Abstract
Background
Osteoporosis and other bone degenerative diseases are among the most challenging non-communicable diseases to treat. Previous works relate bone loss due to osteoporosis with oxidative stress generated by free radicals and inflammatory cytokines. Alternative therapy to hormone replacement has been an area of interest to researchers for almost three decades due to hormone therapy-associated side effects.
Methods
In this study, we investigated the effects of gamma-amino butyric acid (GABA), gamma-oryzanol (ORZ), acylated steryl glucosides (ASG), and phenolic extracts from germinated brown rice (GBR) on the expression of genes related to bone metabolism, such as bone morphogenic protein-2 (BMP-2), secreted protein acidic and rich in cysteine (SPARC), runt-related transcription factor 2 (RUNX-2), osteoblast-specific transcription factor osterix (Osx), periostin, osteoblast specific factor (Postn), collagen 1&2 (Col1&2), calcitonin receptor gene (CGRP); body weight measurement and also serum interleukin-6 (IL-6) and osteocalcin, in serum and bone. Rats were treated with GBR, ORZ, GABA, and ASG at (100 and 200 mg/kg); estrogen (0.2 mg/kg), or remifemin (10 and 20 mg/kg), compared to ovariectomized non-treated group as well as non-ovariectomized non-treated (sham) group. Enzyme-linked immunosorbent assay was used to measure the IL-6 and osteocalcin levels at week 2, 4, and 8, while the gene expression in the bone tissue was determined using the Genetic Analysis System (Beckman Coulter Inc., Brea, CA, USA).
Results
The results indicate that groups treated with GABA (100 and 200 mg/kg) showed significant upregulation of SPARC, calcitonin receptor, and BMP-2 genes (P < 0.05), while the ORZ-treated group (100 and 200 mg/kg) revealed significant (P < 0.05) upregulation of Osx, Postn, RUNX-2, and Col1&2. Similarly, IL-6 concentration decreased, while osteocalcin levels increased significantly (P < 0.05) in the treated groups as compared to ovariectomized non-treated groups.
Conclusion
GABA and ORZ from GBR stimulates osteoblastogenesis by upregulation of bone formation genes, possibly via the activation of GABAB receptors and by inhibiting the activity of inflammatory cytokines and reactive oxygen species. Therefore, it could be used effectively in the management of osteoporosis.
Introduction
Diet plays an important role in modifying gene expression in both healthy and diseased conditions. The changes and modifications in gene expression in bone tissue explain a developmental sequence that has three principal periods: the proliferation stage, extracellular matrix maturation, and mineralization period.Citation1,Citation2 Bone metabolism is a complex mechanism controlled by a number of factors including genetic, environmental, and lifestyle.Citation3 Genetic factors play a significant role in the development of skeletal malformations which have been estimated to account for 70%–80% of the variance in bone density (BMD), and also 25%–35% risk for bone fractures.Citation4–Citation6 Cells in bone tissues are divided into the osteoblast, which are mainly involved in bone formation and mineralization;Citation7 they are derived from pluripotent mesenchymal stem cells which differentiate into chondrocytes, adipocytes, myoblasts, fibroblasts, osteoclasts, and osteocytes.Citation8,Citation9 The osteocytes play a role in responding to mechanical stimuli, which initiate a modeling or remodeling response, while the osteoclasts are bone resorbing cells from hematopoietic precursors of the monocyte/macrophage lineage and are associated with osteoclastogenesis.Citation7,Citation10,Citation11 Bone morphogenic protein-2 (BMP-2) is a potent osteo-inductive factor which induces the osteogenic differentiation of mesenchymal cells,Citation12,Citation13 secreted protein acidic and rich in cysteine (SPARC) is a secreted calcium-binding glycoprotein that regulates mineralization of bone tissues in mammals,Citation14,Citation15 and runt-related transcription factor 2 (RUNX-2) plays an important role in osteogenesis.Citation16,Citation17 Osterix (OSX) has been described as a transcriptional factor for osteoblast differentiation.Citation18,Citation19 Osteocalcin is a major non-collagenous protein of the bone matrix – osteoblasts synthesize osteocalcin and after production, part of the osteocalcin is incorporated into the bone matrix while the other part goes into the circulatory system. Circulating osteocalcin is regarded as a specific marker for bone formation due to its association with the changes in the rate of bone turnover.Citation20 Interleukin-6 (IL-6) is an inflammatory marker that is strongly associated with an increase in estrogen (EST) deficiency, especially in osteoporosis.Citation21 Germinated brown rice, a brown rice subjected to sporulation, has been reported to increase the concentration of its bioactives. The most important is the natural tranquilizer, gamma-amino butyric acid (GABA), which is an amino acid and a neurotransmitter that fosters communication between nerve cells. It improves relaxation and sleep and also plays an active role in seizure prevention and alleviation of chronic pain.Citation22 GABA is known as the major inhibitory neurotransmitter found in the central nervous system. Its major role is the regulation of neuron excitability in the nervous system and it has been directly implicated in the regulation of muscle tone.Citation23 The concentration of GABA in brown rice increases dramatically to about 10-fold after germination.Citation24–Citation28 Oryzanol (ORZ), which as been describe as a powerful antioxidant, has also been shown to increase during the process.Citation25 In the present work, we studied the expression of seven genes related to bone metabolism in ovariectomized (OVX) rats treated with germinated brown rice (GBR)-phenolics, ORZ, GABA, acylated steryl glucosides (ASG), remifemin (REM), or EST to determine the role of these compounds in stimulating bone formation.
Materials and methods
Brown rice, drugs, and chemicals
Brown rice (BR) is from the Malaysian rice variety (MR220) which was supplied by PadiBeras Nasional (BERNAS) factory (Sri Tiram Jaya, Malaysia). The germination procedures were carried out as explained in our previous publication.Citation29 GABA was extracted and quantified using a high performance liquid chromatography diode array detector (Agilent Technologies, Santa Clara, CA, USA) and the same principles as described by Rozan et al,Citation30 while ORZ was analyzed applying the method reported by Azlan et al.Citation31 ASG was extracted and analyzed as reported by Usuki et al.Citation32Cimicifuga racemosa (Remifemin® 20 mg/tab) was purchased from Schaper and Brummer (Salzgitter, Germany). The conjugated EST (Premarin® 0.625 mg/tab) was procured from Wyeth Ireland Newbridge, Co (Kildare, Ireland) and the Rat-MID Osteocalcin enzyme immunoassay (EIA) (Ref no AC-12F1) was from Immunodiagnostic Systems (Boldon Colliery, UK). The Rat IL-6 enzyme-linked immunosorbent assay (ELISA) kit (Ref no ER3IL6), was from Thermo Scientific (Rockford, IL, USA).
Experimental animals, grouping, and dosing
Sprague Dawley rats weighing approximately 250–260 g were used in this study. They were divided into 13 groups of six rats each (78 rats). The rats were acclimatized for 2 weeks before commencement of the experiment. The study was carried out according to the guidelines for the use of animals as approved by the Animal Care and Use Committee Faculty of Medicine with approval number UPM/FPSK/PADS/UUH/F01, University Putra Malaysia. The rats in group 1 were sham operated by exposing the ovaries and returning them back to their anatomical position. Bilateral ovariectomy was performed on the rats in groups 2–13 under general anesthesia. Treatments were given orally, starting from 2 weeks after the surgery, and were given once daily for a period of 8 weeks. Group 2 rats were OVX without treatment; groups 3, 4, and 5 were treated with 0.2 mg/kg EST, 10 and 20 mg/kg REM, respectively; groups 6 and 7 rats were treated with GBR-phenolics at the dose rates of 100 and 200 mg/kg, respectively; groups 8 and 9 were treated with ASG at 100 and 200 mg/kg, respectively; groups 10 and 11 were treated with 100 and 200 mg/kg of GABA, respectively; while groups 12 and 13 were each treated with ORZ at 100 and 200 mg/kg, respectively.
Body weight measurements
Rats were weighed using a weighing scale before the surgery 2 weeks after the surgery just before the commencement of the treatments, and at weeks 2, 4, and 8 after the commencement of the treatments.
IL-6 and osteocalcin ELISA assays Serum IL-6
The Rat IL-6 ELISA kit was used for the assay as per the manufacturer instructions. Briefly 100 μL of the standard or serum samples was added to each well of the 96-well plate, incubated for 2 hours at 20°C–25°C, and washed five times with water. Biotinylated antibody (100 μL) was added to each well, the plate was covered and incubated at room temperature for 1 hour. Streptavidin-horseradish peroxidase solution (100 μL) was then added to each well after washing and incubated at room temperature for 30 minutes. Plates were washed and 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate was added to each well before developing in the dark for 30 minutes; 50 μL of the stop solution was then added to each well, and the absorbance was read at 450 nm using a spectrophotometer, Thermo Labsystem ELISA reader OPSYS MR (Thermo life science, Basingstoke, UK).
Serum osteocalcin level
Rat-MID osteocalcin EIA, was used for osteocalcin quantification, following the manufacturer instructions. Briefly, 100 μL of biotinylated osteocalcin was added to each well of a streptavidin pre-coated 96-well plate, covered using sealing tape, incubated for 30 minutes at 20°C on a microtiter mixing apparatus (300 rpm), and washed five times with washing solution. Twenty milliliters of the standard control, or the unknown serum sample were pipetted into the appropriate wells followed by the addition of the primary antibody (150 μL), which was earlier prepared by mixing the primary antibody and primary incubation buffer in a ratio of 1:100. The strips were covered and incubated for 1 hour at room temperature on a mixing apparatus; the plates were then washed before the addition of the secondary antibody (100 μL). These were covered and incubated for another hour at room temperature. The plates were washed and a substrate solution (100 μL) was added to each well and incubated for 15 minutes at room temperature in the dark on the plate mixing apparatus. The reaction was then stopped by adding 100 μL of the stopping solution to each well and the absorbance was measured using a spectrophotometer at 450 nm using 650 nm as the reference. All solutions were equilibrated to room temperature.
RNA isolation
RNA (ribonucleic acid) was isolated from the bone tissue immediately after sacrifice using the HiYield Total RNA Mini Kit® (Real Biotech, Taipei, Taiwan) according to the manufacturer’s instructions. Briefly, 25 mg of bone tissue from the femur was cracked using a bone cracker and then immediately transferred into a 1 mL Eppendorf tube containing 400 μL of lysis buffer on ice. β-mercaptoethanol (4 μL) was added and a micropestle was used to grind the tissue for about 3 minutes, and then incubated at room temperature for 5 minutes. The sample was transferred to a lysate filter column placed on a 2 mL collection tube and centrifuged at 1000 rpm/4°C for 1 minute. Seventy percent absolute ethanol (400 μL) was added to the filtrate and the mixture was shaken vigorously. The ethanol added mixture was then transferred to an RNA binding column placed in a 2 mL collection tube and centrifuged at 12,000 rpm/4°C for 2 minutes, and the flow-through 25 μL of DNAse was then added to the center of the column and left to stand for 20 minutes.
RNA yield, purity, and integrity
RNA yield is reported based on the absorbance at 260 nm using a Nanodrop spectrophotometer which was further confirmed using the Agilent RNA 6000 Nano Lab-Chip Kit and the Agilent 2100 Bioanalyzer (Agilent Technologies). The quality of the isolated RNA was determined using the Agilent 2100 Bioanalyzer; an absorbance ratio at 260 nm and 280 nm, along with the ratio of the absorbance at 260 nm and 230 nm were used in detecting the quality. Quantification of the RNA quality is reported in an RNA Integrity Number (RIN), which is the total RNA produced in the analysis. A RIN of more than or equal to 7 indicates that the RNA is suitable for gene expression study89
Primer design
Primers stocks were diluted in nuclease-free water to a concentration of 500 nM for the reverse primer, and 200 nM for the forward primer. The size ranged from 145–224 nucleotides, with a 6-nucleotide minimum separation size between polymerase chain reaction (PCR) products. In addition to the seven genes of interest, each panel contained an internal control gene and normalization genes. The reverse primers consisting of 20 nucleotides complementary to the target were tagged to a 19-nucleotide universal reverse sequence, while the forward primers consisted also of 20 nucleotides corresponding to the target gene tagged to an 18-nucleotide universal forward sequence ().
Table 1 Serum IL-6 concentration (pg/mL) in OVX rats treated with GBR-phenolic compounds, ASG, GABA, ORZ, EST, or REM over a period of 8 weeks
Table 2 Body weight (g) in OVX rats treated with GBR-phenolic compounds, ASG, GABA, ORZ, EST, or REM over a period of 8 weeks
Table 3 Serum osteocalcin concentration in OVX rats treated with GBR-phenolic compounds, ASG, GABA, ORZ, EST, or REM over a period of 8 weeks
Table 4 Gene description, accession number, primer sequence, and the product size of the selected genes
cDNA synthesis
From each sample, 250 ng of RNA was reverse transcribed to complementary DNA (cDNA) using multiplex universal reverse primers. Reactions were performed following the protocols stated in the GenomeLab™ Start Kit (Beckman Coulter, Brea, CA, USA) using a thermal-cycler at 48°C for 1 minute; 37°C for 5 minutes; 42°C for 60 minutes; 95°C for 5 minutes, and holding at 4°C.
PCR amplification
The PCR was carried out in a reaction mixture containing the cDNA from the reverse transcription reaction product (9.3 μL), 200 nM forward universal primer set mix (2 μL), 25 mM MgCl2 (4 μL), Thermo Start Taq DNA polymerase (0.7 μL), and 5 × PCR Master Mix buffer (4 μL). Amplification was done initially by denaturation at 95°C for 10 minutes, then by 35 two-step cycles of 94°C for 30 seconds and 55 °C for 30 seconds, ending in a single extension cycle of 68°C for 1 minute in an XP Thermal Cycler.
GeXP multiplex data analysis
The GeXPS machine (Beckman Coulter, Brea, CA, USA) was used to separate PCR products based on size by capillary gel electrophoresis and to measure their dye signal strength in arbitrary units (AU) of optical fluorescence, which is the fluorescent signal minus background. PCR products were diluted in water at a ratio of 2:8, and 1 μL of this solution was added to a 38.5 μL sample loading solution with 0.5 μL of DNA size standard 400.
Fragment analysis and gene expression signature analysis
Data were initially analyzed using the Fragment Analysis module of the gene expression machine before importing into a profiler software. Control genes were tested for result consistency and the cyclophilin A gene gave consistent results, so it was chosen for normalizing the data for all the genes of interest.
Statistical analysis
Data were expressed as mean ± standard deviation, and the means were compared using ANOVA (analysis of variance), while the differences between the means were determined via the Tukey–Kramer post-hoc test using JMP 10 statistical software (SAS Institute, Cary, NC, USA). Values for P < 0.05 were considered statistically significant.
Results
Body weight
There was no significant difference found in terms of weight between the groups just before the initiation of treatments and 2 weeks after the surgery; a steady increase in body weight was observed in all the groups from week 2 to week 8 (). A slight increase in body weight, though not significant (P > 0.05) in all the OVX treated groups was observed at week 2 after treatment, compared to the sham non-OVX group (). A significant increase in weight was observed in the OVX non-treated group compared to sham and all the OVX treated groups at weeks 4 and 8 after treatment (P < 0.05), respectively (). No significant difference (P > 0.05) between the EST 0.2 mg/kg treated group and the sham non-OVX group was found at week 8 of treatment ().
Serum IL-6 and osteocalcin ELISA
Serum IL-6 increased significantly at weeks 2, 4, and 8 in the OVX-non-treated group compared to the sham and other OVX treated groups (P < 0.05) as shown in . IL-6 concentration decreased significantly in groups treated with GABA 200 mg/kg; the sham non-OVX group; and groups treated with EST, ORZ, and ASG at 200 mg/kg; and REM 20 mg/kg (P < 0.05) at weeks 2, 4, and 8 (). The osteocalcin level significantly increased in groups treated with EST and ORZ at 200 mg/kg and the sham non-OVX group compared to the other treated groups, at week 2 (P < 0.05). The concentration also significantly increased (P < 0.05) at weeks 4 and 8 after treatment in groups treated with EST, REM 20 mg/kg, ASG, ORZ, GABA, and GBR at 200 mg/kg ().
Gene expression
Results for the primer sequence generated from the National Center for Biotechnology Information (NCBI) with the genes’ descriptions, their accession numbers, and product sizes are described in . ATP synthase, cyclophilin A, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and Actb serve as the housekeeping genes, with KANr as the internal control, while the other seven genes are related to bone formation/metabolism as shown in . Results for the relative expression of the seven genes are described in –.
Figure 1 Relative mRNA expression of CGRP gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger ribonucleic acid; CGRP, calcitonin receptor.
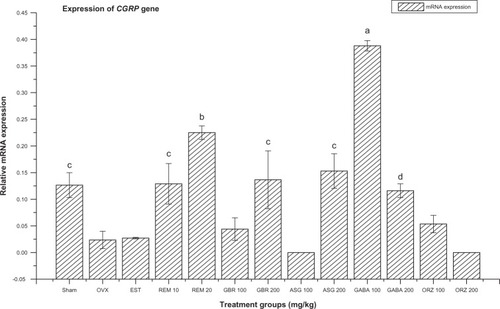
Expression of calcitonin receptor gene (CGRP)
The group treated with GABA at 100 mg/kg gave the highest significant upregulation (P < 0.05) of CGRP compared to the OVX-non-treated group, non-OVX, non-treated (sham) group, and groups treated with ASG, ORZ, and GBR at 100 and 200 mg/kg, EST at 0.2 mg/kg, and GABA at 200 mg/kg (). The groups treated with REM at 20 mg/kg and ASG at 200 mg/kg were significantly upregulated (P < 0.05) compared to OVX non-treated group and groups treated with ORZ at 100 and 200mg/kg, ASG at 100 mg/kg, GBR at 100 mg/kg, and EST at 0.2 mg/kg ().
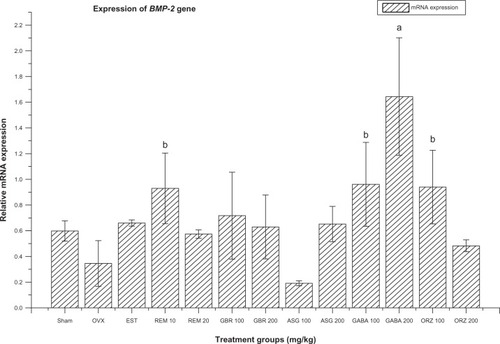
Expression of BMP-2 gene
Group treated with GABA at 200 mg/kg showed the highest significant upregulation in the expression of BMP-2 (P < 0.05) compared to the OVX-non-treated group, non-OVX non-treated (sham), and groups treated with ASG at 100 and 200 mg/kg, ORZ at 200 mg/kg, REM at 20 mg/kg, GBR at 200 mg/kg, and EST at 0.2 mg/kg ().
Figure 2 Relative mRNA expression of BMP-2 gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated groups.
Abbreviations: ASG, acylated steryl glucosides; BMP, bone morphogenic protein; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger ribonucleic acid.
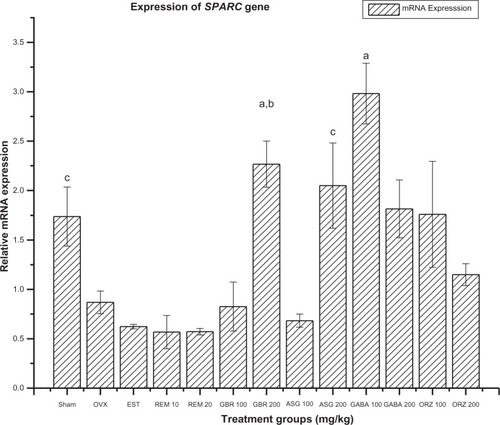
Expression of SPARC gene
The group treated with GABA at 100 mg/kg showed significant (P < 0.05) expression of the SPARC gene compared to the OVX-non-treated group, the non-OVX non-treated (sham), and groups treated with REM at 10 and 20 mg/kg, EST at 0.2 mg/kg, ASG at 100 and 200 mg/kg, GBR at 100 mg/kg, GABA at 200 mg/kg, and ORZ at 100 and 200 mg/kg (). The group treated with GBR at 200 mg/kg was significantly different (P < 0.05) to the OVX-non-treated group and groups treated with GBR at 100 mg/kg, REM at 10 and 20 mg/kg, EST, ASG at 100 mg/kg, and ORZ at 200 mg/kg. The group treated with ORZ at 100 mg/kg showed significant upregulation of the SPARC gene (P < 0.05) compared to groups treated with ASG at 100 mg/kg, REM at 10 and 20 mg/kg, and EST at 0.2 mg/kg (). The group treated with ASG at 200 mg/kg was upregulated (P < 0.05) when compared to the OVX-non-treated group, groups treated with REM at 10 and 20 mg/kg, EST, and ASG at 100 mg/kg, while the OVX-non-treated group and groups treated with EST 0.2 mg/kg, ASG 100 mg/kg, and GBR 100 mg/kg were downregulated compared to the sham (non-OVX, non-treated) groups ().
Figure 3 Relative mRNA expression of SPARC gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger ribonucleic acid; SPARC, secreted protein acidic and rich in cysteine.
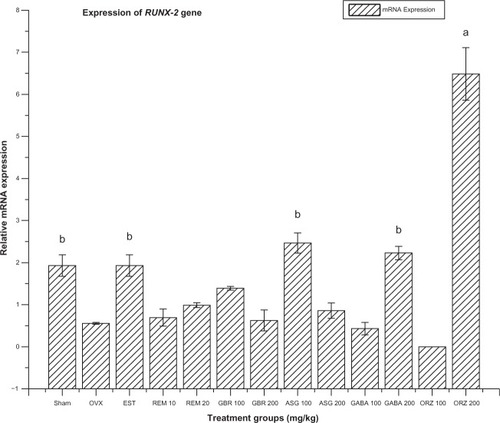
Expression of RUNX-2 gene
The group treated with ORZ at 200 mg/kg was highly upregulated (P < 0.05) in the expression of the RUNX-2 gene compared to the OVX-non-treated group, sham, EST, and those groups treated with ORZ at 100 mg/kg, GABA at 100 and 200 mg/kg, GBR at 100 and 200 mg/kg, REM at 100 and 200 mg/kg, and ASG at 100 and 200 mg/kg (). The group treated with ASG at 100 mg/kg also differed significantly (P < 0.05) from the OVX-non-treated group and the groups treated with GBR at 100 and 200 mg/kg, REM at 10 and 20 mg/kg, ASG at 200 mg/kg, and GABA and ORZ at 100 mg/kg (). The group treated with EST at 0.2 mg/kg showed significant upregulation compared with the OVX-non-treated group, ORZ at 100 mg/kg, REM at 10 mg/kg, GBR at 200 mg/kg, and ASG at 200 mg/kg (P < 0.05). The OVX-non-treated group and groups treated with ORZ and GABA at 100 mg/kg, GBR and ASG at 200 mg/kg, and REM at 10 mg/kg were significantly downregulated compared to the non-OVX, non-treated (sham) group (P < 0.05). The GABA 200 mg/kg treated group was also upregulated and significantly different to the OVX-non-treated group, as well as groups treated with GABA at 100 mg/kg, GBR and ASG at 200 mg/kg, and REM at 10 and 20 mg/kg. Lastly, the groups treated with GBR at 100 mg/kg and REM at 20 mg/kg were significantly upregulated (P < 0.05) compared to the group treated with ORZ at 100 mg/kg ().
Figure 4 Relative mRNA expression of RUNX-2 gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger ribonucleic acid; RUNX-2, runt-related transcription factor 2.
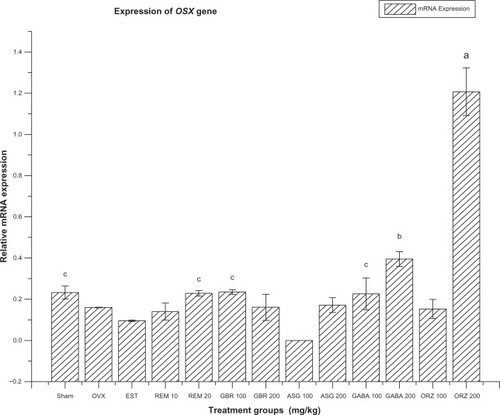
Expression of OSX gene
The OSX gene expression was significantly upregulated (P < 0.05) in the group treated with ORZ at 200 mg/kg when compared to the OVX-non-treated group, ORZ at 100 mg/kg group, sham, and groups treated with GBR at 100 and 200 mg/kg, REM at 10 and 20 mg/kg, and GABA at 100 and 200 mg/kg (). The group treated with GABA at 200 mg/kg showed a significant upregulation compared to the OVX-non-treated group and the groups treated with ASG at 100 and 200 mg/kg, ORZ at 100 mg/kg, EST at 0.2 mg/kg, REM at 10 mg/kg, and GBR at 200 mg/kg. The group treated with ASG at 100 mg/kg was significantly (P < 0.05) downregulated compared to the sham (non-OVX, non-treated) group, and groups treated with REM at 20 mg/kg and GABA at 100 mg/kg ().
Figure 5 Relative mRNA expression of Postn gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger RNA; Postn, periostin, osteoblast specific factor.
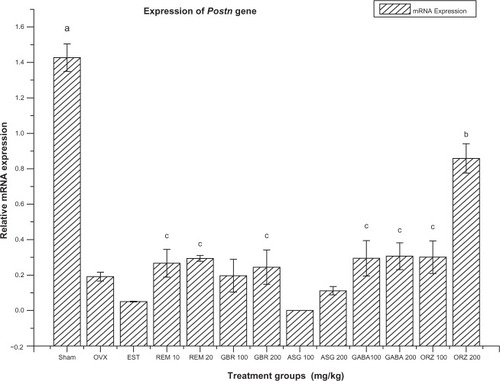
Expression of Postn gene
The OVX-non-treated group, and groups treated with ASG at 100 and 200 mg/kg, GBR at 100 and 200 mg/kg, REM at 10 and 20 mg/kg, ORZ at 100 and 200 mg/kg, GABA at 100 and 200 mg/kg, and EST 0.2 mg/kg were significantly downregulated in the expression of the Postn gene compared to the sham (non-OVX, non-treated) group (). The group treated with ORZ at 200 mg/kg showed upregulation (P < 0.05) compared to the OVX-non-treated group and groups treated with ASG at 100 and 200 mg/kg, GBR at 100 and 200 mg/kg, REM at 10 and 20 mg/kg, GABA at 100 and 200 mg/kg, EST at 0.2 mg/kg, and ORZ at 100 mg/kg. The groups treated with GABA at 100 and 200 mg/kg, ORZ at 100 mg/kg, ORZ at 100 mg/kg and REM at 20 mg/kg showed significant upregulation compared to the group treated with ASG at 100 mg/kg ().
Figure 6 Relative mRNA expression of OSX gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger ribonucleic acid; OSX, osterix.
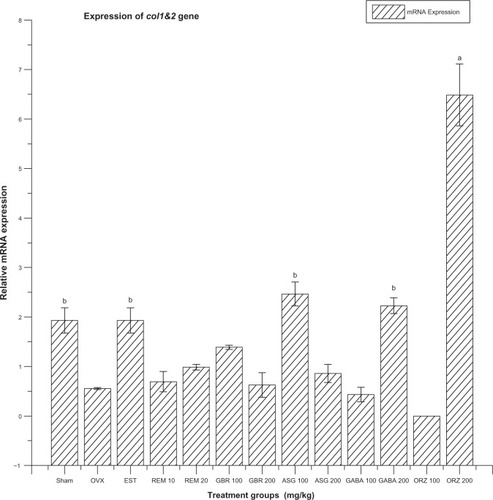
Expression of collagen 1&2 (col1&2) gene
The OVX-non-treated group, and groups treated with GABA at 100 and 200 mg/kg, REM at 10 and 20 mg/kg, ASG at 200 mg/kg, EST at 0.2 mg/kg, and ORZ at 200 mg/kg were significantly downregulated (P < 0.05) in the expression of col1&2 compared to the sham group (non-OVX, non-treated). The group treated with ORZ at 100 mg/kg was upregulated compared to the OVX-non-treated group, and groups treated with GABA at 200 mg/kg, REM at 10 and 20 mg/kg, GBR at 100 mg/kg, ASG at 200 mg/kg, EST at 0.2 mg/kg, and ORZ at 200 mg/kg (). The group treated with ASG at 100 mg/kg was significantly upregulated (P < 0.05) compared to groups treated with GBR at 100 mg/kg, REM at 10 and 20 mg/kg, and EST at 0.2 mg/kg. The OVX-non-treated group and groups treated with GBR at 100 mg/kg, REM at 10 and 20 mg/kg, EST at 0.2 mg/kg, and GABA at 200 mg/kg were significantly downregulated (P < 0.05) compared to groups treated with GABA at 100 mg/kg and ORZ at 200 mg/kg. The group treated with ASG at 200 mg/kg differed significantly (P < 0.05) to the group treated with EST at 0.2 mg/kg ().
Figure 7 Relative mRNA expression of col1&2 gene in OVX rats treated with EST, REM, GBR, GABA and ORZ in different doses compared to sham and OVX non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GABA, gamma-amino butyric acid; GBR, phenolic extracts from germinated brown rice; ORZ, gamma-oryzanol; OVX, ovariectomized; REM, remifemin; mRNA, messenger ribonucleic acid; col1&2, collagen 1&2.
Discussion
This study, examines the relative expression of genes related to bone metabolism, osteocalcin, and IL-6 levels in OVX rats treated with GBR-phenolics, ORZ, ASG, and GABA extracted from GBR, in comparison to groups treated with EST and REM. Total body weight increased in the OVX rats compared to the sham non-OVX group; as shown in , there was a difference of 52 g between the sham and the OVX group. Ovariectomies have been reported to increase body mass during the first 3 weeks after surgery.Citation33 Decreases in ovarian hormones in rats are associated with increased food intake and decreased motor activity, which leads to increased body mass.Citation34,Citation35 Treatment with EST and GBR bioactives for 8 weeks significantly reduced the increase in body weight induced by ovariectomy with EST given the most significant effect; this shows that GBR and its bioactive compounds has weaker estrogenic effect. Our data show that GABA and ORZ at 200 mg/kg decreased serum IL-6 and increased osteocalcin concentration. ORZ, a mixture of ferulic acid esters of triterpene alcohols, is known to possess powerful antioxidant and anti-inflammatory effects.Citation36–Citation38 In this study, we found that the calcitonin gene was upregulated in groups treated with GABA at 100 mg/kg when compared to the other treatment groups; likewise BMP-2 and SPARC were both upregulated in groups treated with GABA at 200 and 100 mg/kg, respectively. The neuroprotective, antioxidant, and memory enhancement properties attributed to the high content of GABA in GBR have been documented in other studies.Citation39–Citation41 It has been reported that GABA increases the level of human growth hormone, reduces fat mass, and increases bone mass density.Citation42–Citation44 The GABA receptor antagonist picrotoxin inhibits bone formation and decreases calcium content in rats at various growth sites of the skull.Citation45 Similarly, GABA may play a role in mechanisms associated with cellular proliferation, differentiation, and development through GABA(B) receptors (GABABR) expressed in cultured osteoblasts.Citation46 In the same vein, Takahata et alCitation47 showed that functional GABABR is predominantly expressed by osteoblasts rather than osteoclasts during bone remodeling as well as in skeletogenesis, and GABAergic signaling could be a novel target for the treatment of bone diseases. The upregulation of BMP-2, S PARC, and osteocalcin genes by GABA therefore may be attributed to the stimulation of GABAB receptors in the osteoblasts, which initiates the GABAergic signaling that in turn stimulates the expression of BMP-2, S PARC, and osteocalcin. It has been reported that functional GABABR are predominantly expressed by osteoblasts rather than osteoclasts to regulate cellular maturation related to BMP-2 expression.Citation47 Groups treated with ORZ at 200 and 100 mg/kg gave the highest upregulation in terms of expression of OSX, Postn, RUNX-2, and col1&2 compared to other treated and non-treated groups. ORZ is known for its anticholesteremic, antioxidant, anti-inflammatory, and anti-platelet aggregation properties.Citation48–Citation50 Ovariectomies have been shown to induce oxidative stress and impair bone antioxidant systems in adult rats.Citation51 The upregulation of these bone formation genes by ORZ may be attributed to their anti-inflammatory and antioxidant effects. Decreases in EST level in osteoporosis have been linked to upregulation of osteoclastogenesis through the activation of receptor activator of nuclear factor kappa-B ligand (RANKL) and a decrease in osteoprotegerin production by osteoblasts, as well as increased expression of cytokines such as tumor necrosis factor alpha, IL-1, and IL-6, in osteoblasts.Citation52–Citation54 ORZ has been shown to have an upregulatory effect on antioxidant genes.Citation55 In another study, nicotine was linked to oxidative stress which in turn led to downregulation of the expression of bone formation genes such as RUNX-2.Citation56
Conclusion
GABA-treated groups had the highest expression of the calcitonin gene, BMP-2, and SPARC compared to groups treated with GBR-phenolics, ORZ, ASG, EST, or REM. This is possibly due to the activation of GABAB-receptors. ORZ had the highest upregulation of the expression of RUNX-2, Osx, Postn, and col1&2 than the other groups due to its antioxidative, as well as anti-inflammatory effects. More studies are underway to evaluate the effects of these compounds on GABA receptors, and to elucidate the effect of the combination of these bioactives on the studied genes, to properly ascertain their clinical relevance in the management of osteoporosis and other related diseases.
Acknowledgments/disclosure
We wish to thank Padiberas National (BERNAS) rice company in Sri Tiram Jaya, Malaysia for funding this research. We also wish to acknowledge Dr Ibrahim Anka for his guidance on the statistical analysis. The authors report no conflicts of interest in this work.
References
- SteinGSLianJBOwenTARelationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiationFASEB J1990413311131232210157
- OwenTAAronowMShalhoubVProgressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrixJ Cell Physiol199014334204301694181
- RubinC-JLindbergJFitzsimmonsCDifferential gene expression in femoral bone from red jungleflowl and domestic chicken, differing for bone phenotypic traitsBMC genomics20078120817605776
- ArdenNBakerJHoggCBaanKSpectorTThe heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twinsJ Bone Miner Res19961145305348992884
- HarrisMNguyenTHowardGKellyPEismanJGenetic and environmental correlations between bone formation and bone mineral density: a twin studyBone19982221411459477237
- DengHWChenWMReckerSGenetic determination of Colles’ fracture and differential bone mass in women with and without Colles’ fractureJ Bone Miner Res20001571243125210893672
- CompstonJESex steroids and bonePhysiol Rev200181141944711152762
- SchroederAMuellerOStockerSThe RIN: an RNA integrity number for assigning integrity values to RNA measurementsBMC Mol Biol200671316448564
- CarterLEKilroyGGimbleJMFloydZEAn improved method for isolation of RNA from boneBMC Biotechnol2012121522260224
- Auf’mkolkBHauschkaPVSchwartzERCharacterization of human bone cells in cultureCalcif Tissue Int19853732282352990642
- OwenMFriedensteinAStromal stem cells: marrow-derived osteogenic precursorsCiba Found Symp198813642603068016
- WangEARosenVD’AlessandroJSRecombinant human bone morphogenetic protein induces bone formationProc Natl Acad Sci1990876222022242315314
- Ghosh-ChoudhuryNHarrisMAFengJQMundyGRHarrisSEExpression of the BMP 2 gene during bone cell differentiationCrit Rev Eukaryot Gene Expr199442–33453557881166
- RennJSchaedelMVolffJ-NGoerlichRSchartlMWinklerCDynamic expression of sparc precedes formation of skeletal elements in the Medaka (Oryzias latipes)Gene200637220821816545530
- KasugaiSNagataTSodekJTemporal studies on the tissue compartmentalization of bone sialoprotein (BSP), osteopontin (OPN), and SPARC protein during bone formation in vitroJ Cell Physiol199215234674771510790
- KomoriTYagiHNomuraSTargeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblastsCell19978957557649182763
- OttoFThornellAPCromptonTCbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone developmentCell19978957657719182764
- CaoYZhouZde CrombruggheBOsterix, a transcription factor for osteoblast differentiation, mediates antitumor activity in murine osteosarcomaCancer Res20056541124112815734992
- ZhangCTangWLiYYangFDowdDRMacDonaldPNOsteoblast-specific transcription factor Osterix increases vitamin D receptor gene expression in osteoblastsPloS one2011610e2650422028889
- GaumetNSeibelMCoxamVDaviccoMLebecquePBarletJInfluence of ovariectomy and estradiol treatment on calcium homeostasis during aging in ratsArch Physiol Biochem199710554354449439780
- PfeilschifterJKöditzRPfohlMSchatzHChanges in proinflammatory cytokine activity after menopauseEndocr rev20022319011911844745
- BabuPDSubhasreeRBhakyarajRVidhyalakshmiRBrown rice – beyond the color reviving a lost health food – a reviewAmerican-Eurasian Journal of Agronomy2009226772
- WatanabeMMaemuraKKanbaraKTamayamaTHayasakiHGABA and GABA receptors in the central nervous system and other organsInt Rev Cytol200221314711837891
- OhC-HOhS-HEffects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosisJ Med Food200471192315117548
- LeeJHChanges in nutritional components throughout germination in paddy rice and brown riceJ Food Sci Nutr2010152113119
- RoohinejadSOmidizadehAMirhosseiniHEffect of pregermination time on amino acid profile and gamma amino butyric acid (GABA) contents in different varieties of Malaysian brown riceInt J Food Propert201114613861399
- ImamMUMusaSNAzmiNHIsmailMEffects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 diabetic ratsInt J Mol Sci20121310129521296923202932
- SongtipPJangchudKJangchudATungtrakulPPhysicochemical property changes in germinated brown rice flour from different storage periods of paddy riceInt J Food Sci Technol2012474682688
- SaniIMIqbalSChanKWIsmailMEffect of acid and base catalyzed hydrolysis on the yield of phenolics and antioxidant activity of extracts from germinated brown rice (GBR)Molecules20121767584759422713349
- RozanPKuoY-HLambeinFFree amino acids present in commercially available seedlings sold for human consumption. A potential hazard for consumersJ Agric Food Chem200048371672310725139
- AzlanAIsmailMAbdul HamidAExtraction and determination of oryzanol in rice bran of mixed herbarium UKMB: AZ 6807: MR 185, AZ 6808: MR 211, AZ6809: MR 29ASEAN Food J20081518996
- UsukiSItoYMorikawaKEffect of pre-germinated brown rice intake on diabetic neuropathy in streptozotocin-induced diabetic ratsNutr Metab (Lond)2007412518036220
- ChenYHeimanMLIncreased weight gain after ovariectomy is not a consequence of leptin resistanceAm J Physiol Endocrinol Metab20012802E315E32211158936
- MookDGKenneyNJRobertsSNussbaumAIRodierWI3rdOvarian-adrenal interactions in regulation of body weight by female ratsJ Comp Physiol Psychol19728121985084436
- RoyEJWadeGNRole of food intake in estradiol-induced body weight changes in female ratsHorm Behav197783265274881167
- ChandrasekaraAShahidiFInhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen speciesJ Agric Food Chem201059142843621133411
- Lerma-GarcíaMHerrero-MartínezJSimó-AlfonsoEMendonçaCRRamis-RamosGComposition, industrial processing and applications of rice bran γ-oryzanolFood Chem20091152389404
- PatelMNaikSGamma-oryzanol from rice bran oil: a reviewJ Sci Ind Res India200463569578
- IsmailNIsmailMFarhana FathySNeuroprotective effects of germinated brown rice against hydrogen peroxide induced cell death in human SH-SY5Y cellsInt J Mol Sci20121389692970822949825
- ZhangRLuHTianSProtective effects of pre-germinated brown rice diet on low levels of Pb-induced learning and memory deficits in developing ratChem Biol Interact2010184348449120138853
- MamiyaTAsanumaTKiseMEffects of pre-germinated brown rice on beta-amyloid protein-induced learning and memory deficits in miceBiol Pharm Bull20042771041104515256737
- CavagniniFInvittiCPintoMEffect of acute and repeated administration of gamma aminobutyric acid (GABA) on growth hormone and prolactin secretion in manActa Endocrinol (Copenh)19809321491547376786
- DriskellJANutrition and Exercise Concerns of Middle AgeBoca RatonCRC Press2009
- PowersMEYarrowJFMcCoySCBorstSEGrowth hormone isoform responses to GABA ingestion at rest and after exerciseMed Sci Sports Exerc200840110418091016
- Ben-ShacharDLauferDLivneESilbermannMPicrotoxin, a gamma-aminobutyric acid-receptor antagonist, retards craniofacial development in the weaning rat: I. Effect on mandibular bone growthJ Craniofac Genet Dev Biol1988843512851612
- FujimoriSHinoiEYonedaYFunctional GABA(B) receptors expressed in cultured calvarial osteoblastsBiochem Biophysl Res Commun2002293514451452
- TakahataYTakaradaTHinoiENakamuraYFujitaHYonedaYOsteoblastic γ-aminobutyric acid, type B receptors negatively regulate osteoblastogenesis toward disturbance of osteoclastogenesis mediated by receptor activator of nuclear factor κB ligand in mouse boneJ Biol Chem201128638329063291721828041
- CiceroAGaddiARice bran oil and γ-Oryzanol in the treatment of hyperlipoproteinaemias and other conditionsPhytother Res200115427728911406848
- KimSJHanDMoonKDRheeJSMeasurement of superoxide dismutase-like activity of natural antioxidantsBiosci Biotechnol Biochem19955958227787296
- UedaTArmstrongDPreventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eyeOphthalmic Res19962831841928829176
- MuthusamiSRamachandranIMuthusamyBOvariectomy induces oxidative stress and impairs bone antioxidant system in adult ratsClin Chim Acta20053601–2818615970279
- Eghbali-FatourechiGKhoslaSSanyalABoyleWJLaceyDLRiggsBLRole of RANK ligand in mediating increased bone resorption in early postmenopausal womenJ Clin Invest200311181221123012697741
- HofbauerLCKhoslaSDunstanCRLaceyDLSpelsbergTCRiggsBLEstrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cellsEndocrinology199914094367437010465311
- RiggsBLKhoslaSMeltonLJSex steroids and the construction and conservation of the adult skeletonEndocr Rev200223327930212050121
- IsmailMAl-NaqeebGMamatWAhmadZGamma-oryzanol rich fraction regulates the expression of antioxidant and oxidative stress related genes in stressed rat’s liverNutr Metab2010723113
- AbukhadirSSAMohamedNMakpolSMuhammadNEffects of palm vitamin e on bone-formation-related gene expression in nicotine-treated ratsEvid Based Complement Alternat Med2012201265602523049610