Abstract
Heart failure is a common and disabling condition with morbidity and mortality that increase dramatically with advancing age. Large observational studies, retrospective subgroup analyses and meta-analyses of clinical trials in systolic heart failure, and recently published randomized studies have provided data supporting the use of beta-blockers as a baseline therapy in heart failure in the elderly. Despite the available evidence about beta-blockers, this therapy is still less frequently used in elderly compared to younger patients. Nebivolol is a third-generation cardioselective beta-blocker with L-arginine/nitric oxide-induced vasodilatory properties, approved in Europe and several other countries for the treatment of essential hypertension, and in Europe for the treatment of stable, mild, or moderate chronic heart failure, in addition to standard therapies in elderly patients aged 70 years old or older. The effects of nebivolol on left ventricular function in elderly patients with chronic heart failure (ENECA) and the study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure (SENIORS) have been specifically aimed to assess the efficacy of beta-blockade in elderly heart failure patients. The results of these two trials demonstrate that nebivolol is well tolerated and effective in reducing mortality and morbidity in older patients, and that the beneficial clinical effect is present also in patients with mildly reduced ejection fraction. Moreover, nebivolol appears to be significantly cost-effective when prescribed in these patients. However, further targeted studies are needed to better define the efficacy as well as safety profile in frail and older patients with comorbid diseases.
Introduction
Heart failure shows an age-related increasing prevalence (affecting more than 10% of individuals over 75 years old), as a consequence of the aging of the population and the improvement in survival of patients with ischemic heart disease and hypertension.Citation1–Citation3 As the mean age of patients in the community is about 76 years old,Citation2–Citation5 heart failure is considered a typical disorder of the elderly and is the most frequent reason for hospital admissions among older people.Citation5,Citation6 The lifetime risk of developing heart failure is increasing and is currently estimated at 20%.Citation7 Despite the recent advances in diagnosis and treatment, and although recent observations suggest an improvement of prognosis in the last decades,Citation8 the mortality of older unselected patients remains significantly high, ranging from 26% to 38% at 1 year.Citation4,Citation6,Citation9,Citation10
Heart failure: age-related changes
Clinical assessments and the management of older patients are often more difficult than in younger ones and heterogeneity is the main clinical feature. Heterogeneity is based on the dynamic definition of aging itself, as it is well known that biological age often does not equal demographic age. Epidemiological studies in geriatrics as well as in cardiology settings have demonstrated that the essence of older individuals is complex, with many clinical and nonclinical factors determining different clinical presentation and prognosis.Citation11,Citation12 At advanced age, the cardiovascular status and, as a consequence global health, is the result of a complex and dynamic interaction between three different areas: the changes related to “normal” aging of the cardiovascular system; the evolution of cardiovascular disease; and concomitant comorbid conditions, social factors, and lifestyle.Citation11–Citation14 Age-related changes throughout the cardiovascular system in combination with the high prevalence of cardiovascular diseases predispose older adults to the development of heart failure. These changes are mainly a consequence of increased left ventricular afterload secondary to increased aortic impedance, and diminished sympathetic modulation.Citation15
Clinical features that distinguish heart failure at advanced age from that occurring during middle age include an increasing proportion of women and multiple etiologies with a shift from coronary heart disease to hypertension as the most common one, more severe clinical manifestations, comorbid diseases, and age-related conditions.Citation13,Citation14 Furthermore, as many as 30%–50% of elderly patients with heart failure may have normal systolic function.Citation3 About 30%–40% of older patients with heart failure not only have hypertension, diabetes, renal failure, chronic obstructive pulmonary disease (COPD), and anemia, but also cognitive impairment, incontinence, psychological problems, and limitations in activities of daily living.Citation11,Citation12
Overt heart failure in older persons is frequently associated with a worse prognosis. Data from the Italian Network on Congestive Heart Failure (CHF) registry suggest that age is an independent and powerful predictor of mortality, with an increase in risk of about 3% per year of age,Citation6 and a high mortality rate of older outpatients followed up by cardiologists (up to 26% in the first year in patients >75 years old). Prognosis worsens with increasing New York Heart Association (NYHA) functional class, but a variety of medical, functional, social, and psychocognitive factors may have significant effects on survival. Moreover, predictors of mortality vary by age and by the presence of preserved or reduced left ventricular ejection fraction (LVEF), and traditional predictors of mortality in patients with reduced LVEF may not apply to elderly patients with preserved LVEF. Finally, the importance of reducing mortality in older patients may be questioned because of the reduced life expectancy and poor quality of life.
Pharmacologic treatment of elderly patients with heart failure
The quality of care of older heart failure patients is often far from satisfactory in clinical practice.Citation5,Citation6,Citation11,Citation16 Thus, the relative “under use” of evidence based treatments largely appears to depend on the higher complexity and the lack of definite evidence on efficacy and safety of nonpharmacological and pharmacological treatments in the very elderly.Citation12 Indeed, effective heart failure treatments such as angiotensin-converting enzyme (ACE) inhibitors, aldosterone antagonists, or beta-blockers may be considered not indicated in the elderly because of the high prevalence of renal vascular disease, renal impairment, diabetes, COPD and other various reasons. Multidrug therapy is a common feature in older patients, with multiple cardiovascular and noncardiovascular medications used for several associated diseases. Drug interactions and adverse reactions are common when multiple medications are prescribed for elderly patients. Thus the older heart failure population, which in fact comprises the majority of all patients, is in general less well studied, both experimentally and clinically, than younger populations.
Older patients are generally underrepresented in randomized clinical trials because only a few of them have addressed the impact of therapy in patients aged more than 70-years-old and virtually none included patients aged more than 85-years-old. These observations are likely dependent on the eligibility criteria of clinical trials, in which only patients with a poor LVEF and without significant comorbidities are included, whereas preserved systolic function and comorbidities frequently characterize elderly people.
Thus, ACE-inhibitors, beta-blockers, angiotensin receptor antagonists and aldosterone antagonists have shown a benefit in terms of mortality and rehospitalization only in patients with a mean age of 63 and reduced LVEF, and the evidence on the effects in elderly patients and those with preserved systolic function are still limited. Recent guidelines pointed out the lack of adequate knowledge on heart failure treatment in the elderly.Citation17 It is evident that targeted clinical trials and rigorous observational studies are needed, aiming at developing more effective treatments and favoring the implementation of specific guidelines into clinical practice.
Beta-blockers in older heart failure patients
During the past decade, randomized clinical trials have shown that carvedilol, bisoprolol and metoprolol significantly reduce mortality and hospital admissions, improve symptoms and slow the progression of the disease.Citation18–Citation20 However, these trials enrolled highly selected patients who were middle-aged, prevalently male, and with reduced systolic function. As a consequence, in clinical practice, older, complex patients have been undertreated with beta-blockers in comparison to younger ones, with doses approximately half the target of clinical trials. Indeed, the most frequent reasons for the limited use of beta-blockers and prescription of suboptimal doses are advanced age, concern about the potential risk of adverse events or worsening of symptoms, and the sizeable proportion of patients with preserved systolic function.
The subgroup analysis of randomized trials showed that beta-blockers reduce mortality also in older subgroups of patients (aged 60–80 years old) with systolic heart failure, and that the benefit was similar to that observed in younger ones (aged < 60 years).Citation21,Citation22 A meta-analysis of all-cause mortality from five completed beta-blocker trials confirmed that elderly and nonelderly chronic heart failure patients derived considerable prognostic benefit from beta-blocker therapy without a statistically significant difference in mortality reduction between the two groups. The relative risks of the elderly subgroup are reported in .
Figure 1 Der Simonian and Laird relative risks (random effects) plot of beta-blocker versus placebo in the subgroup of elderly patients with heart failure. Point estimates and 95% CIs represented next to box plot.
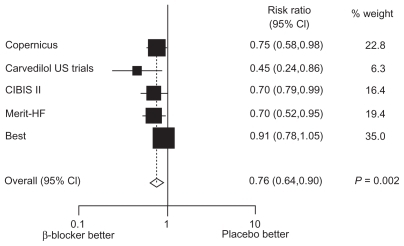
Observational studies have assessed the effects of beta-blockers in elderly patients from clinical practice, suggesting that beta-blockers may also be beneficial in these patients.Citation23–Citation25 Sin and McAlister evaluated the associations between beta-blocker use and outcomes in a population-based cohort of 11,942 older (age ≤ 65 years, mean 79 years old) patients between 1994 and 1999, with a propensity score adjusted analysis. Beta-blocker use was associated with substantial reductions in all-cause mortality (hazard ratio [HR] = 0.72; 95% confidence interval [CI]: 0.65–0.80), mortality due to heart failure (HR = 0.65; 95% CI: 0.47–0.90), and hospitalizations for heart failure (HR = 0.82; 95% CI: 0.74–0.92). These endpoints were less frequent in patients treated with beta-blockers than in untreated patients in all examined subgroups. All doses of beta-blockers were associated with benefit, but there was a trend towards greater benefit in patients prescribed higher doses. This observational study confirmed that the benefits of beta-blockers seen in randomized trials extend to older patients and to those with conditions that would have led to their exclusion from the trials.
Recently, another observational study examined the associations between beta-blocker therapy and outcomes among elderly patients hospitalized for heart failure in the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF).Citation25 Among patients with left ventricular systolic dysfunction (n = 3,001), beta-blockers were associated with adjusted HR of 0.7 (95 CI: 0.68–0.87) for mortality, 0.89 (95% CI: 0.80–0.99) for rehospitalization, and 0.87 (95% CI: 0.79–0.96) for mortality–rehospitalization. Patients with preserved systolic function had poor outcomes, and beta-blockers did not significantly influence the mortality and rehospitalization risks.
Nebivolol
Nebivolol is a lipophilic, third-generation, highly cardioselective, beta1-adrenergic receptor antagonist characterized by endothelium nitric oxide (NO)-dependent vasodilation.Citation26–Citation28 Unlike other third-generation beta-blockers, such as carvedilol and labetalol, which cause vasodilatation via alpha1-mediated receptor antagonism, nebivolol is unique in that it causes peripheral vasodilatation via L-arginine/NO-induced release from endothelial cells and subsequent increased nitric oxide bioavailability in the endothelium.Citation29–Citation31 In healthy subjects, brachial artery infusion of nebivolol significantly increases forearm blood flow, which is reduced by NC-monomethyl-L-arginine (L-NMMA), an inhibitor of NO synthase, and is restored by infusion of L-arginine.Citation32 These findings indicate that nebivolol vasodilatory activity is dependent on the L-arginine/NO pathway. NO is a major endothelium-derived vasodilatory compound that is also reported to have antithrombotic, antiproliferative, and antiinflammatory effects as well as lead to decreased myocardial oxygen demands.
Nebivolol is a racemic mixture of equal parts d- and l-nebivolol. The d-isomer is responsible for beta1-adrenergic receptor antagonism, while the l-isomer is primarily responsible for vasodilatation, with some contribution from the d-isomer. Nebivolol demonstrates beta1-receptor selectivity that is 321-fold higher than for the beta2-receptor at doses less than or equal to 10 mg in extensive metabolizers, which is a majority of the population. Thus, it is the most cardioselective beta-blocker currently available. However, at daily doses >10 mg or in poor metabolizers, it is reported to lose this potent cardioselectivity. Nebivolol is devoid of membrane-stabilizing activity, intrinsic sympathomimetic activity, and alpha1 antagonist properties at therapeutic concentrations.Citation33
Recent studies suggest that the endothelium-dependent vasodilation through activation of the NO pathway by nebivolol may be mediated via a beta3-adrenergic receptor agonist,Citation34 and that in the myocardium this stimulation may induce a NO-dependent negative inotropic effect that potentially could improve the energetic balance in the heart.Citation35
Nebivolol lowers heart rate and blood pressure, and improves systolic and diastolic function. The hemodynamic profile of nebivolol is different from traditional beta-blockers in that it increases stroke volume while reducing peripheral vascular resistance and increasing left-ventricular ejection fraction. Nebivolol has a neutral effect on cardiac output and may increase exercise capacity.Citation36–Citation39 A significant improvement in LVEF with nebivolol vs placebo was also seen in the study elderly heart failure patients in the efficacy of nebivolol in the treatment of elderly patients with chronic heart failure (ENECA) as add-on therapy to ACE inhibitors or angiotensin II receptor blockers, diuretics, and/or digitalis, and also in a small echocardiographic substudy of the study of the effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure (SENIORS).Citation40,Citation41 In patients with hypertension, nebivolol, compared with atenolol, has been shown to improve diastolic function by means of a decrease in isovolumic relaxation time, deceleration time of the mitral flow velocity, and increase in the early and late (atrial) (E/A) ratio.Citation42 Unlike nonselective beta-blockers, which may cause airway obstruction due to antagonist activity at beta2-adrenoceptors, nebivolol did not affect airway patency in patients with asthma or chronic obstructive pulmonary disease.Citation43
In the SENIORS trial, changes in fasting serum glucose for nondiabetic patients were 0.54 mg/dL and 0.9 mg/dL for nebivolol and placebo, respectively. For diabetic patients, there were reductions in fasting serum glucose of 5.76 mg/dL and 1.98 mg/dL for nebivolol and placebo, respectively. Although not statistically significant, nebivolol was associated with fewer cases of new onset diabetes mellitus than placebo (1.8% nebivolol vs 2.1% placebo).Citation44
Caution and consideration for dose-adjustment of nebivolol is recommended for patients with severe renal impairment (creatinine clearance < 30 mL/min), as the apparent clearance of nebivolol was decreased by 53% in this patient population. Nebivolol should be used with caution in patients receiving dialysis, as no formal studies have been conducted in these patients. Nebivolol is also contraindicated in patients with severe hepatic impairment because of a lack of data in these patients.
Clinical aspects of nebivolol in older patients with heart failure
Comparative studies
One randomized, single-blinded, open-label, parallel-group, 6-month studyCitation45 compared the effects of nebivolol vs carvedilol on left ventricular function in 70 patients in NYHA Class II or III and with LVEF ≤ 40% (mean age 67-years-old, mean LVEF 34%). Patients were randomized 1:1 to carvedilol 3.125 mg twice daily, titrated to target 25 mg twice daily if systolic blood pressure (SBP) > 110 mmHg and heart rate > 60 beats per minute (bpm), or nebivolol 1.25 mg daily titrated to target 5 mg daily if SBP > 110 mmHg and heart rate > 60 bpm. Carvedilol target dose was achieved in 77% of patients, while nebivolol target dose was achieved in 83% of patients. Compared with baseline, LVEF increased in both carvedilol arm (33% ± 6% to 37% ± 11%) and nebivolol arm (34% ± 7% to 38% ± 10%), with nonsignificant between-group differences. NYHA Class improved slightly in both arms, although only the carvedilol arm reached statistical significance (P < 0.05). Adverse effects occurred in 20% of carvedilol and 26% of nebivolol recipients, with one patient drop-out in each treatment arm. The most common adverse effects in each arm were fatigue and dizziness.
Another randomized, prospective, double-blinded, parallel-group study compared the efficacy of nebivolol vs carvedilol on LVEF and exercise capacity in 72 heart failure patients with NYHA Classes II–III and non-ischemic dilated cardiomyopathy.Citation46 After a titration phase to target doses of 5 mg daily of nebivolol and 25 mg twice daily of carvedilol, patients were followed for 12 months. LVEF was shown to significantly increase at 3 and 12 months from baseline in both nebivolol and carvedilol arms (P < 0.05). An intergroup-analysis revealed that carvedilol was associated with a greater effect on LVEF at 3 months (32.1% ± 34.9% vs 15.3% ± 15.9%, mean difference −16.7 ± 16.5, P = 0.04) and 12 months (35.5% ± 31.9% vs 20.7% ± 19.1%, mean difference −14.7 ± 6.4, P = 0.002) compared with nebivolol. Exercise duration significantly improved at 12 months in both the nebivolol (P = 0.01) and carvedilol arm (P = 0.01), with no significant between-group differences. An initial deterioration in exercise capacity was seen after 3 months in nebivolol-treated patients but was not observed in carvedilol-treated patients. Although nebivolol was likely under-dosed in these two studies, they are currently the only published prospective comparator trials and helped to pave the way for two larger-scale, placebo-controlled trials.
ENECA study
The ENECA study evaluated the effects of nebivolol vs placebo on ventricular remodeling as well as its safety and tolerability, in elderly heart failure patients.Citation40 In this randomized, prospective, multicenter, placebo-controlled, double-blinded, parallel-group study, 260 patients, aged more than 65-years-old (mean age 72-years-old) in NYHA Class II to IV and LVEF ≤ 35%, were randomized to either nebivolol (mean dose 7.2 mg; 64.2% achieved target 10 mg daily) or placebo, as an add-on to usual therapy. The primary end-point of the study was the absolute change in LVEF in comparison with baseline value. Secondary end-points were total mortality, change in NYHA Class, hospitalization rates and quality of life, assessed with the Minnesota Living with Heart Failure Questionnaire (MLHFQ). A total of 124 and 112 patients in the nebivolol and placebo groups, respectively, completed the study. Improvement in LVEF was significantly greater in nebivolol-treated vs placebo-treated patients (6.51% vs 3.97%; P = 0.027). A subgroup analysis revealed that nebivolol-treated males with no prior myocardial infarction history or with heart rate >75 bpm demonstrated the highest relative improvement in LVEF. In terms of NYHA Class changes, 33 patients in the nebivolol group improved by one class compared to 34 patients in the placebo group. The overall difference in functional status between the two groups was not statistically significant. Following 8 months of treatment, there was no difference in mean value of the total score of the MLHFQ between nebivolol and placebo (−9.1% ± 13.8% vs −11.0% ± 14.6% placebo; P = not significant [ns]).
Nebivolol-treated patients (baseline: 76.9 ± 10.8 bpm vs 8 month: 67.1 ± 9.2 bpm) had a significantly lower heart rate compared to placebo (baseline: 75.3 ± 9.9 bpm vs 8 month: 75.0 ± 9.6 bpm, P < 0.0001). Nebivolol was well tolerated, as 64% of patients achieved the maximum dose of 10 mg, and the incidence of adverse events was no different from the placebo group. Bradycardia, hypotension, and dizziness were the most frequent drug-related adverse effects in patients treated with nebivolol. The results of the ENECA study indicated that in elderly heart failure patients nebivolol is well tolerated and may significantly improve LVEF.
SENIORS study
The SENIORS study evaluated the safety, efficacy, and tolerability of nebivolol in the management of heart failure in the elderly. This was a randomized, prospective, multinational, multicenter, placebo-controlled, double-blinded, parallel-group study to evaluate the effects of nebivolol on mortality and hospitalization in clinically stable patients aged ≥ 70 years in NYHA Classes I–IV.Citation47,Citation48 The study enrolled 2128 patients with documented heart failure admission within the previous 12 months or documented LVEF less than 35% within the previous 6 months. Patients that were excluded from the study included those treated with beta-blocker therapy; patients with heart failure secondary to valvular disease; those that had severe coronary artery disease and had a revascularization procedure planned; contraindications or previous intolerance to beta-blockers, or change in cardiovascular therapy in the 2 weeks before randomization. The mean age of patients was 76-years-old, and most were in NYHA class II (56%) and III (39%). All patients underwent echocardiography after entry to the study, prior to administration of the study drug. LVEF was ≤35% in 64% of subjects and >35% in 36%. Prior hypertension was present in 61%, coronary artery disease in 69% and previous myocardial infarction in 44% of patients. Patients were randomized to placebo (n = 1061) or nebivolol (n = 1067) at a starting dose of 1.25 mg once daily titrated to 10 mg once daily over a 4–16 week period, as tolerated. Patients were followed from 12 to 39 months (average follow-up 21 months).
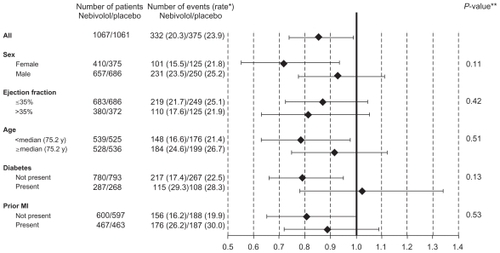
Treatment with nebivolol resulted in a statistically significant 14% decrease in the primary composite endpoint (all-cause death or cardiovascular hospitalization) vs placebo. The primary endpoint occurred in 332 (31.1%) nebivolol-treated patients vs 375 (35.3%) placebo-treated patients (HR = 0.86; 95% CI: 0.74–0.99; P = 0.039) (). The absolute risk reduction was 4.2%, suggesting a number needed to treat (NNT) of 24 patients for 21 months to avoid one event. The difference between nebivolol and placebo was evident after 6 months and gradually increased during the follow-up. The interaction between the primary outcome and some demographics [gender, age] and clinical factors [prior acute myocardial infarction, diabetes] was not statistically significant. The decrease in incidence of the primary end-point was similar in patients with reduced or preserved LVEF ().
Figure 2 Time to all-cause mortality or cardiovascular hospital admission (primary endpoint) in SENIORS.
Abbreviations: NEB, nebivolol; PL, placebo. Copyright© 2005. Modified with permission from Oxford University Press. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225.Citation47
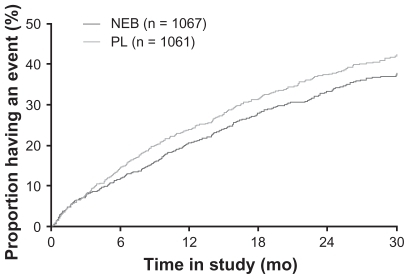
Figure 3 Prespecified sub-group analysis of SENIORS study. No interaction was found in subgroups with respect to the primary end-point.
*Number of events per 100 patient-years of follow-up at risk. **P-value for interaction: age and left ventricular ejection fraction considered as continuous variables.
Among secondary outcomes, the incidence of cadiovascular mortality or cardiovascular hospitalization was also significantly lower in patients treated with nebivolol than in those receiving placebo (28.6 vs 33.0%; HR 0.90; 95% CI: 0.72–0.98; P = 0.02). By contrast, there were no significant between-group differences for the other secondary endpoints. In particular, all cause mortality was 15.8% in the nebivolol group and 18.1% in the placebo group (HR 0.88; 95% CI: 0.71–1.08; P = ns). Results for functional assessment (NYHA mean class and the 6-minute walk test) have not yet been reported.Citation48
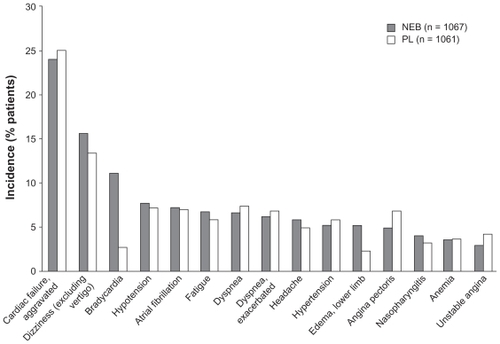
The proportion of patients reaching a dose of nebivolol greater than or equal to 5 and 10 mg at the end of the titration period was 80 and 68% of subjects, respectively (similar to the placebo group rates) and the mean maintenance dose was 7.7 mg per day. Nebivolol was generally well-tolerated, as compared to other approved beta-blockers.Citation18–Citation20 Premature discontinuation for any reason other than death occurred in 27% and 25% in the nebivolol and placebo groups, respectively. There was an increased incidence of bradycardia in nebivolol-treated patients (11.1% vs 2.6% placebo). Bradycardia was the cause for study withdrawal in 18 nebivolol-treated patients and four placebo-treated patients (no statistical analysis reported) (). Hypotension incidence was similar in the nebivolol (7.7%) and placebo (7.2%) groups. In summary, the SENIORS study showed that nebivolol is well tolerated and effective in reducing mortality and morbidity in elderly patients with heart failure.
Figure 4 Tolerability profile of nebivolol in SENIORS.
SENIORS substudies
A subgroup of SENIORS patients underwent complete echocardiographic recording in order to assess the effect of nebivolol treatment on systolic and diastolic ventricular function. Citation41 The substudy randomized 112 patients in 29 European centers, of whom 104 were evaluable for the study; 43 had an ejection fraction (EF) ≤ 35% and 61 had an EF > 35%. Left ventricular end-systolic volume, LVEF, mitral valve E/A ratio, and E-wave deceleration time were assessed at baseline and after 12 months. Nebivolol significantly increased LVEF (4.6%; P = 0.008) and decreased end-systolic volume (P = 0.016) in patients with systolic left ventricular dysfunction (<35%), confirming the results of the ENECA. On the other hand, no significant changes were observed in left ventricular structure and function in patients with preserved or slightly reduced systolic function (EF > 35%).
In another prespecified substudy the effects of nebivolol in the subgroups with impaired EF (<35%) and preserved EF (> 35%) were explored. Forty-nine of the 2,111 patients, 1,359 (64%) had impaired LVEF (mean 28.7%) and 752 (36%) had preserved LVEF (mean 49.2%). The effect of nebivolol was investigated in these two groups, and it was compared to explore the interaction of LVEF with outcome. Follow-up was 21 months; the primary end-point was all-cause mortality or cardiovascular hospitalizations. Patients with preserved LVEF were more often women (49.9% vs 29.8%) and had less advanced heart failure, more hypertension, and fewer prior myocardial infarctions (all P < 0.001). During follow-up, the primary end-point occurred in 465 patients (34.2%) with impaired LVEF and in 235 patients (31.2%) with preserved LVEF. The effect of nebivolol on the primary end-point HR of nebivolol vs placebo was 0.86 (95% CI: 0.72–1.04) in patients with impaired EF and 0.81 (95% CI: 0.63–1.04) in preserved LVEF (P = 0.720 for subgroup interaction). Effects on all secondary end-points were similar between groups (HR for all-cause mortality 0.84 and 0.91, respectively), and no P value for interaction was <0.48. The authors concluded that the effect of beta-blockade with nebivolol in elderly patients in this study was similar in those with preserved and impaired LVEF. However, it should be noted that although the primary outcome composite end-point was similar in low and preserved LVEF groups, there was only a 1.1% absolute (difference n = 3) reduction in all-cause mortality in those with LVEF > 35% versus a 2.8% (difference n = 20) absolute difference for those with LVEF ≤ 35%.
More recently, a substudy of SENIORS evaluated the safety and efficacy of nebivolol in patients with renal dysfunction.Citation50 Patients (n = 2112) were divided by tertile of estimated glomerular filtration rate (eGFR). The eGFR was strongly associated with outcomes and nebivolol was similarly efficacious across eGFR tertiles. The primary outcome rate (all-cause mortality or cardiovascular hospital admission) and adjusted HR for nebivolol use in those with low eGFR was 40% and 0.84 (95% CI: 0.67–1.07), 31% and 0.79 (0.60–1.04) in the middle tertile, and 29% and 0.86 (0.65–1.14) in the highest eGFR tertile. There was no interaction between renal function and the treatment effect (P = 0.442). Nebivolol use in patients with moderate renal impairment (eGFR < 60) was not associated with major safety concerns, apart from higher rates of drug discontinuation due to bradycardia. The authors concluded that nebivolol is safe and has a similar effect in elderly patients with mild or moderate renal impairment.
SENIORS post-hoc analyses
Three post-hoc analyses have been carried out in subgroups of SENIORS patients. The all-cause mortality relative risk reduction of nebivolol vs placebo in SENIORS was 12% compared with risk reductions of 34%–35% for bisoprolol, carvedilol and metoprolol CR/XL versus placebo in the cardiac insufficiency bisoprolol study-(CIBIS) II, carvedilol prospective randomized cumulative survival (COPERNICUS), and metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF) trials.Citation18–Citation20 In order to compare the SENIORS results to those of the other trials, the authors of SENIORS conducted a not prespecified exploratory analysis in one subgroup that more closely resembled patient groups from the other beta-blocker trials.Citation47 In the subgroup of nebivolol patients aged <75.2-years-old who had an LVEF ≤ 35%, the risk reduction for all-cause mortality was 38% ( and ).
Figure 5 Time to all-cause mortality in patients aged <75.2 years (median age) with left ventricular ejection fraction (LVEF) ≤35% in SENIORS. The hazard ratio was 0.62 (95% CI: 0.43, 0.89; P = 0.011).
Abbreviations: NEB, nebivolol; PL, placebo. Copyright© 2006. Modified with permission from Wolters Kluwer. Moen MD, Wagstaff AJ. Nebivolol: a review of its use in the management of hypertension and chronic heart failure. Drugs. 2006;66(10): 1389–1409.Citation27
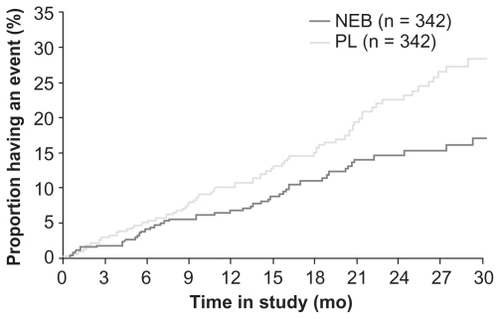
Figure 6 Hazard ratio plots (with 95% CIs) for total mortality for comparable patient subgroups from the four main beta-blockers mortality trials, ie, SENIORS [nebivolol]; COPERNICUS [carvedilol], MERIT-HF [metoprolol] and CIBIS II [bisoprolol], using data derived from the trial reports. These data are from published patient subgroups reported by the authors themselves for each trial, and the criteria, therefore, differ between trials. The reported patient age subgroups chosen here are those most similar to each other across the four trials. For nebivolol, this is left ventricular ejection fraction (LVEF) ≤35% and age less than median (70–75.2 years); for carvedilol, LVEF ≤ 25% and age ≥65 years; for metoprolol LVEF ≤ 40% and age >69 years; and for bisoprolol LVEF ≤ 35% and age ≥71 years. Copyright© 2005. Modified with permission from Coats AJS. Coats AJS, The modern tailored management of chronic heart failure: SENIORS. Proceedings of the Annual Congress of the European Society of Cardiology; 2005 Sep 3–7; Stockholm.Citation52
![Figure 6 Hazard ratio plots (with 95% CIs) for total mortality for comparable patient subgroups from the four main beta-blockers mortality trials, ie, SENIORS [nebivolol]; COPERNICUS [carvedilol], MERIT-HF [metoprolol] and CIBIS II [bisoprolol], using data derived from the trial reports. These data are from published patient subgroups reported by the authors themselves for each trial, and the criteria, therefore, differ between trials. The reported patient age subgroups chosen here are those most similar to each other across the four trials. For nebivolol, this is left ventricular ejection fraction (LVEF) ≤35% and age less than median (70–75.2 years); for carvedilol, LVEF ≤ 25% and age ≥65 years; for metoprolol LVEF ≤ 40% and age >69 years; and for bisoprolol LVEF ≤ 35% and age ≥71 years. Copyright© 2005. Modified with permission from Coats AJS. Coats AJS, The modern tailored management of chronic heart failure: SENIORS. Proceedings of the Annual Congress of the European Society of Cardiology; 2005 Sep 3–7; Stockholm.Citation52](/cms/asset/e6cfdbb8-b0cd-4ea8-8197-717512f55764/dcia_a_4482_f0006_b.jpg)
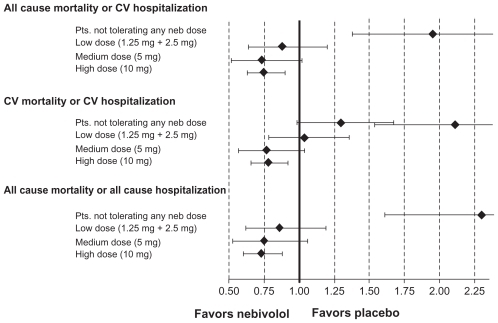
A second post-hoc analysis assessed the tolerability and dose-related effects of nebivolol.Citation51 Patients assigned to nebivolol (n = 1031) were classified into 4 groups, according to the dose achieved at the end of titration phase (maintenance dose): 0 mg (n = 74), low dose (1.25 or 2.5 mg, n = 142), medium dose (5 mg, n = 127), and target dose (10 mg, n = 688) and compared with those allocated to placebo (n = 1030). Age, sex and LVEF were similar between the groups, but prior myocardial infarction, coronary revascularization, and serum creatinine levels were lower in patients who achieved higher maintenance doses of nebivolol. After adjustment, all-cause mortality or cardiovascular hospitalization was significantly reduced in the 10 mg dose group compared with placebo (HR 0.75, 95% CI: 0.63–0.90) which was similar to the medium dose group (HR 0.73, 95% CI: 0.52–1.02). The low dose group had an apparently lower benefit (HR 0.88, 95% CI: 0.64–1.20), whereas patients unable to tolerate any dose of nebivolol had an increased risk of death or cardiovascular hospitalization (HR 1.95, 95% CI: 1.38–2.75) (). The authors concluded that the benefits of nebivolol in elderly patients with heart failure appear to be related to the maintenance dose achieved. Patients unable to tolerate any dose have the worst prognosis. However, the reasons for 32% of patients not reaching the 10 mg daily dosage were not reported.
Figure 7 Primary and secondary outcomes (HR with 95% CI) in patients receiving placebo versus nebivolol at different maintenance doses.
Another post-hoc analysis for the endpoint of sudden cardiac death reported an HR of 0.62 for nebivolol versus placebo (95% CI: 0.42, 0.91; P = 0.014). These nonprespecified analyses should be considered exploratory and hypothesis-generating and may suggest possible areas for future research.Citation52
Economic aspects of nebivolol therapy
In the SENIORS trial the cost-effectiveness of nebivolol compared with standard medical therapy was evaluated using a Markov Monte Carlo simulation model developed to assess the cost, survival, quality adjusted survival and cost effectiveness of nebivolol over the patient’s life time.Citation53 Health states were defined as stable condition, cardiovascular hospitalization events, death in hospital, sudden death, and death due to other causes, based on monthly cycles. Patients’ characteristics, time to sudden death, time to hospitalization with standard medical therapy, the hazard ratios with nebivolol, and resource used data were derived from the SENIORS clinical trial. Utility scores for each NYHA class were derived from a large heart failure trial. The economic analysis was conducted from the UK health care perspective including costs of hospitalization, drug cost, cost of treatment for severe adverse effects and general practitioner visit cost. A fully probabilistic sensitivity analysis for all input values to explore uncertainty derived from the model parameters was conducted. Costs and outcomes were discounted at 3.5% annually. The model predicted that the total cost per patient for the nebivolol group was $18,120 compared with $14,298 for standard medical treatment respectively. The mean life-years were 8.49 and 7.16 and quality-adjusted life years (QALYs) were 5.69 and 4.80 for nebivolol and medical standard treatment respectively. The probabilistic sensitivity analysis gave an incremental cost of $3,822, a QALYs score of 0.88 and a life year estimate of 1.32. This gives incremental cost-effectiveness ratios (ICERs) of $4,322 (95% CI: $3,975–$4,731) per QALY gained and $2,888 (95% CI: $2,663–$3,170) per life year gained. This model-based analysis indicates that nebivolol is highly cost-effective, achieving an incremental cost-effectiveness ratio well below a standard benchmark used for resource allocation decisions in elderly people with heart failure, when compared to standard medical therapy.
Discussion
Subgroup analyses,Citation21,Citation22 meta-analysesCitation23 and observational studiesCitation24,Citation25 showed a beneficial effect of beta-blockers in elderly populations, including those with depressed and preserved LVEF. Approximately two-thirds of elderly patients with heart failure tolerate a beta-blocker, but only 40%–70% of the target doses recommended in randomized trials are achieved. Moreover, the effect of beta-blockers on all-cause mortality may be lower in very elderly and frail patients.Citation23 In other words, the level of evidence regarding beta-blocker therapy in the elderly is not regarded as high as that in younger patients.
There is also evidence that beta-blockers are less frequently prescribed in elderly patients in clinical practice, and that this lack of treatment is associated with impaired outcomes. Establishing which beta-blockers are effective in the elderly is therefore of importance. The elderly have a reduced cardiovascular reserve and may be less tolerant to a vasoconstricting beta-adrenoceptor antagonist. In addition, the higher proportion of elderly heart failure patients with relatively preserved systolic function (for which no treatment has been proven to reduce mortality and morbidity) and with multiple comorbidities and age-related impairments means that we cannot say with certainty that beta-blockers have been proven to be effective in a general elderly heart failure population.
Third-generation beta-adrenoceptor antagonists with vasodilating properties may offer several theoretical advantages. Three of this class (carvedilol, bucindolol and nebivolol) have been evaluated in heart failure, and only two of these (carvedilol and nebivolol) had a proven outcome benefit in a properly powered randomized, controlled trial. In SENIORS, nebivolol was more effective than placebo in reducing the risk of the composite endpoint of all-cause mortality or cardiovascular hospitalization and was generally well tolerated in elderly patients with heart failure with reduced or preserved systolic function.Citation47
Despite the beneficial results of SENIORS, some uncertainty or disagreement about whether beta-blockers are equally beneficial and well tolerated in elderly heart failure patients as in younger ones still remain. First, the HR for the primary outcome in the SENIORS was 0.86,Citation47 a lesser risk reduction compared with previous large beta-blocker trials.Citation18–Citation20 As suggested, there are several possible reasons for this:Citation27,Citation54,Citation55 1) nebivolol, at the dose used in the trial, might be inferior to the other beta-blockers tested; 2) the marked differences in populations enrolled (older and with less compromised left ventricular systolic function) and/or the different duration of follow-up in SENIORS compared with other beta-blocker trials in heart failure might account for the differences in outcomes; and 3) older patients may respond differently to drugs in terms of efficacy and tolerability.
Older patients enrolled in SENIORS may not fully reflect the clinical profile of the “real world” elderly. Indeed, SENIORS enrolled patients selected for low comorbidities (and age-related impairments) and probably at low-risk of mortality and morbidity. The event rate in SENIORS was unexpectedly low, because all cause mortality at a mean follow-up of 21 months in the placebo group (18.1%) was significantly lower than that previously reported. For example, in the observational study beta-blockers in patients with congestive heart failure: guided use in clinical practice (BRING-UP), patients older than 70 years enrolled in cardiology heart failure clinics and not treated with beta-blockers had the same mortality rate (18%) at 12 months.Citation56 If we consider unselected community-living older patients with multiple comorbidities and age-related impairments enrolled at discharge from hospital in a disease management program, the 24-month all-cause mortality rises up to 34.1% (18.3% in patients tolerating and 52.5% in those not tolerating beta-blockers). Citation57
Although SENIORS demonstrated a clear benefit of nebivolol, it is not possible to directly compare outcomes between SENIORS and other beta-blockers trials because of the differences in trial design.Citation48 The benefit of nebivolol on mortality in older adults may be attenuated by competing contributors to death not modifiable by nebivolol. Moreover, although the prespecified component of the primary end-point, that is, cardiovascular hospitalization, was reduced by nebivolol, all-cause hospitalization was unchanged.
Other trials included younger patients (and excluded very old patients) with low LVEF (≤40%) and used different study endpoints.Citation18–Citation20 The authors of SENIORS therefore conducted exploratory analyses (not prespecified) in subgroups that more closely resembled patient groups from other studies.Citation52 The risk reduction for all-cause mortality (the primary endpoint in CIBIS-II and COPERNICUS and one of the primary endpoints in MERIT-HF) for nebivolol compared with placebo was 12% in SENIORS compared with risk reductions of 34%–35% for bisoprolol, carvedilol and metoprolol CR/XL versus placebo. However, in the subgroup of nebivolol recipients from SENIORS aged < 75.2 years who had an LVEF ≤ 35%, the risk reduction for all-cause mortality was 38% ().
When analyzed according to age strata, the oldest patients (above the median age of 75.2 years) derived somewhat a less benefit (not statistically significant) than younger patients. It may be argued that the increased risk of death from other causes in the elderly may compete with the potential benefits of treatment. Thus, it is plausible that there is a threshold of biological age, beyond which the benefit of any treatment is difficult to demonstrate. Although the benefits of nebivolol appeared to be reduced in patients aged greater than 75 years, age as a continuous variable did not significantly affect the treatment effect.Citation47,Citation54,Citation55
The results of SENIORS also extend the benefit of beta-blocker therapy to patients with preserved left ventricular systolic function, a sizable proportion of heart failure patients. However, these patients represented only a third of the patients enrolled in the SENIORS trial, and the LVEF cut-off was 35%, far different from that of 45%–50% usually considered in epidemiological studies as “preserved” LVEF. Indeed, the exact percentage of patients with normal LVEF (ie, >50%) was not reported in the studyCitation47 and that of patients with LVEF > 40% was 30.4%.Citation49 Therefore, this is just a hypothesis that requires confirmation in properly designed and powered studies. Theoretically, there are several reasons why nebivolol might improve diastolic function. The decrease in heart rate by prolonging the diastolic filling time more than the ejection time should improve myocardial perfusion and metabolism. The increased NO release caused by nebivolol might also improve early relaxation. Previously, a small echocardiographic substudy of the SENIORS trial failed to show any improvement in diastolic performance.Citation41 However, in the long term of a progressive condition such as heart failure, the subtle changes in diastolic function might not be captured by a technique sensitive to multiple factors, including the loading conditions, such as standard Doppler echocardiography. The question of whether or not nebivolol can improve left ventricular diastolic function remains unanswered.Citation55
With respect to the dose achieved in the SENIORS trial, only the highest doses of nebivolol were associated with a significant event reduction. During the titration phase, 7% of patients could not tolerate any nebivolol, and 33% were not at the dose at which mortality benefit was clear.Citation47,Citation51 Post-hoc analyses from SENIORS suggesting that nebivolol may reduce sudden cardiac death and that greater benefits are achieved in those who reach the target maintenance dose of 10 mg/day require further investigation. Patients unable to tolerate target doses were older and were more likely to be receiving other medications that alter heart rate and conduction (antiarrhythmic agents and calcium blockers). This underscores the challenges of the generalizability of this trial to older adults in clinical practice, where polypharmacy, pre-existing frailty, and conditions affecting tolerability of beta-blockers in maximal doses are more prevalent. Thus, the open question is whether we should use the same target dose in the elderly as that in younger patients. Theoretically, the most effective dose is the highest dose tolerated, which may differ across different age groups and may not be applicable to the frail, older population. In these complex and vulnerable patients it is therefore time to shift from the paradigm of the “target dose” to that of the “highest dose tolerated”.Citation55 On the other hand, data from observational studies suggest that “low dose” is better than “no dose”, because the prognosis of patients intolerant to beta-blockers is worse.Citation24,Citation25
Finally, the prespecified secondary outcomes of functional capacity by NYHA functional class and 6-min walk test in the SENIORS trial have never been reported: these data would greatly assist clinicians in applying the overall result.Citation48
In summary, SENIORS is the first and only trial that has prospectively investigated beta-blocker treatment of heart failure elderly patients, including those with relatively preserved systolic function, and demonstrated a significant reduction in the risk of death or cardiovascular hospitalization. Thus, nebivolol should be considered as an alternative first-line treatment option in selected elderly patients with heart failure. Moreover nebivolol appears to be significantly cost-effective when prescribed in these patients. However, in order to better define the profile of efficacy and safety of beta-blockers in older patients, further data are needed from targeted clinical trials and rigorous observational studies, showing definite improvement in outcomes as well as clearly favorable benefit-risk analysis in typical older heart failure patients irrespective of comorbidity, frailty and polypharmacy.
Disclosure
The authors report no conflicts of interest in this work.
References
- CowieMRWoodDACoatsAJIncidence and aetiology of heart failure; a population-based studyEur Heart J19992042142810213345
- HoKKPinskyJLKannellWBLevyDThe epidemiology of heart failure: the Framingham StudyJ Am Coll Cardiol199322Suppl A6A14A8509564
- SenniMTribouilloyCMRodehefferRJCongestive heart failure in the community: trends in incidence and survival in a 10-year periodArch Intern Med199915929349892327
- LevyDKenchaiahSLarsonMGLong-term trends in the incidence of and survival with heart failureN Engl J Med20023471397140212409541
- HavranekEPMasoudiFAWestfallKASpectrum of heart failure in older patients: results from the National Heart Failure projectAm Heart J200214341241711868045
- PulignanoGDel SindacoDTavazziLClinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (Italian Network on Congestive Heart Failure [IN-CHF] Registry)Am Heart J2002143455511773911
- Lloyd-JonesDMLarsonMGLeipEPLifetime risk for developing congestive heart failure: the Framingham Heart StudyCirculation20021063068307212473553
- SenniMDi LenardaACacciatoreGfor IN-CHF InvestigatorsTemporal trends in survival and hospitalizations among outpatients with chronic heart failure, in 1995 and 1999: IN-CHFJ Card Fail20051127027815880335
- HoKKLAndersonKMKannelWBSurvival after onset of congestive heart failure in Framingham Heart Study subjectsCirculation1993881071158319323
- CroftJBGilesWHPollardRAHeart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare populationArch Intern Med199915950551010074960
- RichMWHeart failure in the 21st century: a cardiogeriatric syndromeJ Gerontol A Biol Sci Med Sci200156M88M9611213282
- PulignanoGDel SindacoDDi LenardaASinagraGThe evolving care of the elderly with heart failure: from the ‘high-tech’ to the ‘high-touch’ approachJ Cardiovasc Med (Hagerstown)2006784184617122668
- LienCTGillespieNDStruthersADMcMurdoMEHeart failure in frail elderly patients: diagnostic difficulties, co-morbidities, polypharmacy and treatment dilemmasEur J Heart Fail20024919811812669
- De GeestSScheurweghsLReyndersIDifferences in psychosocial and behavioral profiles between heart failure patients admitted to cardiology and geriatric wardsEur J Heart Fail2003555756712921819
- LakattaEGLevyDArterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart diseaseCirculation200310734635412538439
- ClelandJGSwedbergKCohen-SolalAThe Euro heart failure survey of the EUROHEART survey programme. A survey on the quality of care among patients with heart failure in Europe: The Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology; The Medicines Evaluation Group Centre for Health Economics University of YorkEur J Heart Fail2000212313210856724
- European Society of Cardiology (ESC), Heart Failure Association (HFA) of the ESC, European Society of Intensive Care Medicine (ESICM), et alESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of CardiologyEur J Heart Fail20081093398918826876
- PackerMCoatsAJFowlerMBCarvedilol prospective randomized cumulative survival study group. Effect of carvedilol on survival in severe chronic heart failureN Engl J Med20013441651165811386263
- CIBIS II investigators and CommitteesThe Cardiac lnsufficiency Bisoprolol Study II (CIBIS ll); a randomised trialLancet199935391310023943
- MERIT-HF Study GroupEffect of metoprolol CR/XL in chronic heart failure; metoprolol CR/XL randomised lntervention trial in congestive heart failure (MERIT-HE)Lancet19993532001200710376614
- DeedwaniaPCGottliebSGhaliJKWaagsteinFWikstrandJCMGroup MERIT-HF StudyEfficacy, safety and tolerability of B-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failureEur Heart J2004251300130915288157
- ErdmannELechatPVerkennePWiemannHResults from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failureEur J Heart Fail2001346947911511434
- DulinBRHaasSJAbrahamWTKrumHDo elderly systolic heart failure patients benefit from beta blockers to the same extent as the non-elderly? Meta-analysis of >12,000 patients in large-scale clinical trialsAmerican J Cardiol200595896898
- SinDDMcAlisterFAThe effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failureAm J Med2002113865065612505115
- HernandezAFHammillBGO’ConnorCMSchulmanKACurtisLHFonarowGCClinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Life-saving Treatment in Hospitalized Patients with Heart Failure) registryJ Am Coll Cardiol20095318419219130987
- de BoerRAVoorsAAvan VeldhuisenDJNebivolol: third generation β-blockadeExpert Opin Pharmacother20078101539155017661735
- MoenMDWagstaffAJNebivolol: a review of its use in the management of hypertension and chronic heart failureDrugs200666101389140916903772
- IgnarroLJByrnsRETrinhKNebivolol: a selective B1-adrenergic receptor antagonist that relaxes vascular smooth muscle by nitric oxide- and cyclic GMP-dependent mechanismsNitric Oxide20027758212223176
- AltweggLAd’UscioLVBarandierCNebivolol induces NO-mediated relaxations of rat small resistance but not of large elastic arteriesJ Cardiovasc Pharmacol20003631632010975588
- IgnarroLJExperimental evidence of vasodilatory activity of nebivolol, a third generation beta-blockerBlood Press200413Suppl 1216
- CosentinoFBonettiSRehorikRNitric oxide-mediated relaxations in salt-induced hypertension: effect of chronic B1-selective receptor blockadeJ Hypertens20022042142811875309
- CockcroftJRChowienczykPJBrettSENebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO dependent mechanismJ Pharmacol Exp Ther199537410071071
- BrixiusKBundkirchenBolckBNebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardiumBr J Pharmacol20011331330133611498519
- RozecBQuangTTNoireaudJGauthierCMixed beta3-adrenoceptor agonist and alpha1-adrenoceptor antagonist properties of nebivolol in rat thoracic aortaBr J Pharmacol2006147769970616474420
- RozecBErfanianMLaurentKTrochuJGauthierCNebivolol, a vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the nonfailing transplanted human heartJ Am Coll Cardiol200953171532153819389564
- StoreluLWijnsWBouvyTEffects of D-nebivolol and L-nebivolol on left ventricular systolic and diastolic functions: comparison with D-L-nebivolol and atenololJ Card Pharmacol199322183190
- RousseauMFChapelleFvan EyllCMedium-term effects of beta-blockade on left ventricular mechanics: a double-blind, placebo controlled comparison of nebivolol and atenolol in patients with ischemic left ventricular dysfunctionJ Card Fail1996215238798100
- BruneSTebbeUSchmidtTHaemodynamic effects of nebivolol, at rest and exertion in patients with heart failureAngiology1990416967011977335
- BoutelantSLechatRKomajdaMNon invasive evaluation of cardiovascular effects of nebivolol in patients with cardiac insufficiencyArch Mal Coeur Vaiss1992858638701358044
- EdesIGasiorZWitaKEffects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA studyEur J Heart Fail2005763163915921805
- GhioSMagriniGSerioAEffects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic sub-placebo-controlled crossover studyEur Heart J200627556256816443607
- KampOSieswerdaGTVisserCAComparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertensionAm J Cardiol20039234434812888152
- Dal NegroRWTognellaSPomariCOnce-daily nebivolol 5 mg does not reduce airway patency in patients with chronic obstructive pulmonary disease and arterial hypertension: a placebo-controlled crossover studyClin Drug Invest200222361367
- Agabiti RoseiERizzoniDMetabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristicsDrugs20076781097110717521213
- LombardoRMReinaCAbrignaniMGEffects of nebivolol versus carvedilol on left ventricular function in patients with chronic heart failure and reduced left ventricular systolic functionAm J Cardiovasc Drugs20066425926316913827
- PatrianakosAPParthenakisFIMavrakisHEComparative efficacy of nebivolol versus carvedilol on left ventricular function and exercise capacity in patients with nonischemic dilated cardiomyopathy: a 12-month studyAm Heart J2005150985e.9985e.1816290981
- FlatherMDShibataMCCoatsAJRandomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS)Eur Heart J20052621522515642700
- ShibataMCFlatherMDBohmMStudy of the effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure (SENIORS): rationale and designInt J Cardiol200286778512243852
- van VeldhuisenDJCohen-SolalABöhmMSENIORS InvestigatorsBeta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure)J Am Coll Cardiol200953232150215819497441
- Cohen-SolalAKotechaDvan VeldhuisenDJSENIORS InvestigatorsEfficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trialEur J Heart Fail200911987288019648605
- DobreDvan VeldhuisenDJMordentiGTolerability and dose-related effects of nebivolol in elderly patients with heart failure: data from the study of the effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure (SENIORS) trialAm Heart J200715410911517584562
- CoatsAJSThe modern tailored management of chronic heart failure: SENIORSProceedings of the Annual Congress of the European Society of Cardiology2005 Sep 3–7Stockholm
- YaoGFreemantleNFlatherMTharmanathanPCoatsAPoole-WilsonPALong-term cost-effectiveness analysis of nebivolol compared with standard care in elderly patients with heart failure: an individual patient-based simulation modelPharmacoeconomics2008261087988918793034
- Mc MurrayJMaking Sense of SENIORSEur Heart J20052620320615647290
- ArmstrongPWAlexanderKPNebivolol in older adults with heart failure: reduced rates for seniorsJ Am Coll Cardiol2009532159216119497442
- OpasichCBoccanelliACafieroMProgramme to improve the use of B-blockers for heart failure in the elderly and in those with severe symptoms: results of the BRING-UP 2 studyEur J Heart Fail2006864965716466962
- Del SindacoDPulignanoGMinardiGTwo-year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failureJ Cardiovasc Med (Hagerstown)2007832432917443097