Abstract
Purpose
Aging is characterized by a gradual functional decrease of all systems including the kidneys. Growing evidence links altered lipid protein redox-homeostasis with renal dysfunction. The effect of sexual dimorphism on the lipid protein redox-homeostasis mechanisms in the aging kidney is obscure. In the current study, we aimed to investigate redox homeostasis as it related to sexual dimorphism on protein oxidation and lipid peroxidation parameters, as protein carbonyl (PCO), total thiol (T-SH), advanced oxidation protein products (AOPP), malondialdehyde, glutathione (GSH), and superoxide dismutase (SOD) activity, as potential aging biomarkers, which may contribute to an analysis of the free radical theory of aging.
Materials and methods
The study was carried out with 16 naturally aged rats (24 months old; eight males and eight females) and their corresponding young rat groups as controls (6 months old; eight males and eight females). All of the aforementioned parameters (PCO, T-SH, AOPP, MDA, GSH, SOD) were measured manually instead of automated devices or ELISA kits.
Results
PCO, AOPP, and malondialdehyde levels in aged rats were significantly higher in the older rat group than in the younger rat group, whereas SOD activities were significantly lower in old rats. T-SH levels were not significantly different in male groups; however, T-SH levels were lower in the aged female group than in the young female control group. In addition, GSH levels were significantly different between the aged rat group and the corresponding young control group for both genders.
Conclusion
With respect to PCO and AOPP, impaired redox homeostasis is substantially more prominent in males than females. The decrease of G-SH levels in male groups could be attributed to stabilizing the redox status of protein thiol groups by the depletion of the GSH groups. Considering the results, the renal tissue proteins and lipids in different genders may have different susceptibilities to oxidative damage.
Introduction
The entire world is undergoing a progressive phenomenon where society has a larger number of older adults demanding to live healthy lives, which is occurring together with increasing public health costs. The aging process affects all organs, including the kidneys. As part of this inevitable process, progressive scarring and a measurable decline in renal function occur in most people over time.Citation1,Citation2 A number of structural changes occur in the kidneys with aging. The aging kidney is characterized by a loss of renal mass, arterial sclerosis, arteriolar hyalinosis, an increased number of sclerotic glomeruli, a loss of tubules, and interstitial fibrosis. The pathogenesis of aging-associated structural changes is not completely understood.Citation2
Clinical factors, including hypertension, diabetes mellitus, obesity, abnormal lipid levels and vitamin D deficiency have been associated with increasing renal sclerosis with aging.Citation1,Citation2 In addition to the aforementioned pathologic states, tissue factors such as protein, deoxyribonucleic acid, and lipid oxidation products are associated with renal aging.Citation3,Citation4
Growing evidence has linked altered lipid protein redox-homeostasis (LPRH), antioxidant enzyme expression, and renal dysfunction.Citation3,Citation4 Aging causes decreased gonadal hormone levels in systemic circulation,Citation5,Citation6 as well as altered systemic redox homeostasis of plasma proteins in both genders;Citation7 hence, the possible changes in this function may affect the LPRH of the aging kidney. The effect of gender dimorphism on LPRH mechanisms in the aging kidney is still an unclear issue.
Renal biopsy is only recommended for selected patients with kidney diseases. It is well known that elderly people in both genders, who have generally suffered from various aforementioned clinical morbidities or who may be using some medications that interfere with the accuracy of the result of renal biopsies and other diagnostic tools, compel us to choose other less invasive diagnostic tests other than renal biopsy in aged rats. Our aim was to identify the difference between a broad set of oxidative stress parameters () to enlighten a road map for a possible discovery of aging biomarkers in order to differentiate chronological aging from biological aging, as well as to contribute to the literature of experiment-based evidence on an oxidative stress theory of aging.Citation8
Table 1 Oxidative stress parameters currently being researched and their descriptions
Materials and methods
Experimental animals and procedures
Ten male and 13 female aged Sprague Dawley rats (24 months of age) and corresponding young controls (eight females, eight males; 6 months of age) were kindly provided by Professor Tuncay Altuğ (deceased) from the Experimental Animal Research and Breeding Laboratory, Cerrahpaşa Medical Faculty, Istanbul University, Istanbul, Turkey. All animals were kept in the same unit at a constant temperature (22°C ± 1°C) under a 12-hour light/dark cycle. Food and fresh tap water were supplied ad libitum throughout the experiment. All of the experimental studies were conducted in accordance with “Recommendations on the Establishment of Animal Experimental Guidelines,” and ethical procedures were conducted under Reduction, Replacement and Refinement (the 3 Rs rule). All rats were weighed at the same time and food and water were monitored daily throughout the study period. A total of eleven neoplasms were observed in seven (two males and five females) of the 23 aged rats during the sacrification period. The study was carried out with the remaining 16 aged (eight males, eight female) rats together with the 16 corresponding younger rats.
Chemicals and apparatuses
Chemicals and solvents used in the experiments were of the highest purity and analytical grade. All chemicals and reagents were purchased from Merck KGaA (Darmstadt, Germany) or Sigma-Aldrich (St Louis, MO, USA). Deionized water was used in the analytical procedures. All reagents were stored at +4°C. The reagents were maintained in equilibrium at room temperature for 0.5 hours before use. All centrifugation procedures were performed with a Sigma 3–18 KS centrifuge (SIGMA Laborzentrifugen GmbH, Osterode am Harz, Germany). Protein oxidation and other oxidative stress parameters were measured by using the Biotek Synergy™ H1 Hybrid Multi-Mode Microplate Reader (BioTek US, Winooski, VT, USA).
Preparation of tissue samples
The kidney tissue samples, which were obtained from the rats under deep anesthesia with intraperitoneally administered sodium pentothal, were washed in cooled 0.9% NaCl and placed on an ice-cold plate. The samples were then immediately frozen in liquid N2 until they were homogenized. Tissue (200 mg) samples were homogenized manually in 2 mL of homogenizing buffer (100 mM KH2PO4–K2HPO4, pH 7.4, plus 0.1% [w/v] digitonin). Homogenates that were obtained from the kidney tissues were centrifuged at 5000 g for 10 minutes, and various analytes of the supernatant fraction were examined.
Assay of protein carbonyl groups
Protein carbonyl (PCO) groups were measured spectrophotometrically by using the Reznick and Packer method.Citation14 PCO groups react with 2, 4-dinitrophenylhydrazine (DNPH) to generate chromophoric dinitrophenylhydrazones. DNPH was dissolved in HCl, and after DNPH reaction, proteins were precipitated with an equal volume of 20% (w/v) trichloroacetic acid and washed three times with 4 mL of an ethanol/ethyl acetate mixture (1:1). Washing was done by the mechanical disruption of pellets in the washing solution using a small spatula, and repelleting by centrifugation at 6000 g for 5 minutes. Finally, the protein precipitates were dissolved in 6 M-guanidine-HCl solution and the absorbance values were measured at 360 nm using the molar extinction coefficient of DNPH, ɛ = 22,000 M−1 cm−1.
Assay of total thiol groups
A part (125 μL) of the supernatant fraction that was obtained by the centrifugation of the homogenates was mixed in 5 mL test tubes with 375 μL of 0.2 M Tris buffer, pH 8.2, and 25 μL of 0.01 M 5,5-dithiobis 2-nitrobenzoic acid (DTNB). The volume of the mixture was increased up to 2.5 mL with 1975 μL of absolute methanol. A reagent blank (without sample) and a sample blank (without DTNB) were prepared in a similar manner. The test tubes were covered with rubber caps, color was formed in 15 minutes, and the mixtures were centrifuged at approximately 3000 g at room temperature for 15 minutes. The absorbance values of the supernatant fractions were read at 412 nm with a spectrophotometer.Citation15 The value of molar extinction coefficient of total thiol (T-SH) groups at wavelength 412 nm is approximately ɛ = 13,100 M−1 cm−1.
Assay of advanced oxidation protein products
Spectrophotometric determination of advanced oxidation protein products (AOPP) was performed by a modification of Witko, Nguyen, and Descamps-Latscha’s method.Citation16 Samples were prepared in the following procedure: 100 μL of supernatant was diluted 1:5 in phosphate buffered saline (PBS), 5 μL of 1.16 M potassium iodide was then added to each tube, followed by 10 μL acetic acid two minutes later. The absorbance of the newly formed mixture was immediately measured at 340 nm by using a blank as reference containing 1000 μL of PBS, 50 μL of Potassium Iyodide (KI), and 100 μL of acetic acid. The chloramine-T absorbance at 340 nm was observed to be linear within the range of 0 μmol/L to 100 μmol/L. AOPP concentrations were expressed as micromoles per liter of chloramine-T equivalents.
Assay of malondialdehyde
The rate of lipid peroxidation was determined by the procedure of Buege and Aust.Citation17 One of the major secondary products of lipid peroxidation is malondialdehyde (MDA). MDA, along with other byproducts, react with thiobarbituric acid to generate a colored product, which absorbs maximally at 535 nm, representing the color produced by all the thiobarbituric acid reactive substances.
Assay of glutathione
Cayman’s (Cayman Chemical Company, Ann Arbor, MI, USA) glutathione (GSH) assay kit utilizes an enzymatic recycling method, using GSH reductase, for the quantification of GSH. The thiol group of GSH reacts with DTNB (Ellman’s reagent) and produces a yellow-colored 5-thio-nitrobenzoic acid (TNB). The mixed disulfide, (GSTNB), that is concomitantly produced, is reduced by GSH reductase to recycle the GSH and produce more TNB. The rate of TNB production is directly proportional to the concentration of GSH in the deproteinized sample.
Assay of superoxide dismutase activity
Determination of superoxide dismutase (Cu-Zn SOD) (EC 1.15.1.1) activity was performed in supernatant fractions based on the method developed by Sun et al.Citation18 This assay involves the inhibition of nitroblue tetrazolium reduction, with xanthine oxidase used as a superoxide generator. One unit of Superoxide Dismutase (SOD), is defined as the amount of enzyme needed to exhibit a 50% dismutation of superoxide radical.
Statistical analysis
Data are expressed as the mean ± standard error of the mean for eight animals in each group. Differences between the groups were assessed by one-way analysis of variance using the SPSS software package for Windows (IBM Corporation, Armonk, NY, USA). Post hoc testing was performed for intergroup comparisons using the least significance difference test. Post hoc tests were conducted by the Bonferroni–Dunn test. A probability value of less than 0.05 was considered statistically significant.
Results
In our experimental observations, which have been conducted within the animal husbandry conditions provided by the Experimental Animal Research and Breeding Laboratory, Cerrahpaşa Medical Faculty, Istanbul University, Istanbul, Turkey, the median lifespan of the male rat was found to be 26 months and its maximum lifespan was 30 months. On the other hand, the median lifespan of the female rat turned out to be 28 months and its maximum lifespan was 33 months.
PCO and AOPP levels in the aged male rats were significantly higher than those in the young male group ( and ). T-SH levels were not found to be different between both the young and old male rats (). In aged male rats, MDA levels were found to be higher than among their corresponding young controls (). Cu-Zn SOD activities of the aged male rats were significantly lower compared to those of the young male rats (). GSH levels were found to be different between the aged male group and the corresponding young controls ().
Figure 1 PCO levels in aged male and female rats were significantly higher than those in the young male and female groups (P < 0.001 for males; P < 0.01 for females). There is a statistically significant difference with * representing male and ¥ representing females.
Abbreviations: PCO, protein carbonyl; pr, protein.
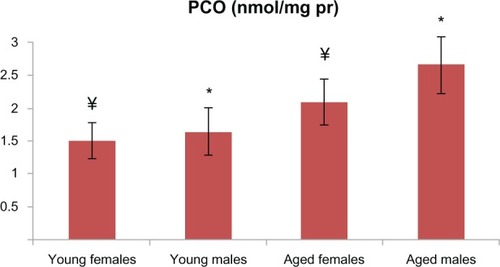
Figure 2 AOPP levels in aged male and female rats were significantly higher than those in the young male and female groups (P < 0.001 for males and P < 0.01 for females). There is a statistically significant difference where * represents males and ¥ represents females.
Abbreviation: AOPP, advanced oxidation protein products.
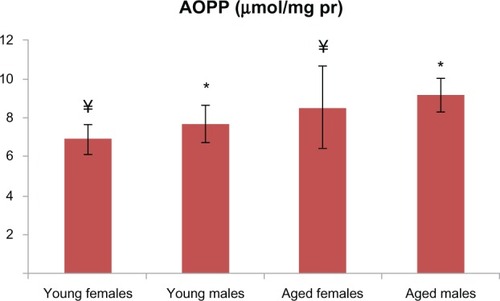
Figure 3 T-SH levels were not found to be different between the male groups.
Note: On the other hand, T-SH levels were found to be lower in the aged female group (P < 0.05 for the female groups). There is a statistically significant difference with * representing male and ¥ representing females.
Abbreviation: T-SH, total thiol.
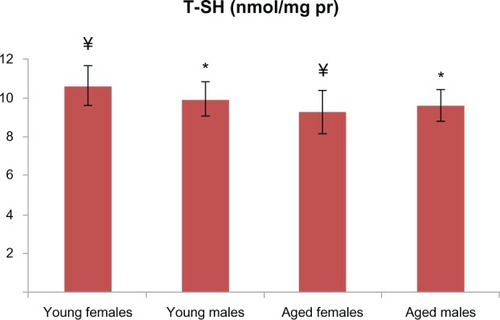
Figure 4 In aged male rats, MDA levels were found to be higher than among the young male rats, which was also evident for females (P < 0.05 for both males and females). There is a statistically significant difference with * representing male and ¥ representing females.
Abbreviations: MDA, malondialdehyde; pr, protein.
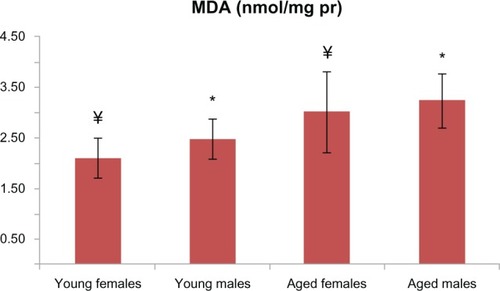
Figure 5 Cu-Zn SOD activities of aged male and female rats were significantly lower when compared with those of the young rats (P < 0.001 for both groups). There is a statistically significant difference with * representing males and ¥ representing females.
Abbreviation: Cu-Zn SOD, superoxide dismutase.
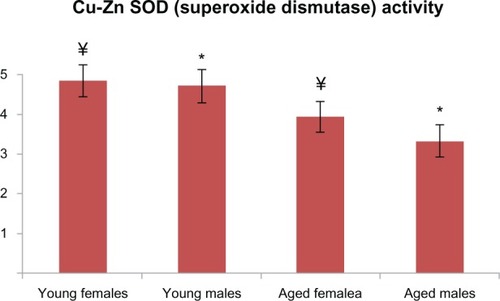
Figure 6 GSH levels were found to be different between the aged male rats and the corresponding young controls (P < 0.01); GSH levels were found to be lower in the aged female rats and the corresponding young controls (P < 0.05). There is a statistically significant difference with * representing male and ¥ representing females.
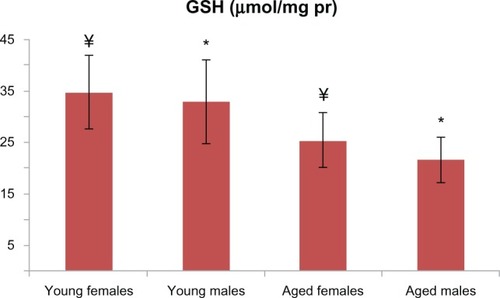
In addition to the male groups, PCO and AOPP levels in the aged female rats were significantly higher than those in the group of young female rats ( and ). T-SH levels were found to be lower among the aged female group (). MDA levels were higher in the aged female rats than in the young female rats (), whereas the GSH levels were found to be lower in the aged female rats and the corresponding young controls (). The activity of Cu-Zn SOD among the aged female rats was significantly lower when compared with that of the young female rats ().
Discussion
The biology of human aging is a highly active and rapidly evolving field. At this point in time, there are as many questions as there are answers; however, an increasing number of inquiries have not resulted in sufficient answers, and these questions are mostly related to the genesis of physiologic aging. Although all organs age in a different time manner, renal aging is an unsolved health problem for the whole society. The kidney is also highly vascular and is impacted by aging of the vascular system with respect to the development of atherosclerosis and hypertension. It has been known for over 50 years that renal functions decline with age, and glomerulosclerosis increases with age even in the absence of comorbidities.Citation19 Females develop lower levels of age-dependent renal function loss than males, which may be in part due to the protective effects of estrogen on cardiorenal function. However, the effect of androgen levels on cardiovascular–renal health is controversial.Citation20
Oxidative stress is linked to the production of reactive lipid aldehydes that nonenzymatically alkylate cysteine, histidine, or lysine residues in a reaction termed protein carbonylation.Citation20 Reactive lipid aldehydes such as MDA and their derivatives are detoxified via a variety of phase 1 and phase 2 systems, and when antioxidant defenses are compromised or oxidative conditions are increased, protein carbonylation (a formation of the PCO groups) is increased. Identifying the carbonylation of proteins is critical for cellular homeostasis, and it could potentially provide important information concerning molecular mechanisms underlying the development and progression of aging and diseases linked to oxidative stress.Citation21
Age-related renal insufficiency has important implications due to the impaired redox homeostasis of proteins.Citation3 Oxidative modifications of cellular proteins, such as PCO and AOPP formation, usually result in a loss of protein function. When aged male and female rats are compared to their young controls with respect to PCO and AOPP, the impaired redox homeostasis on renal tissue proteins is more prominent in males than females. Therefore, the cysteine residues of some proteins act as sensors of redox conditions and can be oxidized in a reversible manner.Citation22,Citation23 Hydrogen peroxide is now considered to act as a second messenger, specifically on the thiol groups, which are the direct targets of the oxidant signal. Central to redox signaling processes are the GSH and thioredoxin systems, which control hydrogen peroxide levels and thus the thiol/disulfide balance.Citation23 GSH inhibits free radical-mediated injury by eliminating reactive oxygen species and protects T-SH groups from oxidation by serving as a biological redox agent. The lack of a significant difference on the levels of the T-SH groups between the aged and young male rats may be related to the compensatory and critical role of GSH on protein redox regulation. The prominent decrease in the GSH levels among the male groups may be related to a change in the redox status of protein thiol groups.
Diseases of chronic oxidative stress and aging involve the accumulation of reactive aldehydes such as MDA. The generation of reactive aldehydes via lipid peroxidation results in protein carbonylation.Citation24 MDA levels were found to be significantly higher in both genders of rats when compared to their corresponding young controls. We are of the conviction that the increased lipid peroxidation rate, which appears to occur in both genders of rats, may be an enhancing factor in the propagation of protein oxidation, as indicated by the PCO and AOPP levels in renal tissue. The variations found between the statistically significant levels of T-SH in the aged male and female rats should be mostly attributed to the role that gender-related factors play in protecting aged female rats against the lipid peroxidation of the kidney tissues. In addition to this finding, we observed that the number of female rats that presented with tumors was almost higher than that of males, of which the difference was accounted for chiefly by the high incidence of mammary tumors in the females. Urinary system tumors were observed in the two males, but not in the females.
It has been previously shown that Cu-Zn SOD activities in the serum and kidney tissues of experimental animals are regulated by the aging process.Citation25,Citation26 Decreased activity of Cu-Zn SOD would be expected to increase the susceptibility of the aging kidney tissues to oxidative injury in male and female rats. On the other hand, there are no established data or explanations in the current literature as to why the plasma Cu-Zn SOD activities among both genders of aged rats differ from their corresponding young controls.
Conclusion
Considering the results of the current study, the renal tissue proteins among the different genders may have different susceptibilities to oxidative protein damage. The observed differences could be attributed to the aging processes, gender-related hormonal status, and altered cellular homeostatic mechanisms. The statistically significant variations found between the aged male and female rats should be mostly referred to by the gender-related factors that could play a role in protecting the aged female rats against oxidative damage of the kidney tissues. Our findings can also support the idea that gender is a crucial feature for redox status of renal proteins in aged rats.
Though there are many answers that have been provided by researchers, there are still questions asking how living cells respond to oxidative stress or manage to adopt it in a deeper and more detailed manner for theurapathic point of view. The gender-related data on these oxidative protein damage parameters in aged rats could be quite beneficial in studies of aging-related disorders like type 2 diabetes mellitus, urinary incontinence, renovascular diseases, or even renal malignancies, and in the future, these data can lead to possible therapeutic interventions developed from a protein oxidation point of view.
Acknowledgments
In memoriam: this paper is dedicated to the memory of Professor Tuncay Altuğ, Experimental Animal-Research and Breeding Laboratory, Istanbul University, Cerrahpaşa Faculty of Medicine, who kindly provided experimental animals for our current research. Duygu Uzun and Tamer Cebe, are medical students at Istanbul University, Turkey, Istanbul.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChoudhuryDLeviMKidney aging – inevitable or preventable?Nat Rev Nephrol201171270671721826079
- PannaraleGCarboneRDel MastroGThe aging kidney: structural changesJ Nephrol201023Suppl 15S37S4020872369
- AydinSYanarKAtukerenPComparison of oxidative stress biomarkers in renal tissues of D-galactose induced, naturally aged and young ratsBiogerontology201213325126022179795
- GomesPSimãoSSilvaEAging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressureOxid Med Cell Longev20092313814520592768
- VeldhuisJDAging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and womenAgeing Res Rev20087318920818343203
- HorstmanAMDillonELUrbanRJSheffield-MooreMThe role of androgens and estrogens on healthy aging and longevityJ Gerontol A Biol Sci Med Sci201267111140115222451474
- ÇakatayUAydinSYanarKUzunHGender-dependent variations in systemic biomarkers of oxidative protein, DNA, and lipid damage in aged ratsAging Male2010131515819883294
- HarmanDAging: a theory based on free radical and radiation chemistryJ Gerontol195611329830013332224
- ÇakatayUProtein redox-regulation mechanisms in agingBondySCMaiseKAging and Age-Related DisordersNew York, NYSpringer2010124
- Witko-SarsatVFriedlanderMCapeillère-BlandinCAdvanced oxidation protein products as a novel marker of oxidative stress in uremiaKidney Int1996495130413138731095
- De ChiaraBSeddaVParoliniMPlasma total cysteine and cardiovascular risk burden: action and interactionScientific World Journal2012201230365422593672
- ErgünYKurutaşEBAtalayFAliciTEffects of silibinin and ethanol on skeletal muscle ischemia-reperfusion injuryActa Cir Bras201328317918423503858
- LiYHuangYPiaoYProtective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testisReprod Biol Endocrinol2013112323514035
- ReznickAZPackerLOxidative damage to proteins: spectrophotometric method for carbonyl assayMethods Enzymol19942333573638015470
- SedlakJLindsayRHEstimation of total, protein-bound, and nonprotein sulfhydryl groups in tisue with Ellman’s reagentAnal Biochem19682511922054973948
- WitkoVNguyenATDescamps-LatschaBMicrotiter plate assay for phagocyte derived taurine-chloraminesJ Clin Lab Anal19926147531542083
- BuegeJAAustSDMicrosomal lipid peroxidationMethods Enzymol197852302310672633
- SunYOberleyLWLiYA simple method for clinical assay of superoxide dismutaseClin Chem19883434975003349599
- WigginsJWhy do our kidneys get old?Nephron Exp Nephrol2011119Suppl 1e1e521832856
- BaylisCSexual dimorphism: the aging kidney, involvement of nitric oxide deficiency, and angiotensin II overactivityJ Gerontol A Biol Sci Med Sci201267121365137222960474
- BaraibarMALiuLAhmedEKFriguetBProtein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseasesOxid Med Cell Longev2012201291983223125894
- CurtisJMHahnWSLongEKBurrillJSArriagaEABernlohrDAProtein carbonylation and metabolic control systemsTrends Endocrinol Metab201223839940622742812
- BindoliARigobelloMPPrinciples in redox signaling: From chemistry to functional significanceAntioxid Redox Signal201318131557159323244515
- FritzKSPetersenDRExploring the biology of lipid peroxidation-derived protein carbonylationChem Res Toxicol20112491411141921812433
- ComaiSBertazzoARagazziECaparrottaLCostaCVAllegriGInfluence of age on Cu/Zn-superoxide dismutase and indole 2,3-dioxygenase activities in rat tissuesItal J Biochem2005543–423223916688932
- AsgharMGeorgeLLokhandwalaMFExercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old ratsAm J Physiol Renal Physiol20072933F914F91917634393