Abstract
Purpose
The serum lipid level is strongly associated with atherosclerosis. However, research on the relationship between lipid-derived indices and acute ischemic stroke (AIS) occurrence in hemodialysis populations is limited. This study aimed to explore the predictive value of lipid-derived indices, including atherogenic index of plasma (AIP), Non- high density lipoprotein cholesterol (Non-HDL-C), Non-HDL-C/HDL-C, and lipoprotein combine index (LCI) in clinical practice for the occurrence and prognosis of AIS in hemodialysis patients.
Methods
A total of 451 patients undergoing maintenance hemodialysis were screened and 350 were enrolled in this study. The lipid parameters exhibit a progressive increase across the tertiles, with values rising from Q1 through Q3. Enrolled patients were divided into three groups (Q1, Q2, and Q3) based on tertiles of AIP, Non-HDL-C, Non-HDL-C/HDL-C, and LCI values. Kaplan-Meier curves were performed to investigate the association between the AIP, Non-HDL-C, Non-HDL-C/HDL-C, LCI and AIS-free survival in hemodialysis patients. Chi-square analysis was used to explore the association between the AIP, Non-HDL-C, Non-HDL-C/HDL-C, LCI and AIS outcomes in hemodialysis patients. AIS outcomes were assessed using the modified Rankin Scale (mRS).
Results
Kaplan-Meier analysis revealed that the AIS-free survival rates were significantly higher in the Q1 group compared to Q2 and Q3 groups for AIP, Non-HDL-C, Non-HDL-C/HDL-C, and LCI. Log rank tests showed statistically significant differences between the Q1 group and the Q2 and Q3 groups (p < 0.05 for all). The proportion of patients with a good outcome mRS was higher in the Q1 group compared to the Q2-Q3 groups (AIP: 0.818 vs 0.792; Non- HDL-C: 0.866 vs 0.767; Non- HDL-C/HDL-C: 0.867 vs 0.767; LCI: 0.938 vs 0.750).
Conclusion
The four lipid-derived parameters are effective predictors of AIS in patients undergoing hemodialysis, and AIP has a strongest correlation with the risk of AIS. Hemodialysis patients with elevated levels of the four lipid-derived indices had a higher incidence of AIS and poorer functional outcomes compared to those with lower levels. Our conclusions may require confirmation by further research in the future.
Introduction
Progressive decline in renal function in patients with chronic kidney disease (CKD) eventually leads to end-stage renal disease (ESRD).Citation1 ESRD, characterized by the kidneys’ inability to maintain fluid, electrolyte, and waste balance, poses a significant global public health challenge.Citation1 Maintenance hemodialysis, a widely-used and effective renal replacement therapy, is often employed for patients with ESRD.Citation2
Acute ischemic stroke (AIS) is caused by a decrease in blood supply to a certain area of the brain due to vascular obstruction, and has been reported as the most common type of stroke.Citation3 AIS is a common complication in patients on maintenance hemodialysis, with a reported 3 to 7.1-fold increased stroke risk in patients with CKD stages 3 to 5 on dialysis, compared to the general population.Citation4 Furthermore, hemodialysis patients appear to have an elevated stroke risk compared to those undergoing peritoneal dialysis or kidney transplantation.Citation5 Additionally, AIS in hemodialysis patients is associated with higher mortality and disability rates, and worse prognosis than in the general population, significantly impacting their quality of life.Citation6,Citation7 Early identification of patients at high risk for AIS enables proactive prevention and intervention by physicians, which has important clinical implications.Citation8
Atherosclerosis, significantly influenced by dyslipidemia, is a principal traditional risk factor in the pathogenesis of AIS in hemodialysis patients.Citation9,Citation10 Recent studies indicate that dyslipidemia promotes a pre-activated state of platelets, facilitates the role of transforming growth factor-β (TGF-β) in vascular remodeling, and triggers nitration reactions mediated by inducible nitric oxide synthase (iNOS). These processes collectively contribute to the formation of atherosclerosis, thereby increasing the risk of ischemic vascular disease.Citation11–13 The atherogenic index of plasma (AIP) is an effective marker of atherosclerosis,Citation14 and elevated AIP levels correlate with increased cardiovascular events and all-cause mortality in both the general and hemodialysis populations.Citation15,Citation16 Non-high-density lipoprotein cholesterol (non-HDL-C) serves as another composite index of atherogenic lipoproteins.Citation17 In hemodialysis patients, higher non-HDL-C levels are positively correlated with an increased incidence of acute myocardial infarction.Citation15 The lipid complexity index (LCI) shows a stronger correlation with atherosclerotic vascular disease than does any single lipid parameter.Citation18 Furthermore, the non-HDL-C/HDL-C ratio is proposed as a straightforward indicator of cardiovascular disease risk.Citation19 These lipid-derived parameters are strongly associated with atherosclerosis, offering simple and easily obtainable clinical results. However, research on the relationship between these parameters and AIS occurrence in hemodialysis populations is limited. As dyslipidemia in hemodialysis patients differs from that in the general population, the predictive effectiveness of lipid-derived parameters for AIS in hemodialysis patients may contradict findings in the general population.Citation20,Citation21 These are all the more motivating for us to conduct this study to investigate the correlation of AIP, non-HDL-C, non-HDL-C/HDL-C ratio, and LCI with the occurrence of AIS events in hemodialysis patients and their predictive value for the occurrence of AIS. This study aimed to explore whether these lipid-derived parameters (AIP, Non-HDL-C, Non-HDL-C/HDL-C, LCI) have a predictive value for AIS in hemodialysis patients and the relationship between these lipid-derived parameters and outcome of AIS.
Materials and Methods
Study Design and Subject Enrollment
Maintenance hemodialysis patients were screened between January 1, 2015, and April 30, 2023, in the Department of Nephrology, Beijing Chao-Yang Hospital. The inclusion criteria were as follows: 1) Age over 18 years; 2) Renal failure requiring maintenance hemodialysis, hemodialysis duration more than 3 months, with a frequency of three times per week, each session lasting 4 hours; 3) Agreement to participate in the study and voluntary signing of informed consent. The exclusion criteria were as follows: 1) Severe infections, liver failure, malignant tumors, malignant fluid, and other conditions affecting; 2). Absence of laboratory data (lipid indices, including triglycerides, LDL cholesterol, HDL cholesterol, total cholesterol).
From January 1, 2015 to April 30, 2023, a total of 451 patients were screened in the Department of Nephrology, Beijing Chao-Yang Hospital. Among them, 101 patients were excluded and 350 patients were included in this study. Participant recruitment details are presented in . Conducted in compliance with the Declaration of Helsinki for Ethics in Clinical Research, this study was approved by the Ethics Committee of Beijing Chaoyang Hospital (No. 2023-K19). All patients provided written informed consent prior to participation.
Data Collection
Data collected included sex, age, dialysis duration, primary disease diagnosis, smoking history, diabetes mellitus, atrial fibrillation, hypertension, coronary artery disease, previous stroke history, baseline erythropoietin (EPO) dose, antiplatelet drug use, lipid-lowering medication use, and average one-week ultrafiltration volume. This information was sourced from patient interviews and medical records. The endpoint event was defined as the first occurrence of AIS during the follow-up period. Venous blood samples were collected from fasting patients before their second weekly hemodialysis session, typically on a Wednesday or Thursday. To reduce experimental errors, all blood samples were sent to the same laboratory for analysis within one hour of collection. Routine blood tests utilized an automated hematology analyzer (SYSMEX, Japan), while blood biochemistry tests employed an automated biochemistry analyzer (Siemens, Germany). Hemodialysis was conducted using Nikkiso DBB-26 or DBB-27 machines with F7 polysulfone membrane dialyzers (Fersen, Germany), bicarbonate dialysate, a blood flow rate of 250 to 300 mL/min, and a dialysate flow rate of 500 mL/min.
The four lipid-derived parameters are defined as follows: 1) AIP = lg (TG(mmol/l) / HDL(mmol/l)); 2) Non-HDL-C = TC (mmol/l) - HDL (mmol/l); 3) Non-HDL-C/HDL-C = (TC (mmol/l) - HDL (mmol/l)) / HDL (mmol/l); 4) LCI = TC (mmol/l) × TG (mmol/l) × LDL-C (mmol/l) / HDL-C (mmol/l). Patients were categorized into tertiles for AIP, Non-HDL-C, LCI, and Non-HDL-C/HDL-C, resulting in Q1-Q3 groups for each parameter.
Outcome Measures
AIS diagnosis followed the guidelines of the American Heart Association/American Stroke AssociationCitation22 and patient prognosis was assessed using the modified Rankin Scale (mRS). Prognosis was evaluated 3 months post-first stroke using the mRS scale.Citation23 mRS scores were categorized into seven grades: 0 - No symptoms; 1 - No significant disability: able to perform daily tasks and activities despite symptoms; 2 - Minor disability: unable to perform all prior activities, yet capable of managing personal affairs independently; 3 - Moderate disability: requires some help but can walk unassisted; 4 - Moderate to severe disability: requires assistance with walking and physical activities; 5 - Severe disability: bedridden, incontinent, requiring constant care; 6 - Death.Citation24 Scores were categorized as indicating a good (0–2) or poor (3–6) outcome.Citation24
Statistical Analysis
Continuous variables with a normal distribution were expressed as mean ± standard deviation, and compared using independent samples Student’s t-test. Non-normally distributed continuous variables were expressed as median (M25, M75) and compared using the Mann–Whitney U-test. Categorical variables were expressed as numbers (percentages) and compared using chi-square tests. Cox regression analysis was used to explore the risk factors for AIS in hemodialysis patients. Kaplan-Meier curves were used to investigate the relationship between the four lipid-derived indices and the incidence of AIS in hemodialysis patients. The chi-square analysis was used to compare the relationship between the four lipid-derived indices and the prognosis of AIS in hemodialysis patients. Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software version 6 (GraphPad Software Inc., San Diego, CA, USA). The threshold for statistical significance was set at p < 0.05. A two-tailed p-value was used in our analysis.
Results
A total of 451 maintenance hemodialysis patients were screened, with the following exclusions: one for liver failure due to cirrhosis, two for cancer-related cachexia, eight for hemodialysis duration less than 3 months, 28 for follow-up time under 2 years with unclear outcomes, eight for declining participation, six for missing relevant data, and 48 for early dropout, resulting in 350 enrolled patients. The patient selection process is illustrated in .
Comparison of the Medical History and Treatment Between the Two Groups
This study compared the medical history and treatment between two groups. shows that age (p<0.001), diabetes history (p=0.009), AIS history (p<0.001), coronary atherosclerotic heart disease (CAD) history (p=0.026), smoking (p=0.024), and dialysis ultrafiltration volume (p=0.022) were significantly higher in the AIS group compared to the non-AIS group. However, there were no significant differences in sex, dialysis duration, systolic/diastolic/mean arterial blood pressure, atrial fibrillation history, and administration of aspirin, clopidogrel, statins, fibrates, and erythropoietin dosage between the two groups (all p> 0.05).
Table 1 The Baseline Characteristics of the Medical History and Treatment in the Two Groups
Comparison of the Laboratory Test Indicators Between the Two Groups
Laboratory test indicators () showed significantly higher levels of total cholesterol (p<0.001), LDL-C (p=0.008), triglycerides (p=0.001), AIP (p=0.001), Non-HDL-C (p<0.001), Non-HDL-C/HDL-C (p<0.001), LCI (p<0.001), and ferritin (p=0.032) in the AIS group compared to the non-AIS group. Serum albumin (p<0.001) and serum creatinine (p=0.034) levels were lower in the AIS group compared to the non-AIS group. However, levels of HDL-C, hemoglobin, serum urea, serum uric acid, calcium, phosphorus, fasting blood glucose, iPTH, and hsCRP did not significantly differ between the two groups (all p> 0.05).
Table 2 The Baseline Characteristics of the Laboratory Test Indicators in the Two Groups
Univariate Cox proportional hazards analysis () showed that elevated total cholesterol (HR=1.298, p=0.001), LDL-C (HR=1.388, p=0.004), Triglyceride (HR=1.225, p=0.003), AIP (HR=3.052, p=0.001), Non-HDL-C (HR=1.393, p<0.001), LCI (HR=1.012, p<0.001), Non-HDL-C /HDL-C (HR=1.299, p<0.001) were associated with a higher incidence of AIS in the hemodialysis patients. However, elevated HDL-C (HR=0.490, p=0.046) was associated with a lower incidence of AIS in the hemodialysis patients. Multivariate Cox proportional hazards analysis () showed that elevated AIP (HR=4.288, p=0.030) and age (HR=1.052, p<0.001), were associated with a higher incidence of AIS in the hemodialysis patients.
Table 3 Univariate Cox Proportional Hazards Analysis for Four Lipid-Derived Indices to Predict AIS Event in Maintenance Hemodialysis Patients
Table 4 Multivariate Cox Proportional Hazards Analysis for Four Lipid-Derived Indices to Predict AIS Event in Maintenance Hemodialysis Patients
Association Between Four Lipid-Derived Indices and AIS-Free Survival of Hemodialysis Patients
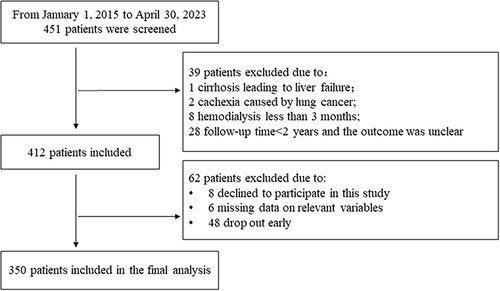
Kaplan-Meier analysis () was performed to investigate the association between four lipid-derived indices and AIS-free survival in hemodialysis patients. Patients were divided into tertiles for AIP, Non-HDL-C, LCI, and Non-HDL-C/HDL-C: AIP Q1 (−0.442 to 0.101), Q2 (0.102 to 0.327), Q3 (0.328 to 1.177) with AIS-free survival rates of 0.875, 0.534, and 0.591, respectively; Non-HDL-C Q1 (1.35 to 2.67), Q2 (2.68 to 3.54), Q3 (3.55 to 9.55) with rates of 0.830, 0.636, and 0.534; LCI Q1 (2.322 to 9.622), Q2 (9.623 to 23.784), Q3 (23.785 to 343.592) with rates of 0.818, 0.602, and 0.580; Non-HDL-C/HDL-C Q1 (0.676 to 2.404), Q2 (2.405 to 3.506), Q3 (3.507 to 10.160) with rates of 0.830, 0.660, and 0.511. Kaplan-Meier analysis showed that the AIS-free survival rates in the AIP, Non-HDL-C, Non-HDL-C/HDL-C, and LCI were significantly higher in the Q1 group compared to Q2 and Q3 groups. Log rank tests revealed statistically significant differences between the Q1 group and the Q2 and Q3 groups (all p < 0.05).
Figure 2 Kaplan–Meier analysis of AIS-free survival in maintenance hemodialysis patients according to: AIP (A), Non-HDL-C (B), LCI, (C), and Non- HDL-C/HDL-C (D).
Association Between of Four Lipid-Derived Indices and Outcome of AIS in Hemodialysis Patients
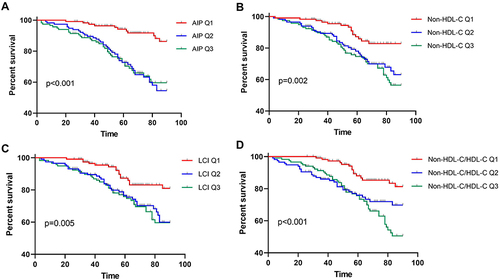
To explore whether AIP, Non-HDL-C, Non-HDL-C/HDL-C, LCI and AIS outcomes are associated with patients undergoing hemodialysis. AIS outcomes were evaluated using mRS scores, with the proportion of patients at each mRS scale being calculated. Comparisons were conducted between patients in the lowest tertile (Q1) and those in the combined higher tertiles (Q2-Q3) of hemodialysis patients (). The proportion of patients with a good outcome (mRS 0–2) was significantly higher in the Q1 group than in the Q2-Q3 group across all lipid-derived parameters (AIP: 0.818 vs 0.792; Non-HDL-C: 0.866 vs 0.767; Non-HDL-C/HDL-C: 0.867 vs 0.767; LCI: 0.938 vs 0.750).
Figure 3 The modified Rankin Scale (mRS) score of AIS patients in different tertiles (Q) group in maintenance hemodialysis patients according to: AIP (A), Non-HDL-C (B), Non- HDL-C/HDL-C (C), and LCI (D).
Discussion
AIS in hemodialysis patients is characterized by high mortality, high disability rate, and an increased need for rehabilitation post-stroke.Citation25 Therefore, identifying simple, cost-effective predictors for high-risk AIS in hemodialysis patients is clinically significant to implement preventive strategies. The KDIGO guidelines recommend that hemodialysis patients need to be monitored for lipid profiles including total cholesterol, LDL-C, HDL-C, and triglycerides.Citation26 Dyslipidemia in hemodialysis patients, differing from the general population, typically features normal serum total cholesterol and LDL levels, alongside reduced HDL-C levels and elevated triglycerides and triglyceride-rich lipoproteins, such as celiac, MDI, and VLDL.Citation21 Previous studies have reported a negative correlation between non-HDL cholesterol, as well as a U-shaped relationship between AIP and all-cause mortality in hemodialysis patients.Citation20,Citation27 The distinct nature of dyslipidemia in hemodialysis patients and previous inconsistencies in lipid-derived parameters’ association with all-cause mortality and cardiovascular prognosis motivated this study.
The study revealed that older hemodialysis patients with an extensive history of diabetes, AIS, CAD, smoking, and greater dialysis ultrafiltration volume were more prone to AIS. Laboratory indicators showed higher levels of total cholesterol, LDL, triglycerides, AIP, Non-HDL-C, Non-HDL-C/HDL-C, LCI, and ferritin in the AIS group compared to the non-AIS group, while serum albumin and creatinine levels were lower. Conversely, higher HDL-C levels were linked to a lower AIS incidence in these patients. This study indicated that dyslipidemia is risk factors for AIS in hemodialysis patients.
The AIP was first proposed by Dobiasova et al in 2001,Citation28 and is regarded as a reliable predictor for CAD.Citation29 The significant association between AIP and cardiovascular risk is primarily attributed to lipoprotein particle size, insulin resistance, and metabolic syndrome.Citation30 In hemodialysis patients, AIP correlated with a 2.59-fold increase in cardiovascular mortality and a 2.15-fold rise in all-cause mortality within the highest quintile.Citation21 This study identified AIP as an independent risk factor for AIS in hemodialysis patients. Among the four lipid parameter-derived indicators, Cox regression analysis results show that the HR value of AIP is the highest, indicating that AIP has the strongest and most sensitive predictive ability for AIS. Elevated AIP results from increased triglycerides (TG) and/or decreased high-density lipoprotein (HDL). In this study, the AIS group exhibited lower HDL and higher TG levels compared to the non-AIS group. Uremic patients with dyslipidemia often present with high levels of TG and low levels of HDL. This is consistent with current guidelines.Citation26 HDL activates the fibrinolytic system, reduces platelet activation, inhibits thromboxane A2 synthesis, decreases endothelial cell damage, and exerts antithrombotic effects.Citation31 Reduced HDL levels are an independent risk factor for restenosis However, HDL-C may be dysfunctional in ESRD patients.Citation32 Elevated levels of TG indicate the presence of celiac remnants that accumulate in arterial walls and induce endothelial dysfunction, attracting numerous mononuclear cells.Citation33 Accumulation of celiac particles in arterial walls and their penetration into endothelial cells leads to dysfunction, attracting monocytes and macrophages. This process, involving phagocytosis and cytokine activation, contributes to sclerotic plaque formation, potentially linked to AIS development.Citation34
Serum non-HDL cholesterol, the difference between total and HDL cholesterol, includes all atherogenic lipoprotein particles such as LDL, LpA, and triglyceride-rich lipoproteins (eg, ML, VLDL, celiac particles). It is a recognized predictor of atherosclerosis and cardiovascular risk.Citation35 Although Shoji et al identified non-HDL-C as an independent risk factor for CAD in both hemodialysis patients and the general population,Citation36 other studies suggest a negative or “U-shaped” association of non-HDL-C with all-cause mortality in hemodialysis patients, possibly due to factors that may contribute to reduced total cholesterol, such as malnutrition, cachexia, and high systemic inflammatory burden.Citation37 In this study, the AIS group had significantly higher non-HDL-C levels compared to the non-AIS group, affirming it as an independent risk factor positively associated with AIS development. Non-HDL-C levels strongly correlate with apolipoprotein B (ApoB) levels, with each pro-atherosclerotic lipoprotein, including VLDLs, VLLDLs, LDLs, and lipoprotein a, containing an ApoB molecule. It has been reported that the atherogenicity of ApoB-containing lipoproteins is associated with subendothelial retention, interaction with arterial wall proteoglycans, and proinflammatory properties.Citation38 In addition, a negative correlation exists between non-HDL-C and LDL particle size; smaller, very low-density lipoprotein particles are more susceptible to oxidation and thus more atherogenic.Citation39 An advantage of non-HDL-C as a predictor is its independence from dietary intake. Desmeules et al showed that non-fasting samples did not alter serum non-HDL-C levels, simplifying its assessment in hemodialysis patients and enhancing clinical practice convenience.Citation40
The non-HDL-C/HDL-C ratio, which represents the ratio of atherogenic factors to anti-atherogenic factors, has been shown to correlate with various dyslipidemia-related diseases, including diabetes mellitus and metabolic syndrome.Citation41,Citation42 Studies have shown that it may be a more accurate predictor of cardiovascular disease than the traditional lipid index,Citation43 and high non-HDL-C/HDL-C ratios are strongly associated with poorer overall survival in peritoneal dialysis patients.Citation44 In addition, an independent association has been found with carotid plaque lipid cores.Citation45 However, studies focusing on the non-HDL-C/HDL-C ratio in hemodialysis patients are limited. To the best of our knowledge, this is the first study to explore the non-HDL-C/HDL-C ratio as a predictor of AIS in hemodialysis patients. Our study found a positive association between the non-HDL-C/HDL-C ratio and AIS occurrence. The non-HDL-C/HDL-C ratio reflects the balance between atherogenic and antiatherogenic lipid particles, potentially explaining its association with plaque stability.
LCI, another frequently used lipid-derived parameter, has been found to be a predictor of cardiovascular risk in patients with acute coronary syndrome.Citation46 It is reported that LCI correlates more strongly with atherosclerotic vascular disease than single lipid parameters.Citation18 Our study found a positive association between LCI and the risk of AIS in hemodialysis patients.
To investigate the association between four lipid-derived indices and the incidence of AIS in hemodialysis patients, this study performed a Kaplan–Meier analysis, and patients enrolled in this study were divided into three groups based on the tertiles of the four lipid-derived indices value (Q1, Q2, and Q3). Four indices in the Q1 group were significantly higher compared to those in the Q2 and Q3 groups.
Indices in the Q1 group were significantly higher compared to those in the Q2 and Q3 groups. AIS outcomes were assessed using the modified Rankin Scale (mRS), a simple, time-efficient tool well-received by patients and evaluators for accurately assessing functional disability in stroke patients. In this study, we calculated the proportion of patients at each mRS level and compared differences between those in the lowest quartile (Q1) and those in the higher quartiles (Q2-Q3) of the lipid-derived indices. Results indicated a significantly higher proportion of patients with a good outcome (mRS 0–2) in the Q1 group compared to the Q2-Q3 group for all lipid-derived parameters. This suggests that higher levels of the four lipid-derived indices are associated with worse functional outcomes of AIS in hemodialysis patients.
This study has several advantages. First, the four lipid-derived indices were obtained from the lipid-related index, which are common indicators in blood biochemical examinations. This approach is noninvasive, cost-effective, and convenient. In addition, these indicators, regularly monitored for medical quality control in hemodialysis patients, offer high detection frequency and produce accurate and reliable results. Second, the study’s experimental design of the study was rigorous. To ensure comparability, the variables might have a potential impact on four lipid-derived indices, such as statin and fibrate administration were not significantly different between the two groups. Finally, the follow-up period ranged from a minimum of 60 to a maximum of 90 months, facilitating a comprehensive analysis of AIS risk predictors in hemodialysis patients and yielding reliable results. However, this study had some disadvantages. This was a single-center study, and multicenter and larger cohort studies are needed to further validate the predictive performance of the four lipid-derived indices for AIS in hemodialysis patients. Additionally, further research should explore the applicability of these findings across different ethnic groups and countries, and the impact of statin intervention on AIS events.
In conclusion, the four lipid-derived indices can be used as a good predictor of AIS in patients undergoing hemodialysis, and AIP has a strongest correlation with the risk of AIS. Hemodialysis patients with elevated levels of the four lipid-derived indices had a higher incidence of AIS and poorer functional outcomes compared to those with lower levels. Our conclusions may require confirmation by further research in the future.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Rosselli D, Rueda JD, Diaz CE. Cost-effectiveness of kidney transplantation compared with chronic dialysis in end-stage renal disease. Saudi J Kidney Dis Transpl. 2015;26(4):733–738. doi:10.4103/1319-2442.160175
- Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573–585. doi:10.1038/s41581-020-0315-4
- Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654–1663. doi:10.1097/CCM.0000000000004597
- Kelly DM, Ademi Z, Doehner W, et al. Chronic kidney disease and cerebrovascular disease: consensus and guidance from a KDIGO controversies conference. Stroke. 2021;52(7):e328–e346. doi:10.1161/STROKEAHA.120.029680
- Findlay M, MacIsaac R, MacLeod MJ, et al. Renal replacement modality and stroke risk in end-stage renal disease-a national registry study. Nephrol Dial Transplant. 2018;33(9):1564–1571. doi:10.1093/ndt/gfx291
- Wetmore JB, Phadnis MA, Ellerbeck EF, Shireman TI, Rigler SK, Mahnken JD. Relationship between stroke and mortality in dialysis patients. Clin J Am Soc Nephrol. 2015;10(1):80–89. doi:10.2215/CJN.02900314
- Findlay MD, Thomson PC, Fulton RL, et al. Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke. 2015;46(9):2477–2481. doi:10.1161/STROKEAHA.115.009095
- Sato K, Konta Y, Furuta K, et al. Prognostic factors for acute ischemic stroke in patients undergoing hemodialysis. Clin Exp Nephrol. 2022;26(3):286–293. doi:10.1007/s10157-021-02146-0
- Hofstra L, Tordoir JH, Kitslaar PJ, Hoeks AP, Daemen MJ. Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts. Does it correlate with flow parameters? Circulation. 1996;94(6):1283–1290. doi:10.1161/01.cir.94.6.1283
- Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res. 2017;120(3):502–513. doi:10.1161/CIRCRESAHA.116.308441
- Yang M, Kholmukhamedov A. Platelet reactivity in dyslipidemia: atherothrombotic signaling and therapeutic implications. Rev Cardiovasc Med. 2021;22(1):67–81. doi:10.31083/j.rcm.2021.01.256
- Jiang H, Ruan Z, Wang Z, et al. Simvastatin reduces atherosclerotic plaques and endothelial inflammatory response in atherosclerosis rats through TGF-beta/Smad pathway. Minerva Med. 2020;111(5):504–507. doi:10.23736/S0026-4806.19.06119-6
- Cai X, Li X, Li L, et al. Adiponectin reduces carotid atherosclerotic plaque formation in ApoE-/- mice: roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol Med Rep. 2015;11(3):1715–1721. doi:10.3892/mmr.2014.2947
- Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43(8):1439–1444. doi:10.1016/j.jacc.2003.11.039
- Zhan Y, Xu T, Tan X. Two parameters reflect lipid-driven inflammatory state in acute coronary syndrome: atherogenic index of plasma, neutrophil-lymphocyte ratio. BMC Cardiovasc Disord. 2016;16:96. doi:10.1186/s12872-016-0274-7
- Yildiz G, Duman A, Aydin H, et al. Evaluation of association between atherogenic index of plasma and intima-media thickness of the carotid artery for subclinic atherosclerosis in patients on maintenance hemodialysis. Hemodial Int. 2013;17(3):397–405. doi:10.1111/hdi.12041
- Wanner C, Krane V. Non-high-density lipoprotein cholesterol: a target of lipid-lowering in dialysis patients. Am J Kidney Dis. 2003;41(3 Suppl 1):S72–S75. doi:10.1053/ajkd.2003.50089
- Oguntola SO, Hassan MO, Duarte R, et al. Atherosclerotic vascular disease and its correlates in stable black South African kidney transplant recipients. Int J Nephrol Renovasc Dis. 2018;11:187–193. doi:10.2147/IJNRD.S160553
- Qin G, Tu J, Zhang C, et al. The value of the apoB/apoAIota ratio and the non-HDL-C/HDL-C ratio in predicting carotid atherosclerosis among Chinese individuals with metabolic syndrome: a cross-sectional study. Lipids Health Dis. 2015;14:24. doi:10.1186/s12944-015-0023-4
- Chang TI, Streja E, Ko GJ, et al. Inverse association between serum non-high-density lipoprotein cholesterol levels and mortality in patients undergoing incident hemodialysis. J Am Heart Assoc. 2018;7(12). doi:10.1161/JAHA.118.009096
- Moradi H, Vaziri ND. Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci. 2018;23(1):146–161. doi:10.2741/4585
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
- Huybrechts KF, Caro JJ, Xenakis JJ, Vemmos KN. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the Athens Stroke Registry. Cerebrovasc Dis. 2008;26(4):381–387. doi:10.1159/000151678
- McArthur K, Fan Y, Pei Z, Quinn T. Optimising outcome assessment to improve quality and efficiency of stroke trials. Expert Rev Pharmacoecon Outcomes Res. 2014;14(1):101–111. doi:10.1586/14737167.2014.870479
- Tariq N, Adil MM, Saeed F, Chaudhry SA, Qureshi AI. Outcomes of thrombolytic treatment for acute ischemic stroke in dialysis-dependent patients in the United States. J Stroke Cerebrovasc Dis. 2013;22(8):e354–e359. doi:10.1016/j.jstrokecerebrovasdis.2013.03.016
- Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303–1309. doi:10.1038/ki.2014.31
- Lee MJ, Park JT, Han SH, et al. The atherogenic index of plasma and the risk of mortality in incident dialysis patients: results from a nationwide prospective cohort in Korea. PLoS One. 2017;12(5):e0177499. doi:10.1371/journal.pone.0177499
- Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–588. doi:10.1016/s0009-9120(01)00263-6
- Cai G, Shi G, Xue S, Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine. 2017;96(37):e8058. doi:10.1097/MD.0000000000008058
- Onat A, Can G, Kaya H, Hergenc G. ”Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4(2):89–98. doi:10.1016/j.jacl.2010.02.005
- Johansen O, Abdelnoor M, Brekke M, Seljeflot I, Hostmark AT, Arnesen H. Predictors of restenosis after coronary angioplasty. A study on demographic and metabolic variables. Scand Cardiovasc J. 2001;35(2):86–91. doi:10.1080/140174301750164691
- Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. 2014;29(8):1554–1562. doi:10.1093/ndt/gfu022
- Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76(4):437–444. doi:10.1038/ki.2009.177
- Peng J, Luo F, Ruan G, Peng R, Li X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017;16(1):233. doi:10.1186/s12944-017-0625-0
- Verbeek R, Hovingh GK, Boekholdt SM. Non-high-density lipoprotein cholesterol: current status as cardiovascular marker. Curr Opin Lipidol. 2015;26(6):502–510. doi:10.1097/MOL.0000000000000237
- Shoji T, Masakane I, Watanabe Y, Iseki K, Tsubakihara Y; Committee of Renal Data Registry JSfDT. Elevated non-high-density lipoprotein cholesterol (non-HDL-C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(5):1112–1120. doi:10.2215/CJN.09961110
- Chiu H, Wu PY, Huang JC, et al. There is a U shaped association between non high density lipoprotein cholesterol with overall and cardiovascular mortality in chronic kidney disease stage 3–5. Sci Rep. 2020;10(1):12749. doi:10.1038/s41598-020-69794-2
- Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134. doi:10.12688/f1000research.9845.1
- El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49(5):547–553. doi:10.1016/j.jacc.2006.09.043
- Desmeules S, Arcand-Bosse JF, Bergeron J, Douville P, Agharazii M. Nonfasting non-high-density lipoprotein cholesterol is adequate for lipid management in hemodialysis patients. Am J Kidney Dis. 2005;45(6):1067–1072. doi:10.1053/j.ajkd.2005.03.002
- Zhang N, Hu X, Zhang Q, et al. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: results from a population-based cohort study. J Diabetes. 2018;10(9):708–714. doi:10.1111/1753-0407.12650
- Kim SW, Jee JH, Kim HJ, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168(3):2678–2683. doi:10.1016/j.ijcard.2013.03.027
- Kouvari M, Panagiotakos DB, Chrysohoou C, Georgousopoulou EN, Tousoulis D, Pitsavos AC. Sex-related differences of the effect of lipoproteins and apolipoproteins on 10-year cardiovascular disease risk; insights from the ATTICA study (2002–2012). Molecules. 2020;25(7). doi:10.3390/molecules25071506
- Xia W, Yao X, Chen Y, Lin J, Vielhauer V, Hu H. Elevated TG/HDL-C and non-HDL-C/HDL-C ratios predict mortality in peritoneal dialysis patients. BMC Nephrol. 2020;21(1):324. doi:10.1186/s12882-020-01993-5
- Virani SS, Catellier DJ, Pompeii LA, et al. Relation of cholesterol and lipoprotein parameters with carotid artery plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) carotid MRI study. Atherosclerosis. 2011;219(2):596–602. doi:10.1016/j.atherosclerosis.2011.08.001
- Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17(1):197. doi:10.1186/s12944-018-0828-z