible to involvement.Citation86 As a risk factor for AS, hypertension serves as a promoting factor to stimulate intimal calcification. Medial calcification is an important step in the pathogenesis of hypertension and hypertension serves as a promoting stressor to create an environment prone to exacerbating Ca deposition by a series of cellular events and alterations in extracellular matrix composition.Citation87 Hypertension is accompanied by arterial wall remodeling, significant changes in extracellular matrix composition, and vascular cell phenotype. The generation of elastin fragments increased protease activity, and activation of the transforming growth factor-β (TGF-β) signaling pathway, as well as the deposition of collagen and proteoglycans, may provide a more favourable environment for VC.Citation86 Few studies have explored the changes in BA metabolism in hypertension and one metabolomic analysis of patients with acute coronary syndrome found that UDCA was significantly downregulated in patients accompanied by hypertension, but how it changed was unclear.Citation78
Current research indicates that the BA network could regulate blood pressure through renal mechanisms and vascular mechanisms. Activation of FXR and TGR5 upregulate aquaporin (AQP2) to restore water and BAs could also bind with the epithelial sodium channel (ENaC) to affect sodium (Na) concentration.Citation88 CDCA, a natural FXR agonist, showed the vasorelaxant and hypotensive capacity to decrease systolic blood pressure in spontaneously hypertensive. These effects have been attributed to the upregulation of endothelial NO synthase (NOS) and the downregulation of endothelin-1 and NF-κB activities.Citation89 Inflammatory cytokines, such as interleukin-1β (IL-1β), are related with blood pressure elevation and end-organ damage resulting from hypertension. FXR ligands could suppress IL-1β stimulated NF-κB activation and inducible NOS in an FXR-SHP dependent manner.Citation39 Inhibition of NLRP3 inflammasome was a potential therapy for hypertension and BAs were armed with this inhibitory effect through the TGR5-cAMP-PKA axis.Citation90 FXR activation may induce an increase in the expression of angiotensin II type 2 receptors, resulting in vasodilation. This means FXR can serve as a novel molecular target for regulating the Ang II signalling pathway in the vascular system.Citation91 The vasodilatory effect of secondary conjugated BAs, including deoxycholyltaurine, deoxycholyltaurine and taurodeoxycholate, was attributed to the regulation of Ca2+ influx.Citation92,Citation93 UDCA treatment in paternal cholestasis has a protective effect on the exacerbation of obesity-related hypertension in male offspring, which suggest that taking UDCA at the time of conception may benefit their offsprings.Citation94 Moreover, dysfunction of glucose and lipid metabolism, obesity, and AS are risk factors for hypertension and the above-mentioned role of the BA network in these disorder states may also serve as mechanisms for anti-hypertensive effects.
Chronic Kidney Disease
CKD is a devastating condition characterized by progressive and irreversible injury to the kidney. VC is the most important pathological process for cardiovascular complications in CKD and it could occur in the aortic arch, iliac artery, pelvic artery, femoral artery, and lower leg arteries. The dysfunction of excretion and reabsorption in the kidney disturbed metabolite balance, leading to inadequate levels of metabolites such as mineralization-associated regulatory molecules, microRNAs, hormones, proteins, enzymes, and inflammatory cytokines, which contributed to the mineral depositions in the media layer of the central larger coronary artery and/or other peripheral arteries.Citation95 With the continuous impairment of renal function, the normal regulatory mechanisms of iron homeostasis are disrupted, typically manifested as hyperphosphatemia, hypocalcemia, and hyperparathyroidism. Hyperphosphatemia and hypocalcemia trigger parathyroid hormone (PTH) secretion, which accelerates bone turnover and Ca deposition in arterial walls.Citation96 Hyperphosphatemia stimulates the upregulation of osteo/chondrogenic genes in VSMCs, causing the cells to undergo a phenotypic trans-differentiation from contractile to osteoblastic/chondroblastic-like phenotype. Under the state of oxidative stress and inflammation, these osteo-/chondroblast-like cells actively induce apoptosis to release matrix vesicles as calcification initiation sites and synthesize a large amount of collagen to promote extracellular matrix remodeling.Citation97 Notably, recent research has indicated that Pi can induce an anti-calcifying action in macrophages, thus compensating for the pathological calcification process induced by itself.Citation98 However, several studies have indicated that magnesium could inhibit VSMCs osteogenic differentiation by downregulating the Wnt/β-catenin signaling pathway and activating the calcium-sensing receptor in VSMCs, as well as regulating oxidative stress in ECs.Citation99 Although VSMCs and ECs are highly involved in VC process separately, more evidence has proved that intercellular interactions between these two cell types, including but not limited to inflammation, exosomes, vasoactive agents, pro-calcification factors, and pro-angiogenic and pro-fibrotic growth factors, are critical in regulating this process.Citation100
Higher serum BA concentration and lower urine BA output could be regarded as early events during the progression of chronic renal failure. The exact mechanism was unclear yet elevated basolateral Mrp3 and Ost-α/β expression may have some effects.Citation101 In human end-stage renal disease, a significant imbalance of BA composition was observed, particularly in the proportion between primary vs secondary and conjugated vs unconjugated BAs.Citation102 Similar changes in BAs were detected in a rat model of CKD.Citation103 These studies collectively suggested that metabolites involved in BA metabolism were tightly connected with the progression of CKD.Citation104 The coronary artery calcification volume score (CACS) was a widely used indicator to predict the volume and density of coronary artery calcification in CKD. Research aimed at moderate to severe CKD patients indicated that higher concentrations of DCA were significantly associated with greater baseline CACS and lower bone mineral density.Citation105 Another study assumed that higher levels of CDCA, DCA and glycolithocholic acids were closely related to higher CACS, which suggested that these three metabolites could be considered potential biomarkers for CACS.Citation106 Conversely, based on a large group of chronic renal insufficiency cohort patients, no association was observed between the serum levels of DCA and coronary artery calcification prevalence, incidence, or progression.Citation107 Further investigations are needed to determine whether specific BAs can be considered relative factors in predicting CACS and to elucidate the reasons for the conflicting results.
Research on how the BA network affects the development of VC in CKD is limited. Takabatake et al pointed out that unsuppressed levels of LCA increased intestinal Ca and Pi absorption to aggravate VC in a VDR-claudin 3 dependent manner.Citation57 Miyazaki demonstrated that DCA induced the osteogenic differentiation and mineralization of VSMCs through activating transcription factor 4 (ATF4) activation and this effect could be mitigated by the FXR agonist, PX20606.Citation58 In previous studies, the ATF4-C/EBP homologous protein (CHOP) axis was proven to influence the expression of osteogenic markers such as sodium-dependent phosphate transporter 1, OCN, OPG, osterix, Runx2, and ALP. Consequently, we hypothesize that the ATF4-CHOP pathway may also play a role in the modulation of VSMCs osteogenic differentiation by DCA.Citation108 Due to the regulatory role of FXR and TGR5 throughout the body, the investigation of natural or synthesized agonists has become a prominent area of research for exploring the specific mechanisms and therapeutic potential. A list of the most widely studied agonists for FXR and TGR5 is provided in . As indicated in this table, FXR and TGR5 have the potential to benefit the kidney by regulating glucose and lipid metabolism, whilst their direct renal effects are more significant. The TGFBR1/TAK1 pathway participated in the calcification process, and FXR activation inhibited the activation of this pathway to retard VC in vascular ECs and VSMCs.Citation109 Moreover, FXR agonists alleviated calcification by activating JNK to downregulate the expression of osteogenic transcription factors MSX2 and osterix.Citation59 These findings suggested the antagonistic effect of FXR on VC in CKD situations. Renal fibrosis was an essential part of CKD, and the Smad family was upregulated during this process. Activation of FXR suppressed Smad3, providing a novel target for the treatment of renal fibrosis.Citation110 Single FXR activation regulated lipid metabolism and reduced fibrosis, inflammation and oxidative stress to protect against renal lesions.Citation111,Citation112 Dual agonists of FXR and TGR5 showed great potential to prevent diabetic nephropathy by modulating inflammation, ERs and mitochondria function, whilst the benefits were credited to FXR more.Citation113 Tauroursodeoxycholic acid (TUDCA) has been shown to alleviate VC by alleviating ERs, decreasing the expression of ATF4 and CHOP, and reducing calcium accumulation.Citation114 Restricted by pathological situations, it is difficult to maximize the use of autologous human mesenchymal stem cells (hMSCs) in CKD patients. Treating CKD-derived hMSCs with TUDCA improved mitochondrial function and served as a promising therapy to address cardiovascular and renal problems in CKD.Citation115 Other research proved that TUDCA inhibited TGF-β1 or P-cresol-induced renal injury by inhibiting ERs.Citation116,Citation117 In a word, targeting the BA network could alleviate renal injury and CKD-induced VC but there is still undiscovered space to explore the specific interaction and latent therapies.
Table 1 Mechanisms of Representative FXR and TGR5 Agonists in Animal Disease Models
Diabetes Mellitus
DM is characterized by a relative decline in insulin secretion accompanied by insulin resistance or not. In diabetic patients, calcification is an independent factor for cardiovascular mortality, and the most common site is medial calcification. Approximately 17–42% of type 2 diabetes mellitus (T2DM) patients have VC, and the most commonly affected arteries are the coronary, carotid and lower limb arteries.Citation134 Calcifications in DM are mediated by excessive proinflammatory and osteogenic re-programming. Hyperglycemia creates an environment conducive to the formation of calcification, including oxidative stress, advanced glycation end products, formation of Ca-Pi crystals, O-GlcnAcylation, and endothelial dysfunction. Under these stimulations, excessive Pi promotes the differentiation of VSMCs into an osteoblast-like phenotype to secrete a series of bone-associated proteins.Citation135 For example, BMP, a protein that promotes bone formation and repair, was proven to increase significantly in DM to stimulate VC.Citation136 With higher levels of serum Ca-Pi product, microcalcification occurs as the initial form of calcification taking place in apoptotic VSMCs and macrophages. Moreover, the research found that the appearance of circulating myeloid-derived calcifying cells could be considered a new biomarker for VC, especially in T2DM.Citation137
In DM, BA metabolism is significantly changed but the result is inconsistent due to different baselines and detection methods. The total abundance of genes encoding BSHs and BA metabolism decreased dramatically in T1DM, and the dysfunction of BA metabolism occurred prior to islet autoimmunity and T1DM onset.Citation138,Citation139 However, in T2DM, research showed that there was an increase in the relative abundance of gut bacteria equipped with BSH and due to the impaired intestinal barrier function, higher serum BA levels appeared.Citation140 Postprandial total BA concentrations were higher than those in healthy controls, and fasting serum total BAs were proven to be connected with worse disease states.Citation141,Citation142 Embodied by higher levels of DCA in T2DM, many studies have indicated that there is a tight connection between the proportion of 12α-OH/non-12α-OH BAs and insulin resistance.Citation143 In addition, in gestational DM, evidence showed that sulfated BAs increased to protect the body against cytotoxicity.Citation144 Bariatric surgery was considered an effective and durable method to address T2DM, and BA profiles were significantly changed after surgery. Higher LCA concentration after surgery could stimulate Slut2 and CA7S to ameliorate diabetic phenotypes in a VDR-dependent manner.Citation52 The increase of CA7S after surgery was also proven to exert systemic glucoregulatory effects through TGR5 activation.Citation145 As previously mentioned, the regulatory effects of BAs on lipid, glucose and energy metabolism are realized by FXR and TGR5 to a great extent. Based on these tight connections, many natural or synthesized receptor ligands were tested to determine their therapeutic potential for DM as listed in . Therefore, the effects of BA on DM could be summarized in the following aspects: decreasing gluconeogenesis and inflammation, as well as promoting GLP-1 secretion, insulin sensitivity, glycogen production and energy expenditure. However, there is no research to explore whether the BA network could alleviate VC caused by DM while treating the disease.
Osteoporosis
Characterized by impaired bone mass and microarchitectural, OP can lead to severe fractures and lower quality of life. Excess bone resorption and insufficient bone formation promote a decrease in bone matrix mineralization.Citation146 In fact, the presence of bone loss in postmenopausal OP is closely associated with an increased incidence of calcifications of the aorta, carotids, and abdominal aorta.Citation147 The coexistence of VC and bone tissue demineralization is not uncommon, giving rise to a new research field known as the calcification paradox, which exists not only in OP but also in CKD-mineral bone disease.Citation148 The paradox is not only due to the pathological state but also a result of the interplay between the bone and vascular axis. VC and OP share many risk factors and pathophysiological changes, such as dysfunction of calcium and phosphorus metabolism, hormonal imbalances, chronic inflammation and oxidative stress.Citation149 Ca deficiency stimulates the secretion of PTH. This, in turn, enhances intestinal Ca absorption, renal Ca reabsorption, and the release of Ca and Pi from bones into the bloodstream, which exacerbates hydroxyapatite crystal deposition in vascular walls.Citation150 However, notable differences exist between vascular and bone calcification processes. For instance, osteoblasts heavily rely on tissue-nonspecific alkaline phosphatase (TNAP) to maintain about 90% of their mineralization capacity. In contrast, VSMCs undergo calcification with minimal dependence on TNAP. Instead, VSMCs differentiate into osteoclast-like cells through the OPG/RANK/RANKL (receptor activator of NF–κB ligand) triad, resulting in a process similar to bone remodeling within plaques.Citation65
Cirrhosis is the most common disease that leads to BA abnormalities, and OP is the typical complication. In patients with cirrhosis, the expression of collagen type 1 alpha 1 chain (COL1A1), OCN, Runx2, and ALP was significantly downregulated and the expression of sclerostin was upregulated, leading to bone loss.Citation151–153 To some extent, this finding suggests that the dysregulated metabolism of BAs in the context of cirrhosis may contribute to the development of OP by affecting the regulation of these osteogenic markers. Previous studies have explored the corresponding changes in BA metabolism in postmenopausal OP patients and ovariectomized mice. A single-centre cross-sectional study aimed at postmenopausal women concluded that the serum levels of BAs were positively correlated with bone density but negatively related to the bone resorption biomarker β-CTX.Citation154 However, another study found that metabolites derived from bile acid biosynthesis were positively correlated with β-CTX.Citation155 These results suggested that the impact of BAs on bone metabolism might be primarily manifested in bone resorption, whilst the mechanism remains unclear. Data from the postmenopausal OP mouse model found that the concentration of DCA was significantly diminished.Citation156 Through the targeted method, HCA was considered a potential marker for the occurrence of osteoporosis.Citation157
The relationship between the activity of BA receptors and bone homeostasis has been extensively studied. Experiments implied that the deletion of FXR in mice seemed to promote osteoclast differentiation and suppress osteoblast formation, probably accompanied by a change in adipogenesis capacity. Moreover, At the same time, exogenous supplementation with an FXR agonist could enhance osteogenesis in vitro and ameliorate bone loss in vivo, owing to the induction of ERK and β-catenin signaling.Citation158 FXR agonists were also proven to stimulate the DNA binding capacity of Runx2 and enhance BMP2 signal expression in BMSCs and mouse BMSC-like ST2 cells. Simultaneously, an excess dose of CDCA completely abrogated lipid vesicle appearance and adipogenesis marker expression in BMSCs.Citation124,Citation159 Knocking out TGR5 had no significant influence on young and middle-aged mice but significantly reduced bone mass in aged and ovariectomized mice. This phenomenon was due to augmented osteoclast differentiation through the AMP-activated protein kinase signaling pathway.Citation160 TGR5 activation also promoted the expression of Runx2, enhanced ALP activity, extracellular matrix mineralization, and the expression of osteoblastic genes (such as ALP, OCN, and osterix).Citation161 FXR and TGR5 dual agonist SH-479 had a more significant effect on promoting osteogenesis but inhibiting osteoclastogenesis than specific agonists.Citation160 As a VDR ligand, LCA diminished the irritating effect of vitamin D on OCN and the expression of RANKL on primary osteoblasts.Citation162 Furthermore, LCA and bilirubin were proven to have detrimental effects on the activity, differentiation and mineralization of osteoblasts, but these impacts could be neutralized by UDCA, suggesting that UDCA could be recognized as a potential therapeutic agent in osteoporotic patients with primary biliary cholangitis.Citation163,Citation164 TUDCA, an FDA-approved hydrophilic BA for the treatment of chronic cholestatic liver disease, was proven to be a potent drug for ovariectomized mice at different concentrations. By administering TUDCA, more small beam structures in the distal femur were preserved, and other indices, such as total bone volume, bone mineral density, and bone volume percentage, were significantly improved compared to the control group.Citation165 These studies further demonstrated that the BA network is important for the regulation of bone metabolic function. Considering the many similarities between bone and vascular calcification, we speculate that the regulatory effect of BA network on bone metabolism, particularly on the expression of specific osteogenic markers, may share similarities with the mechanism by which the BA network impacts VC and further research will provide theoretical basis for this hypothesis. Moreover, considering that treatments for OP may induce unforeseen effects on VC, we should explore more precise targets to avoid relevant adverse events, and vice versa.Citation166
Conclusion
VC is a common pathological phenomenon in aging and aging-related diseases, such as AS, hypertension, CKD, DM and OP, which could lead to the incidence and mortality of cardiovascular diseases. Although research is limited, some BA metabolites and their receptors have been shown to be involved in VC through their participation in Ca-Pi metabolism and VSMC differentiation. Considering the similarity between vascular and bone calcification, studies on the relationship between BA network and bone metabolism will provide references for future exploration of the underlying mechanisms between BA metabolism and VC. Recent research has also provided insights into the potential benefits of regulating the BA network for both these risk factors of VC and vascular lesion itself. These benefits can be achieved through various mechanisms, including adjusting energy metabolism, managing endoplasmic reticulum stress, modulating inflammation response, and regulating cell functions, whilst the specific mechanisms are still not well understood. On the whole, the search for promising therapeutic targets based on the BA network holds great promise in preventing, diagnosing, and treating these VC-associated diseases and VC lesions.
Ethics Approval and Consent to Participate
This review does not contain any studies with human or animal subjects performed by any of the authors.
Consent for Publication
All authors approved the final manuscript and the submission to this journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no potential conflicts of interest in this work.
Acknowledgments
The authors thank the financial support of the National Natural Science Foundation of China (No. 82273294), the Science and Technology Department of Sichuan Province (2022YFS0136), and the Chengdu Bureau of Science and Technology (2022-YF05-01316-SN).
Additional information
Funding
References
- Cai J, Rimal B, Jiang C, et al. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237:108238. doi:10.1016/j.pharmthera.2022.108238
- Kalhan SC, Guo L, Edmison J, et al. Plasma Metabolomic Profile in Non-Alcoholic Fatty Liver Disease. Metabolism. 2011;60(3):404–413. doi:10.1016/j.metabol.2010.03.006
- Grant SM, DeMorrow S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int J Mol Sci. 2020;21(17):5982. doi:10.3390/ijms21175982
- Iijima K. [Bone and calcium update; diagnosis and therapy of bone metabolism disease update. Regulatory Mechanism of Mammalian Sirtuin SIRT1 in Vascular calcification: impact of vascular smooth muscle cell senescence]. Clin Calcium. 2011;21(12):53–60.
- Pan W, Jie W, Huang H. Vascular calcification: molecular mechanisms and therapeutic interventions. MedComm. 2023;4(1):e200. doi:10.1002/mco2.200
- Singh A, Tandon S, Tandon C. An update on vascular calcification and potential therapeutics. Mol Biol Rep. 2021;48(1):887–896. doi:10.1007/s11033-020-06086-y
- Charach L, Charach G, Karniel E, et al. Peripheral Vascular Disease and Carotid Artery Disease Are Associated with Decreased Bile Acid Excretion. Bioengineering. 2023;10(8):935. doi:10.3390/bioengineering10080935
- Shimizu H, Hagio M, Iwaya H, et al. Deoxycholic Acid Is Involved in the Proliferation and Migration of Vascular Smooth Muscle Cells. J Nutr Sci Vitaminol (Tokyo). 2014;60(6):450–454. doi:10.3177/jnsv.60.450
- Schwabl P, Hambruch E, Seeland BA, et al. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J Hepatol. 2017;66(4):724–733. doi:10.1016/j.jhep.2016.12.005
- Kida T, Murata T, Hori M, Ozaki H. Chronic stimulation of farnesoid X receptor impairs nitric oxide sensitivity of vascular smooth muscle. Am J Phys. 2009;296(1):H195–H201. doi:10.1152/ajpheart.00679.2008
- Chiang JYL. Bile Acid Metabolism and Signaling. Compr Physiol. 2013;3:1191–1212. doi:10.1002/cphy.c120023
- Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G554–G573. doi:10.1152/ajpgi.00223.2019
- Shulpekova Y, Shirokova E, Zharkova M, et al. A Recent Ten-Year Perspective: bile Acid Metabolism and Signaling. Molecules. 2022;27(6):1983. doi:10.3390/molecules27061983
- Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18(2):71–87. doi:10.3727/105221618X15156018385515
- Wahlström A, Sayin SI, Marschall H-U, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24(1):41–50. doi:10.1016/j.cmet.2016.05.005
- Fiorucci S, Carino A, Baldoni M, et al. Bile Acid Signaling in Inflammatory Bowel Diseases. Dig Dis Sci. 2021;66(3):674–693. doi:10.1007/s10620-020-06715-3
- Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2019;11(2):158–171. doi:10.1080/19490976.2019.1674124
- Shin D-J, Wang L. Bile Acid-Activated Receptors: a Review on FXR and Other Nuclear Receptors. In: Fiorucci S, Distrutti E, editors. Bile Acids and Their Receptors. Cham: Springer International Publishing; 2019:51–72.
- Asami J, Kimura KT, Fujita-Fujiharu Y, et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022;606(7916):1021–1026. doi:10.1038/s41586-022-04845-4
- Chiang JYL, Ferrell JM. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu Rev Nutr. 2019;39(1):175–200. doi:10.1146/annurev-nutr-082018-124344
- Sayin SI, Wahlström A, Felin J, et al. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013;17(2):225–235. doi:10.1016/j.cmet.2013.01.003
- Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi:10.1016/s1097-2765(00)80348-2
- Kong B, Wang L, Chiang JYL, et al. Mechanism of Tissue-specific Farnesoid X Receptor in Suppressing the Expression of Genes in Bile-acid Synthesis in Mice. Hepatology. 2012;56(3):1034–1043. doi:10.1002/hep.25740
- Chiang JYL, Ferrell JM. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol. 2022;548:111618. doi:10.1016/j.mce.2022.111618
- Davis RA, Miyake JH, Hui TY, Spann NJ. Regulation of cholesterol-7alpha-hydroxylase: bAREly missing a SHP. J Lipid Res. 2002;43(4):533–543. doi:10.1016/S0022-2275(20)31482-6
- Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–1109. doi:10.1172/JCI25604
- Zheng X, Chen T, Jiang R, et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33(4):791–803.e7. doi:10.1016/j.cmet.2020.11.017
- Kim D-H, Xiao Z, Kwon S, et al. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J. 2015;34(2):184–199. doi:10.15252/embj.201489527
- Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi:10.1016/s0092-8674(00)00062-3
- Clifford BL, Sedgeman LR, Williams KJ, et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33(8):1671–1684.e4. doi:10.1016/j.cmet.2021.06.012
- Seok S, Fu T, Choi S-E, et al. Transcriptional regulation of autophagy by an FXR/CREB axis. Nature. 2014;516(7529):108. doi:10.1038/nature13949
- Lee JM, Wagner M, Xiao R, et al. Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 2014;516(7529):112–115. doi:10.1038/nature13961
- Han SY, Song HK, Cha JJ, et al. Farnesoid X receptor (FXR) agonist ameliorates systemic insulin resistance, dysregulation of lipid metabolism, and alterations of various organs in a type 2 diabetic kidney animal model. Acta Diabetol. 2021;58(4):495–503. doi:10.1007/s00592-020-01652-z
- Owen BM, Milona A, van Mil S, et al. Intestinal Detoxification Limits the Activation of Hepatic Pregnane X Receptor by Lithocholic Acid. Drug Metab Dispos. 2010;38(1):143–149. doi:10.1124/dmd.109.029306
- Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–165. doi:10.1038/nm.3760
- Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68(4):1574–1588. doi:10.1002/hep.29857
- Dehondt H, Marino A, Butruille L, et al. Adipocyte-specific FXR-deficiency protects adipose tissue from oxidative stress and insulin resistance and improves glucose homeostasis. Mol Metab. 2023;69:101686. doi:10.1016/j.molmet.2023.101686
- Yang J, de Vries HD, Mayeuf-Louchart A, et al. Role of bile acid receptor FXR in development and function of brown adipose tissue. Biochim Biophys Acta Mol Cell Biol Lipids. 2023;1868(2):159257. doi:10.1016/j.bbalip.2022.159257
- Yty L, Swales KE, Thomas GJ, et al. Farnesoid X Receptor Ligands Inhibit Vascular Smooth Muscle Cell Inflammation and Migration. Arterioscler Thromb Vasc Biol. 2007;27(12):2606–2611. doi:10.1161/ATVBAHA.107.152694
- Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. doi:10.1016/j.cmet.2009.08.001
- Ding L, Yang Q, Zhang E, et al. Notoginsenoside Ft1 acts as a TGR5 agonist but FXR antagonist to alleviate high fat diet-induced obesity and insulin resistance in mice. Acta Pharm Sin B. 2021;11(6):1541–1554. doi:10.1016/j.apsb.2021.03.038
- Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi:10.1038/nature04330
- Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fénelon VS, et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021;33(7):1483–1492.e10. doi:10.1016/j.cmet.2021.04.009
- Sasaki T, Kuboyama A, Mita M, et al. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice. J Biol Chem. 2018;293(26):10322–10332. doi:10.1074/jbc.RA118.002733
- Abrigo J, Gonzalez F, Aguirre F, et al. Cholic acid and deoxycholic acid induce skeletal muscle atrophy through a mechanism dependent on TGR5 receptor. J Cell Physiol. 2021;236(1):260–272. doi:10.1002/jcp.29839
- Ichikawa R, Takayama T, Yoneno K, et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology. 2012;136(2):153–162. doi:10.1111/j.1365-2567.2012.03554.x
- Biagioli M, Carino A, Cipriani S, et al. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J Immunol. 2017;199(2):718–733. doi:10.4049/jimmunol.1700183
- Pols TWH, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14(6):747–757. doi:10.1016/j.cmet.2011.11.006
- Sorrentino G, Perino A, Yildiz E, et al. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. 2020;159(3):956–968.e8. doi:10.1053/j.gastro.2020.05.067
- Ma X, Chen J, Tian Y. Pregnane X receptor as the “sensor and effector” in regulating epigenome. J Cell Physiol. 2015;230(4):752–757. doi:10.1002/jcp.24838
- Han S, Li T, Ellis E, et al. A Novel Bile Acid-Activated Vitamin D Receptor Signaling in Human Hepatocytes. Mol Endocrinol. 2010;24(6):1151–1164. doi:10.1210/me.2009-0482
- Chaudhari SN, Luo JN, Harris DA, et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe. 2021;29(3):408–424.e7. doi:10.1016/j.chom.2020.12.004
- Pols TWH, Puchner T, Korkmaz HI, et al. Lithocholic acid controls adaptive immune responses by inhibition of Th1 activation through the Vitamin D receptor. PLoS One. 2017;12(5):e0176715. doi:10.1371/journal.pone.0176715
- Yao B, He J, Yin X, et al. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-κB dependent mechanism in Caco-2 cells. Toxicol Lett. 2019;316:109–118. doi:10.1016/j.toxlet.2019.08.024
- Durham AL, Speer MY, Scatena M, et al. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114(4):590–600. doi:10.1093/cvr/cvy010
- Sutton NR, Malhotra R, St. Hilaire C, et al. Molecular Mechanisms of Vascular Health: insights From Vascular Aging and Calcification. Arterioscler Thromb Vasc Biol. 2023;43(1):15–29. doi:10.1161/ATVBAHA.122.317332
- Hashimoto N, Matsui I, Ishizuka S, et al. Lithocholic acid increases intestinal phosphate and calcium absorption in a vitamin D receptor dependent but transcellular pathway independent manner. Kidney Int. 2020;97(6):1164–1180. doi:10.1016/j.kint.2020.01.032
- Miyazaki-Anzai S, Masuda M, Shiozaki Y, et al. Free Deoxycholic Acid Exacerbates Vascular Calcification in CKD through ER Stress-Mediated ATF4 Activation. Int J Med. 2021;360(2):857–868. DOI:10.34067/KID.0007502020
- Miyazaki-Anzai S, Levi M, Kratzer A, et al. Farnesoid X Receptor Activation Prevents the Development of Vascular Calcification in ApoE −/− Mice With Chronic Kidney Disease. Circ Res. 2010;106(12):1807–1817. doi:10.1161/CIRCRESAHA.109.212969
- Pacifico L, Andreoli GM, D’Avanzo M, et al. Role of osteoprotegerin/receptor activator of nuclear factor kappa B/receptor activator of nuclear factor kappa B ligand axis in nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24(19):2073–2082. doi:10.3748/wjg.v24.i19.2073
- Allison MA, Criqui MH, Wright CM. Patterns and Risk Factors for Systemic Calcified Atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–336. doi:10.1161/01.ATV.0000110786.02097.0c
- New SEP, Aikawa E. Cardiovascular Calcification – an Inflammatory Disease –. Circulation Journal. 2011;75(6):1305–1313. doi:10.1253/circj.CJ-11-0395
- Bessueille L, Magne D. Inflammation: a culprit for vascular calcification in atherosclerosis and diabetes. Cell Mol Life Sci. 2015;72(13):2475–2489. doi:10.1007/s00018-015-1876-4
- Shioi A, Ikari Y. Plaque Calcification During Atherosclerosis Progression and Regression. J Atheroscler Thromb. 2018;25(4):294–303. doi:10.5551/jat.RV17020
- Akers EJ, Nicholls SJ, Di Bartolo BA. Plaque Calcification. Arterioscler Thromb Vasc Biol. 2019;39(10):1902–1910. doi:10.1161/ATVBAHA.119.311574
- Yahagi K, Kolodgie FD, Otsuka F, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13(2):79–98. doi:10.1038/nrcardio.2015.164
- Charach G, Grosskopf I, Rabinovich A, et al. The association of bile acid excretion and atherosclerotic coronary artery disease. Therap Adv Gastroenterol. 2011;4(2):95–101. doi:10.1177/1756283X10388682
- Liu S, He F, Zheng T, et al. Ligustrum robustum Alleviates Atherosclerosis by Decreasing Serum TMAO, Modulating Gut Microbiota, and Decreasing Bile Acid and Cholesterol Absorption in Mice. Mol Nutr Food Res. 2021;65(14):2100014. doi:10.1002/mnfr.202100014
- Liu Y, Dou C, Wei G, et al. Usnea improves high-fat diet- and vitamin D3-induced atherosclerosis in rats by remodeling intestinal flora homeostasis. Front Pharmacol. 2022;13:1064872. doi:10.3389/fphar.2022.1064872
- Ma Y, Li D, Liu W, et al. Resveratrol on the Metabolic Reprogramming in Liver: implications for Advanced Atherosclerosis. Front Pharmacol. 2021;12:747625. doi:10.3389/fphar.2021.747625
- Byun S, Jung H, Chen J, et al. Phosphorylation of hepatic farnesoid X receptor by FGF19 signaling–activated Src maintains cholesterol levels and protects from atherosclerosis. J Biol Chem. 2019;294(22):8732–8744. doi:10.1074/jbc.RA119.008360
- Fu Y, Feng H, Ding X, et al. Alisol B 23-acetate adjusts bile acid metabolisim via hepatic FXR-BSEP signaling activation to alleviate atherosclerosis. Phytomedicine. 2022;101:154120. doi:10.1016/j.phymed.2022.154120
- Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am J Phys. 2009;296(2):H272–H281. doi:10.1152/ajpheart.01075.2008
- Zhang Y, Wang X, Vales C, et al. FXR Deficiency Causes Reduced Atherosclerosis in Ldlr −/− Mice. Arterioscler Thromb Vasc Biol. 2006;26(10):2316–2321. doi:10.1161/01.ATV.0000235697.35431.05
- Wu Q, Sun L, Hu X, et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J Clin Invest. 2021;131(9):e142865. doi:10.1172/JCI142865
- Qi S, Luo X, Liu S, et al. The Critical Effect of Bile Acids in Atherosclerosis. J Cardiovasc Pharmacol. 2022;80(4):562. doi:10.1097/FJC.0000000000001320
- Miyazaki-Anzai S, Masuda M, Levi M, et al. Dual Activation of the Bile Acid Nuclear Receptor FXR and G-Protein-Coupled Receptor TGR5 Protects Mice against Atherosclerosis. PLoS One. 2014;9(9):e108270. doi:10.1371/journal.pone.0108270
- Miyazaki-Anzai S, Masuda M, Kohno S, et al. Simultaneous inhibition of FXR and TGR5 exacerbates atherosclerotic formation. J Lipid Res. 2018;59(9):1709–1713. doi:10.1194/jlr.M087239
- Kida T, Tsubosaka Y, Hori M, et al. Bile Acid Receptor TGR5 Agonism Induces NO Production and Reduces Monocyte Adhesion in Vascular Endothelial Cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1663–1669. doi:10.1161/ATVBAHA.113.301565
- Schnatz PF, Nudy M, O’Sullivan DM, et al. Coronary Artery Vitamin D Receptor Expression and Plasma Concentrations of Vitamin D: their Association with Atherosclerosis. Menopause. 2012;19(9):967–973. doi:10.1097/gme.0b013e31824cfa8f
- Oh J, Riek AE, Darwech I, et al. Deletion of Macrophage Vitamin D Receptor Promotes Insulin Resistance and Monocyte Cholesterol Transport to Accelerate Atherosclerosis in Mice. Cell Rep. 2015;10(11):1872–1886. doi:10.1016/j.celrep.2015.02.043
- Sui Y, Meng Z, Park S-H, et al. Myeloid-specific deficiency of pregnane X receptor decreases atherosclerosis in LDL receptor-deficient mice[S]. J Lipid Res. 2020;61(5):696–706. doi:10.1194/jlr.RA119000122
- Zhou C, King N, Chen KY, Breslow JL. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J Lipid Res. 2009;50(10):2004–2013. doi:10.1194/jlr.M800608-JLR200
- Lin LM, Peng F, Liu YP, et al. Coadministration of VDR and RXR agonists synergistically alleviates atherosclerosis through inhibition of oxidative stress: an in vivo and in vitro study. Atherosclerosis. 2016;251:273–281. doi:10.1016/j.atherosclerosis.2016.06.005
- Meissner M, Wolters H, de Boer RA, et al. Bile acid sequestration normalizes plasma cholesterol and reduces atherosclerosis in hypercholesterolemic mice. No additional effect of physical activity. Atherosclerosis. 2013;228(1):117–123. doi:10.1016/j.atherosclerosis.2013.02.021
- Rattazzi M, Bertacco E, Puato M, et al. Hypertension and vascular calcification: a vicious cycle? J Hypertens. 2012;30(10):1885. doi:10.1097/HJH.0b013e328356c257
- Kalra SS, Shanahan CM. Vascular calcification and hypertension: cause and effect. Ann Med. 2012;44(sup1):S85–S92. doi:10.3109/07853890.2012.660498
- Ishimwe JA, Dola T, Ertuglu LA, Kirabo A. Bile acids and salt-sensitive hypertension: a role of the gut-liver axis. Am J Physiol Heart Circ Physiol. 2022;322(4):H636–H646. doi:10.1152/ajpheart.00027.2022
- Li C, Li J, Weng X, et al. Farnesoid X receptor agonist CDCA reduces blood pressure and regulates vascular tone in spontaneously hypertensive rats. J Am Society oHypertension. 2015;9(7):507–516.e7. doi:10.1016/j.jash.2015.04.006
- Guo C, Xie S, Chi Z, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45(4):802–816. doi:10.1016/j.immuni.2016.09.008
- Zhang Q, He F, Kuruba R, et al. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res. 2008;77(3):560–569. doi:10.1093/cvr/cvm068
- Khurana S, Yamada M, Wess J, et al. Deoxycholyltaurine-induced vasodilation of rodent aorta is nitric oxide- and muscarinic M3 receptor-dependent. Eur J Pharmacol. 2005;517(1–2):103–110. doi:10.1016/j.ejphar.2005.05.037
- Jadeja RN, Thounaojam MC, Bartoli M, Khurana S. Deoxycholylglycine, a conjugated secondary bile acid, reduces vascular tone by attenuating Ca2+ sensitivity via rho kinase pathway. Toxicol Appl Pharmacol. 2018;348:14–21. doi:10.1016/j.taap.2018.04.012
- Pataia V, Papacleovoulou G, Nikolova V, et al. Paternal cholestasis exacerbates obesity-associated hypertension in male offspring but is prevented by paternal ursodeoxycholic acid treatment. Int J Obes. 2019;43(2):319–330. doi:10.1038/s41366-018-0095-0
- Ren S-C, Mao N, Yi S, et al. Vascular Calcification in Chronic Kidney Disease: an Update and Perspective. Aging Dis. 2022;13(3):673–697. doi:10.14336/AD.2021.1024
- Vervloet MG, van Ballegooijen AJ. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018;93(5):1060–1072. doi:10.1016/j.kint.2017.11.036
- Zununi Vahed S, Mostafavi S, Hosseiniyan Khatibi SM, et al. Vascular Calcification: an Important Understanding in Nephrology. Vasc Health Risk Manag. 2020;16:167–180. doi:10.2147/VHRM.S242685
- Villa-Bellosta R, Hamczyk MR, Andrés V. Novel phosphate-activated macrophages prevent ectopic calcification by increasing extracellular ATP and pyrophosphate. PLoS One. 2017;12(3):e0174998. doi:10.1371/journal.pone.0174998
- Dai Z, Zhang X. Pathophysiology and Clinical Impacts of Chronic Kidney Disease on Coronary Artery Calcification. J Cardiovasc Dev Dis. 2023;10(5):207. doi:10.3390/jcdd10050207
- Zhang Y-X, Tang R-N, Wang L-T, Liu B-C. Role of crosstalk between endothelial cells and smooth muscle cells in vascular calcification in chronic kidney disease. Cell Prolif. 2021;54(3):e12980. doi:10.1111/cpr.12980
- Gai Z, Chu L, Hiller C, et al. Effect of chronic renal failure on the hepatic, intestinal, and renal expression of bile acid transporters. Am J Phys Renal Physiol. 2014;306(1):F130–F137. doi:10.1152/ajprenal.00114.2013
- Wang X, Yang S, Li S, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut. 2020;69(12):2131–2142. doi:10.1136/gutjnl-2019-319766
- Feng Y-L, Cao G, Chen D-Q, et al. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cellular and molecular life sciences. CMLS. 2019;76(24):4961–4978. doi:10.1007/s00018-019-03155-9
- Zhang Z-M, Yang L, Wan Y, et al. Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced CKD rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1174:122728. doi:10.1016/j.jchromb.2021.122728
- Jovanovich A, Isakova T, Block G, et al. Deoxycholic Acid, a Metabolite of Circulating Bile Acids, and Coronary Artery Vascular Calcification in CKD. Am J Kidney Dis. 2018;71(1):27–34. doi:10.1053/j.ajkd.2017.06.017
- Chen W, Fitzpatrick J, Sozio SM, et al. Identification of Novel Biomarkers and Pathways for Coronary Artery Calcification in Nondiabetic Patients on Hemodialysis Using Metabolomic Profiling. Kidney360. 2020;2:279–289. doi:10.34067/KID.0004422020
- Jovanovich A, Cai X, Frazier R, et al. Deoxycholic Acid and Coronary Artery Calcification in the Chronic Renal Insufficiency Cohort. J Am Heart Assoc. 2022;11(7):e022891. doi:10.1161/JAHA.121.022891
- Masuda M, Miyazaki‐Anzai S, Levi M, et al. PERK‐eIF2α‐ATF4‐CHOP Signaling Contributes to TNFα‐Induced Vascular Calcification. J Am Heart Assoc. 2013;2(5):e000238. doi:10.1161/JAHA.113.000238
- Li C, Zhang S, Chen X, et al. Farnesoid X receptor activation inhibits TGFBR1/TAK1-mediated vascular inflammation and calcification via miR-135a-5p. Commun Biol. 2020;3(1):327. doi:10.1038/s42003-020-1058-2
- Zhao K, He J, Zhang Y, et al. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci Rep. 2016;6(1):37234. doi:10.1038/srep37234
- Hu Z, Ren L, Wang C, et al. Effect of Chenodeoxycholic Acid on Fibrosis, Inflammation and Oxidative Stress in Kidney in High-Fructose-Fed Wistar Rats. Kidney Blood Press Res. 2012;36(2):85–97. doi:10.1159/000341485
- Wang XX, Jiang T, Shen Y, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297(6):F1587–1596. doi:10.1152/ajprenal.00404.2009
- Wang XX, Wang D, Luo Y, et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J Am Soc Nephrol. 2018;29(1):118–137. doi:10.1681/ASN.2017020222
- Panda DK, Bai X, Sabbagh Y, et al. Defective interplay between mTORC1 activity and endoplasmic reticulum stress-unfolded protein response in uremic vascular calcification. Am J Phys Renal Physiol. 2018;314(6):F1046–F1061. doi:10.1152/ajprenal.00350.2017
- Yoon YM, Kim S, Han Y-S, et al. TUDCA-treated chronic kidney disease-derived hMSCs improve therapeutic efficacy in ischemic disease via PrPC. Redox Biol. 2019;22:101144. doi:10.1016/j.redox.2019.101144
- Li L, Guo Z-Y, Wang J, et al. Tauroursodeoxycholic acid inhibits TGF-β1-induced renal fibrosis markers in cultured renal mesangial cells by regulating endoplasmic reticulum stress. Exp Ther Med. 2022;23(6):432. doi:10.3892/etm.2022.11359
- Yun SP, Yoon YM, Lee JH, et al. Tauroursodeoxycholic Acid Protects against the Effects of P-Cresol-Induced Reactive Oxygen Species via the Expression of Cellular Prion Protein. Int J Mol Sci. 2018;19(2):352. doi:10.3390/ijms19020352
- Hartmann P, Hochrath K, Horvath A, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67(6):2150–2166. doi:10.1002/hep.29676
- Zheng T, Kim N-Y, Yim M. Fexaramine Inhibits Receptor Activator of Nuclear Factor-κB Ligand-induced Osteoclast Formation via Nuclear Factor of Activated T Cells Signaling Pathways. J Bone Metab. 2017;24(4):207–215. doi:10.11005/jbm.2017.24.4.207
- Jiang T, Wang XX, Scherzer P, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56(10):2485–2493. doi:10.2337/db06-1642
- Li L, Zhao H, Chen B, et al. FXR activation alleviates tacrolimus-induced post-transplant diabetes mellitus by regulating renal gluconeogenesis and glucose uptake. J Transl Med. 2019;17(1):418. doi:10.1186/s12967-019-02170-5
- Kim D-H, Park JS, Choi H-I, et al. The critical role of FXR is associated with the regulation of autophagy and apoptosis in the progression of AKI to CKD. Cell Death Dis. 2021;12(4):320. doi:10.1038/s41419-021-03620-z
- Hu T, Chouinard M, Cox AL, et al. Farnesoid X Receptor Agonist Reduces Serum Asymmetric Dimethylarginine Levels through Hepatic Dimethylarginine Dimethylaminohydrolase-1 Gene Regulation*. J Biol Chem. 2006;281(52):39831–39838. doi:10.1074/jbc.M606779200
- Fujimori K, Iguchi Y, Yamashita Y, et al. Synthesis of Novel Farnesoid X Receptor Agonists and Validation of Their Efficacy in Activating Differentiation of Mouse Bone Marrow-Derived Mesenchymal Stem Cells into Osteoblasts. Molecules. 2019;24(22):4155. doi:10.3390/molecules24224155
- Wang XX, Jiang T, Shen Y, et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59(11):2916–2927. doi:10.2337/db10-0019
- Lin C, Yu B, Liu X, et al. Obeticholic acid inhibits hepatic fatty acid uptake independent of FXR in mouse. Biomed Pharmacother. 2022;150:112984. doi:10.1016/j.biopha.2022.112984
- Kumar DP, Rajagopal S, Mahavadi S, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427(3):600–605. doi:10.1016/j.bbrc.2012.09.104
- Kumar DP, Asgharpour A, Mirshahi F, et al. Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis. J Biol Chem. 2016;291(13):6626–6640. doi:10.1074/jbc.M115.699504
- Wang XX, Edelstein MH, Gafter U, et al. G Protein-Coupled Bile Acid Receptor TGR5 Activation Inhibits Kidney Disease in Obesity and Diabetes. J Am Soc Nephrology. 2016;27(5):1362. doi:10.1681/ASN.2014121271
- Rizzo G, Passeri D, De Franco F, et al. Functional Characterization of the Semisynthetic Bile Acid Derivative INT-767, a Dual Farnesoid X Receptor and TGR5 Agonist. Mol Pharmacol. 2010;78(4):617–630. doi:10.1124/mol.110.064501
- Comeglio P, Cellai I, Mello T, et al. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. J Endocrinol. 2018;238(2):107–127. doi:10.1530/JOE-17-0557
- McMahan RH, Wang XX, Cheng LL, et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem. 2013;288(17):11761–11770. doi:10.1074/jbc.M112.446575
- Jadhav K, Xu Y, Xu Y, et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol Metab. 2018;9:131–140. doi:10.1016/j.molmet.2018.01.005
- Lanzer P, Hannan FM, Lanzer JD, et al. Medial Arterial Calcification: JACC State-of-The-Art Review. J Am Coll Cardiol. 2021;78(11):1145–1165. doi:10.1016/j.jacc.2021.06.049
- Yahagi K, Kolodgie FD, Lutter C, et al. ATVB in Focus series on Vascular Calcification in Diabetes. Arterioscler Thromb Vasc Biol. 2017;37(2):191–204. doi:10.1161/ATVBAHA.116.306256
- Boström KI, Jumabay M, Matveyenko A, et al. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ Res. 2011;108(4):446–457. doi:10.1161/CIRCRESAHA.110.236596
- Fadini GP, Albiero M, Menegazzo L, et al. Widespread Increase in Myeloid Calcifying Cells Contributes to Ectopic Vascular Calcification in Type 2 Diabetes. Circ Res. 2011;108(9):1112–1121. doi:10.1161/CIRCRESAHA.110.234088
- Yuan X, Wang R, Han B, et al. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat Commun. 2022;13(1):6356. doi:10.1038/s41467-022-33656-4
- Lamichhane S, Sen P, Dickens AM, et al. Dysregulation of secondary bile acid metabolism precedes islet autoimmunity and type 1 diabetes. Cell Rep Med. 2022;3(10):100762. doi:10.1016/j.xcrm.2022.100762
- Zhao L, Lou H, Peng Y, et al. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res Clin Pract. 2020;169:108418. doi:10.1016/j.diabres.2020.108418
- Sonne DP, van Nierop FS, Kulik W, et al. Postprandial Plasma Concentrations of Individual Bile Acids and FGF-19 in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101(8):3002–3009. doi:10.1210/jc.2016-1607
- Wang X-H, Xu F, Cheng M, et al. Fasting serum total bile acid levels are associated with insulin sensitivity, islet β-cell function and glucagon levels in response to glucose challenge in patients with type 2 diabetes. Endocr J. 2020;67(11):1107–1117. doi:10.1507/endocrj.EJ20-0201
- Sonne DP, Hansen M, Knop FK. MECHANISMS IN ENDOCRINOLOGY: bile acid sequestrants in type 2 diabetes: potential effects on GLP1 secretion. Eur J Endocrinol. 2014;171(2):R47–R65. doi:10.1530/EJE-14-0154
- Gao J, Xu B, Zhang X, et al. Association between serum bile acid profiles and gestational diabetes mellitus: a targeted metabolomics study. Clinica Chimica Acta. 2016;459:63–72. doi:10.1016/j.cca.2016.05.026
- Chaudhari SN, Harris DA, Aliakbarian H, et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat Chem Biol. 2021;17(1):20–29. doi:10.1038/s41589-020-0604-z
- Roschger P, Misof B, Paschalis E, et al. Changes in the Degree of Mineralization with Osteoporosis and its Treatment. Curr Osteoporos Rep. 2014;12(3):338–350. doi:10.1007/s11914-014-0218-z
- Lampropoulos CE, Kalamara P, Konsta M, et al. Osteoporosis and vascular calcification in postmenopausal women: a cross-sectional study. Climacteric. 2016;19(3):303–307. doi:10.3109/13697137.2016.1164134
- Hou Y-C, Lu C-L, Lu K-C. Mineral bone disorders in chronic kidney disease. Nephrology. 2018;23(S4):88–94. doi:10.1111/nep.13457
- Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34(4):715–723. doi:10.1161/ATVBAHA.113.302070
- Reid IR, Bristow SM. Calcium and Bone. In: Stern PH, editor. Bone Regulators and Osteoporosis Therapy. Springer International Publishing, Cham; 2020:259–280.
- Bihari C, Lal D, Thakur M, et al. Suboptimal Level of Bone‐Forming Cells in Advanced Cirrhosis are Associated with Hepatic Osteodystrophy. Hepatol Commun. 2018;2(9):1095–1110. doi:10.1002/hep4.1234
- Guañabens N, Ruiz-Gaspà S, Gifre L, et al. Sclerostin Expression in Bile Ducts of Patients With Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J Bone Minera Res. 2016;31(9):1725–1733. doi:10.1002/jbmr.2845
- Fonseca V, Epstein O, Gill DS, et al. Hyperparathyroidism and Low Serum Osteocalcin Despite Vitamin D Replacement in Primary Biliary Cirrhosis. J Clin Endocrinol Metab. 1987;64(5):873–877. doi:10.1210/jcem-64-5-873
- Zhao Y-X, Song Y-W, Zhang L, et al. Association between bile acid metabolism and bone mineral density in postmenopausal women. Clinics. 2020;75:e1486. doi:10.6061/clinics/2020/e1486
- Bellissimo MP, Roberts JL, Jones DP, et al. Metabolomic Associations with Serum Bone Turnover Markers. Nutrients. 2020;12(10):3161. doi:10.3390/nu12103161
- Wen K, Tao L, Tao Z, et al. Fecal and Serum Metabolomic Signatures and Microbial Community Profiling of Postmenopausal Osteoporosis Mice Model. Front Cell Infect Microbiol. 2020;10:535310. doi:10.3389/fcimb.2020.535310
- Deng D, Pan C, Wu Z, et al. An Integrated Metabolomic Study of Osteoporosis: discovery and Quantification of Hyocholic Acids as Candidate Markers. Front Pharmacol. 2021;12:725341. doi:10.3389/fphar.2021.725341
- Cho SW, An JH, Park H, et al. Positive regulation of osteogenesis by bile acid through FXR. J Bone Minera Res. 2013;28(10):2109–2121. doi:10.1002/jbmr.1961
- Id Boufker H, Lagneaux L, Fayyad-Kazan H, et al. Role of farnesoid X receptor (FXR) in the process of differentiation of bone marrow stromal cells into osteoblasts. Bone. 2011;49(6):1219–1231. doi:10.1016/j.bone.2011.08.013
- Li Z, Huang J, Wang F, et al. Dual Targeting of Bile Acid Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) Prevents Estrogen-Dependent Bone Loss in Mice. J Bone Minera Res. 2019;34(4):765–776. doi:10.1002/jbmr.3652
- Wang Q, Wang G, Wang B, Yang H. Activation of TGR5 promotes osteoblastic cell differentiation and mineralization. Biomed Pharm. 2018;108:1797–1803. doi:10.1016/j.biopha.2018.08.093
- Ruiz-Gaspà S, Guañabens N, Enjuanes A, et al. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur J Clin Invest. 2010;40(1):25–34. doi:10.1111/j.1365-2362.2009.02230.x
- Ruiz-Gaspà S, Guañabens N, Jurado S, et al. Bilirubin and bile acids in osteocytes and bone tissue. Potential role in the cholestatic-induced osteoporosis. Liver Int. 2020;40(11):2767–2775. doi:10.1111/liv.14630
- Ruiz-Gaspà S, Guañabens N, Jurado S, et al. Bile acids and bilirubin effects on osteoblastic gene profile. Implications in the pathogenesis of osteoporosis in liver diseases. Gene. 2020;725:144167. doi:10.1016/j.gene.2019.144167
- Ahn T-K, Kim K-T, Joshi HP, et al. Therapeutic Potential of Tauroursodeoxycholic Acid for the Treatment of Osteoporosis. Int J Mol Sci. 2020;21(12):4274. doi:10.3390/ijms21124274
- Chandran M, Tay D, Mithal A. Supplemental calcium intake in the aging individual: implications on skeletal and cardiovascular health. Aging Clin Exp Res. 2019;31(6):765–781. doi:10.1007/s40520-019-01150-5