Abstract
Objective
The number of elderly patients being diagnosed with cervical cancer is increasing, and the outcome of cervical cancer related to age is controversial. We conducted a retrospective analysis in patients treated for advanced cervical cancer in order to investigate patient characteristics and prognosis of older patients.
Methods
Medical records were collected of 159 patients with cervical cancer who had been treated with radiotherapy or combined radiotherapy and chemotherapy from January 2007 to January 2009. The patients were divided into two age groups: (1) patients ≥65 years old, and (2) patients <65 years old. There were 52 women in group 1, 107 in group 2. Prognosis, patient characteristics, treatment, and toxicities were evaluated.
Results
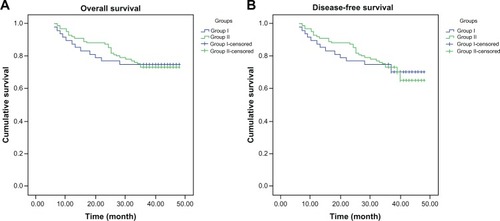
With a median follow-up of 36.5 months, local control for groups 1 and 2 was 88.5% and 79.4%, respectively. Disease-free survival for the two groups was 71.2% and 67.3%; overall survival was 73.1% and 72.9%. As shown by univariate analyses, there was no statistically significant difference between the two groups (P > 0.05). Seventy-six patients had human papillomavirus (HPV) at diagnosis (twelve women ≥65 years, 64 women ≤65 years; P = 0.000). Forty-two women tested positive for HPV 16, while 32 women tested positive for HPV 18 respectively. Pelvic and/or paraaortic lymph-node metastasis was found in 25 patients (eight in group 1, 17 in group 2; P = 0.960) on computed tomography scan. Of the 159 patients analyzed, sixteen patients (16/52) in group 1 received concurrent chemotherapy, while 96 (96/107) in group 2 completed that treatment.
Conclusions
Cervical cancer has the same prognosis in old and young women. Age may not be an independent increased risk of death in women with cervical cancer, and the age-group is at lower risk for virulent HPV strands (HPV 16/18) compared to younger patients. Treatment recommendations were implemented less often for older patients. Radiotherapy remained the most common treatment chosen for elderly patients. This confirms that there is a stronger need to pay attention to the elderly patient.
Keywords:
Introduction
Cervical cancer is the second most common major malignancy among women worldwide.Citation1 Research has established the incidence of cervical cancer peaks in the fourth decade of life, with a median age at diagnosis of 48 years. The number of elderly patients being diagnosed with cervical cancer is increasing in Europe, and older women account for more than 40% of the deaths from cervical cancer annually.Citation2 In an increasingly older population (on the basis of recent figures for population growth, it is estimated that the number of women over 65 years will increase by 23%),Citation3 comorbid conditions and other factors that determine frailty – such as performance status – will probably play an increasing role in clinical decision-making and outcomes.
However, the impact of age on survival of patients with cervical cancer remains uncertain. Some older studies suggested that cervical cancer has the same prognosis in old and young women.Citation4 Others suggested that younger age is an unfavorable prognostic factor, especially in more advanced stages.Citation5 In contrast, Wright et al demonstrated that age is a poor prognostic factor for cervical cancer.Citation6 Moreover, it has been shown that younger patients may have improved outcome compared to older patients,Citation7–Citation9 and that advanced age is linked to decreased survival in a variety of cancers.Citation10,Citation11
Since the outcome of cervical cancer related to age is controversial, and the determination of the effect of age on outcome is complicated by several related issues, including the risk of death from competing age-related illnesses, stage of disease, method of treatment, and histologic type,Citation12,Citation13 we conducted a retrospective analysis in patients treated in our institution in order to investigate the prognosis, patient characteristics, treatment, and toxicities of older patients with advanced carcinoma of the uterine cervix.
Materials and methods
Patients
The Ethics Committee of the First Affiliated Hospital, Medical College, Xi’an Jiao Tong University of China, People’s Republic of China, approved this study. Data were collected retrospectively from the records of 159 consecutive patients with locally advanced cervical cancer who had been treated with radiotherapy or combined radiotherapy and chemotherapy from January 2007 to January 2009. All patients had provided written informed consent for treatment.
The patients included in this study presented with International Federation of Gynecology and Obstetrics (FIGO) stages I–IIIB, good performance status (Eastern Cooperative Oncology Group 0 [asymptomatic], or 1 [symptomatic but ambulatory]), no uncontrolled concomitant disease, no connective tissue disease, and no prior irradiation. For evaluation, we divided our cohort into two age-groups: (1) patients ≥65 years old, and (2) patients <65 years old. There were 52 women in group 1, 107 in group 2.
Human papillomavirus (HPV) DNA testing for 13 carcinogenic HPV genotypes (HPV 16, 18, 3 1, 33, 35, 39, 45, 51, 52, 56, 58, and 68) was performed using HC2 according to the manufacturer’s instructions. Conventional cytology was used.
Radiation therapy
External beam radiation therapy was delivered in a conventional fraction (1.8 Gy/fraction, five fractions/week) using a 10 MV photon beam from a linear accelerator. A total dose of 50.4 Gy was administered to the entire pelvis. This was followed by intracavitary brachytherapy at a dose of 5 Gy in four fractions to point A (the paracervical triangle on the medial edge of the broad ligament where the uterine vessels cross the ureter), which was delivered by a remote afterloading system. External beam radiation therapy was interrupted if the white blood cell count fell below 1,000/mm3 or if platelets fell below 50,000/mm3, and was resumed once counts rose above these levels.
Chemotherapy
Cisplatin and fluorouracil were the chemotherapy agents administered. Cisplatin was given in a dose of 40 mg/m2/day for 3 days; fluorouracil was given at 500 mg/m2/day continuous infusion from the first to the fifth day with radiotherapy.
Patients were seen weekly by a physician for a physical examination and a complete blood count test. Chemotherapy was stopped if creatinine clearance was <30 mL/minute, and interrupted if patients had ≥grade 3 gastrointestinal toxicity, the total white blood cell count was ≤4,000/mm3, or platelets were ≤100,000/mm3. Granulocyte colony-stimulating factors were used when absolute neutrophil count fell ≤500/mm3 or total white blood cell count fell ≤1,000/mm3.
Toxicity
During treatment, toxicities were assessed weekly and graded in accordance with the National Cancer Institute Common Terminology Criteria of Adverse Events: 1, mild; 2, moderate; 3, severe; and 4, life-threatening or disabling.Citation14
Statistical analyses
Data were stored and analyzed using SPSS version 17.0 software (IBM, Armonk, NY, USA). The Kaplan–Meier approach and the log-rank test were used to compare survival profiles between the two patient groups. P <0.05 was considered significant for all statistical analyses.
Results
Patient characteristics
In total, 159 patients were included from January 2007 to January 2009. The median age of the patients at the time of diagnosis was 49.2 years (range 21–78 years). They all had squamous cell carcinoma. Among them, 76 patients had HPV at diagnosis, 42 tested positive for HPV 16 at enrolment, and 34 tested positive for HPV 18 and other carcinogenic HPV types (twelve women ≥65 years, 64 women ≤65 years; P=0.000). Forty-eight patients were diagnosed in FIGO stage IIB (16 in group 1, 32 in group 2), and 100 patients were diagnosed in FIGO stage IIIB (32 in group 1, 68 in group 2). Pelvic and/or paraaortic lymph-node metastasis was found in 25 patients (eight in group 1, 17 in group 2) on computed tomography (CT) scan. Of the 159 patients analyzed, 16 patients (16/52) in group 1 received concurrent chemotherapy, while 36 patients refused the treatment. Ninety-six (96/107) in group 2 completed that treatment.
During the follow-up interval, 14 patients in group 1 and 29 patients in group 2 died. Thirty-six patients died of tumor-related disease, and 74 patients died of causes other than cancer. Tumor recurrence was observed in 28 patients (six in group 1, 22 in group 2). In the entire group, metastasis occurred in 17 patients (four in group 1, 13 in group 2). Patient characteristics are outlined in .
Table 1 Clinicopathology of patients by treatment group
Survival and local control
With a median follow-up time of 36.5 months (range 6–52 months), local control for groups 1 and 2 was 88.5% and 79.4%, respectively. Disease-free survival for the two groups was 71.2% and 67.3%; overall survival was 73.1% and 72.9%. As shown by univariate analyses, there was no statistically significant difference between the two groups (P>0.05) ( and ).
Table 2 Three-year local control, disease-free survival, and overall survival rates stratified by patient group (n, %)
Toxicities
Anemia and neutropenia were found in eleven and 20 patients, respectively. In group 1, 14 and 17 patients developed anemia and neutropenia, while in group 2, 58 and 77 patients developed these side effects. The differences in acute hematologic toxicity between the two patient groups was significant (P = 0.001 for anemia, P = 0.000 for neutropenia). Fewer cases of hematologic toxicity happened in group 1 than in group 2. Ten patients developed thrombocytopenia. The incidence of this was 3.8% and 7.5% in groups 1 and 2, respectively. There was no statistical difference between the patient groups in terms of the incidence of subcutaneous, gastrointestinal, or genitourinary toxicities ().
Table 3 Toxicities stratified by patient group
Discussion
Cancer of the uterine cervix is mainly a disease of middle-aged and older women.Citation15,Citation16 Multiple studies have demonstrated that older women have advanced-stage disease at the time of diagnosis. Brun et al reported that older women present with more advanced disease in France.Citation17 Similarly, Ioka et al reported older women in Japan to present with a later stage at diagnosis and have a poorer outcome, likely from underutilization of Pap smears.Citation11 We discovered a majority of older patients in our study had advanced-stage disease (62.9%) at diagnosis. This percentage of patients with advanced-stage cervical cancer appears to be significantly higher than the percentage of advanced disease found in large population studies.
Although large population-based studies have also demonstrated that survival for cervical cancer is inversely correlated with stage, survival among older women regardless of stage has been reported to be worse than women in their 40s and 50s.Citation17,Citation18 But in our study, there was no statistically significant difference between the two groups in disease-free survival and overall survival in spite of older patients being treated less aggressively than younger women. This is in accordance with Lindegaard et al, who found that age was not a significant variable in any of the investigated end points when standard treatment protocols were completed by reviewing radiotherapy treatment in 114 women with a median age of 75.5 years.Citation19 Our data support the view that outcomes in older women may not be independently correlated with age alone.
Since it is well established that lymph-node metastases are a poor prognostic factor in cervical cancer,Citation20–Citation23 several studies evaluated surgically staged patients of all ages with locally advanced cervical cancer and reported lymph-node metastases ranging from 26.7% to 71%.Citation20,Citation22–Citation24 Some study found 65.2% of patients over age 60 years with stage IB2–IVB who were surgically staged had positive lymph nodes. Larger reviews and multiple series report approximately 20%–50% of pelvic lymph nodes and 10%–30% of paraaortic lymph nodes will contain metastases with locally advanced tumors.Citation3 In our study, we investigated the incidence of lymph-node metastases in women aged 65 years and older with advanced cervical cancer and found only eight of 48 (16.7%) patients with pelvic and/or paraaortic lymph-node metastasis on CT scan.
Survival and lymph-node metastasis incidence of very elderly patients seems to be similar with patients younger than 65 years; different etiologies should be sought in order to explain these findings. Well-known prognostic factors, such as performance status, relapse in an irradiated field, and metastases, were similar among young and old women. Since there were no differences in the disease characteristics or the treatment allocated between age-groups, a possible explanation could be a difference in the biological characteristics of cervical cancer among patients of different ages. According to some reports, one in younger and one in older women, this bimodal incidence may be related to a difference in the etiology, biology, or risk factors between age-groups.Citation25,Citation26 Maybe the biological characteristics of cervical cancer are related to HPV. HPV has been reported as a necessary cause of invasive cervical cancer, and is detectable in virtually all patients with cervical cancer.Citation27 It also plays a role in the etiology of noncervical cancers.Citation28–Citation32 The fact is that HPV infection with oncogenic strains is necessary for the development of cervical cancer and that more than 70% of the cervical cancers are attributed to types 16/18. Our results match this (42 women tested positive for HPV 16, while 32 women tested positive for HPV 18). This age-group may be at higher risk for more virulent HPV strands, resulting in more aggressive tumor histology secondary to physiologic changes of the cervix. Castle et al have demonstrated that physiologic changes of the cervix that occur with aging alter the HPV subtypes found in older women.Citation25 But in our study, this age-group is at lower risk for more virulent HPV strands. Older patients who did not complete concurrent chemotherapy were also able to achieve 3-year local control, disease-free survival, and overall survival that was comparable to younger patients. This might be associated with less aggressive tumor biological characteristics of cervical cancer, since HPV is important to biological characteristics.
Some trials also found that age is an important factor for the selection and allocation of treatment, especially in advanced disease. Elderly cervical cancer patients are usually treated with less aggressive treatments than their younger counterparts, because of considerations concerning patient safety.Citation33 Based on research, age especially influenced the therapy of choice: radiotherapy or chemoradiation. It is known that the elderly are less likely to receive aggressive therapy. Older women are more likely than their younger counterparts to refuse aggressive treatment.Citation34,Citation35 Our study showed that for elderly patients, radiotherapy remained the most common treatment chosen. This fact might not influence the survival of elderly patients, which may reflect different biological behavior of the tumor in the case of older patients. What is more, it reduced acute hematologic toxicity. We also found that younger women had anemia and neutropenia more frequently than older women, which may be related to older women being more likely to get radiotherapy instead of chemo.
In conclusion, cervical cancer has the same prognosis in old and young women. Age may not be an independent increased risk factor for death in women with cervical cancer, and the older age-group is at lower risk for virulent HPV strands (HPV 16/18) compared to younger patients. Treatment recommendations were implemented less for older patients. Radiotherapy remained the most common treatment chosen for elderly patients. The findings of this study confirm our clinical impressions and provide some important information with which to move forward in developing research for elderly patients. This confirms that increased attention should be given to the elderly patient, and the development of age-specific guidelines may therefore be warranted.
However, the study has some limitations. First, the study’s results are limited by its retrospective design and the fact that the data are from a single institution, and we cannot exclude the possibility that other factors correlated with survival. Secondly, older people are less likely to get chemo than younger women, and are obviously less likely to have hematologic toxicities. Thus, that may possibly introduce more bias and skew results. Finally, decreased immunity due to aging should be discussed, as it is highly relevant to the target population. Therefore, further research about older patients with cervical cancer should be undertaken in the future.
Acknowledgment
This work is supported by the Foundation of the First Affiliated Hospital, Medical College, Xi’an Jiaotong University (2010YK3). People’s Republic of China.
Disclosure
The authors declare that they have no competing interests.
References
- WaggonerSECervical cancerLancet200336193762217222512842378
- US National Institutes of HealthCancer of the cervix uteri2005 Available from: http://www.cancer.govAccessed March 20, 2013
- FoxKVShahCASwisherEMAn evaluation of cervical cancer in women age sixty and overGynecol Oncol20081091535818255127
- SpanosWJJrKingAKeeneyEWagnerRSlaterJMAge as a prognostic factor in carcinoma of the cervixGynecol Oncol198935166682792904
- HuangHJChangTCHongJHPrognostic value of age and histologic type in neoadjuvant chemotherapy plus radical surgery for bulky (>/=4 cm) stage IB and IIA cervical carcinomaInt J Gynecol Cancer200313220421112657125
- WrightJDGibbRKGeevargheseSCervical carcinoma in the elderly. An analysis of patterns of care and outcomeCancer20051031859115540239
- ChenRJLinYHChenCAHuangSCChowSNHsiehCYInfluence of histologic type and age on survival rates for invasive cervical carcinoma in TaiwanGynecol Oncol199973218419010329032
- JunorEJSymondsRPWatsonERLamontDWSurvival of younger cervical carcinoma patients treated by radical radiotherapy in the west of Scotland 1964–1984Br J Obstet Gynaecol19899655225282757979
- van der GraafYPeerPGZielhuisGAVooijsPGCervical cancer survival in Nijmegen region, the Netherlands, 1970–1985Gynecol Oncol198830151563366394
- WimbergerPLehmannNKimmigRImpact of age on outcome in patients with advanced ovarian cancer treated within a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR)Gynecol Oncol2006100230030716199079
- IokaATsukumaHAjikiWInfluence of age on cervical cancer survival in JapanJpn J Clin Oncol200535846446916006573
- KasuyaGOgawaKIrahaSPostoperative radiotherapy for uterine cervical cancer: impact of lymph node and histological type on survivalAnticancer Res20133352199220423645776
- RazakNAMnKZubairiYZNaingNNZakiNMEstimating the five-year survival of cervical cancer patients treated in Hospital Universiti Sains MalaysiaAsian Pac J Cancer Prev201314282582823621246
- National Cancer InstituteCommon Terminology Criteria for Adverse Events v3.0 (CTCAE)Bethesda (MD)National Cancer Institute2006 Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed May 31, 2013
- BulkSVisserORozendaalLVerheijenRHMeijerCJCervical cancer in the Netherlands 1989–1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger womenInt J Cancer200511361005100915515017
- KastritisEBamiasABozasGThe impact of age in the outcome of patients with advanced or recurrent cervical cancer after platinum-based chemotherapyGynecol Oncol2007104237237617030353
- BrunJLStoven-CamouDTrouetteRLopezMCheneGHockéCSurvival and prognosis of women with invasive cervical cancer according to ageGynecol Oncol200391239540114599872
- CokerALDuXLFangSEgglestonKSSocioeconomic status and cervical cancer survival among older women: findings from the SEER-Medicare linked data cohortsGynecol Oncol2006102227828416434087
- LindegaardJCThranovIREngelholmSARadiotherapy in the management of cervical cancer in elderly patientsRadiother Oncol200056191510869749
- GoffBAMuntzHGPaleyPJTamimiHKKohWJGreerBEImpact of surgical staging in women with locally advanced cervical cancerGynecol Oncol199974343644210479506
- TakedaNSakuragiNTakedaMMultivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomyActa Obstet Gynecol Scand200281121144115112519111
- HertelHKöhlerCElhawaryTMichelsWPossoverMSchneiderALaparoscopic staging compared with imaging techniques in the staging of advanced cervical cancerGynecol Oncol2002871465112468341
- MarnitzSKöhlerCRothCFüllerJHinkelbeinWSchneiderAIs there a benefit of a pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer?Gynecol Oncol200599353654416126259
- SakuragiNSatohCTakedaNIncidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stage IB, IIA, IIB cervical carcinoma treated with radical hysterectomyCancer19998571547155410193945
- CastlePEJeronimoJSchiffmanMAge-related changes of the cervix influence human papillomavirus type distributionCancer Res20066621218122416424061
- HoGYBiermanRBeardsleyLChangCJBurkRDNatural history of cervicovaginal papillomavirus infection in young womenN Engl J Med199833874234289459645
- WalboomersJMJacobsMVManosMMHuman papillomavirus is a necessary cause of invasive cervical cancer worldwideJ Pathol19991891121910451482
- GillisonMLShahKVChapter 9: Role of mucosal human papillomavirus in nongenital cancersJ Natl Cancer Inst Monogr200331576512807947
- ChuangLCChenHCYouSLAssociation between human papillomavirus and adenocarcinoma of rectum and recto-sigmoid junction: a cohort study of 10,612 women in TaiwanCancer Causes Control201021122123212820721617
- D’SouzaGKreimerARViscidiRCase-control study of human papillomavirus and oropharyngeal cancerN Engl J Med2007356191944195617494927
- GotoALiCPOtaSHuman papillomavirus infection in lung and esophageal cancers: analysis of 485 Asian casesJ Med Virol20118381383139021678442
- SmithJSBackesDMHootsBEKurmanRJPimentaJMHuman papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursorsObstet Gynecol2009113491792419305339
- GoodheartMJacobsonGSmithBJZhouLChemoradiation for invasive cervical cancer in elderly patients: outcomes and morbidityInt J Gynecol Cancer20081819510317466049
- van der AaMASieslingSv d Poll-FranseLVSchutterEMLybeertMLCoeberghJWAge-specific differences in the treatment of cervical cancer in the east and the south of the Netherlands 1989–2004Eur J Obstet Gynecol Reprod Biol20091471788219713027
- RoseJHO’TooleEEEinstadterDPatient age, well-being, perspectives, and care practices in the early treatment phase for late-stage cancerJ Gerontol A Biol Sci Med Sci200863996096818840801