Abstract
Background
Frailty epitomizes the most complex consequence of an aging population. This study aimed to evaluate the impact of frailty, measured using the Clinical Frailty Scale (CFS), on outcomes of older people in an emergency department (ED).
Methods
We conducted a prospective observational study enrolling patients aged 65 years and older in a medical center of Taiwan between March 8, 2021, and November 30, 2021. The primary outcome was 90-day mortality rate. Individuals were categorized into three groups based on the CFS scores. Logistic regression was employed to examine the influence of frailty on clinical outcomes following covariate adjustment. Survival analysis was conducted using Kaplan–Meier curves and Log rank tests.
Results
A total of 473 individuals were included in the study, with a mean age of 82.1 years, and 60.5% of them were males. The 90-day mortality rate was 10.6%. Among these groups, the CFS score 7–9 group had the highest 90-day mortality rate (15.9%), followed by the CFS score 4–6 group (8.0%) and the CFS score 1–3 group (7.1%). The multiple logistic regression analyses demonstrated a significant impact of CFS score on prognosis, with adjusted odd ratios of 1.24 (95% CI 1.06–1.47) for 90-day mortality, 1.18 (95% CI 1.06–1.31) for hospitalization, and 1.30 (95% CI 1.12–1.52) for 180-day mortality. The Kaplan–Meier curves revealed a significantly higher 90-day mortality rate for patients with high CFS scores (Log rank tests, p = 0.019).
Conclusion
In the older ED population, the severity of frailty assessed by the CFS emerged as a significant and important prognostic factor for hospitalization, 90-day mortality, and 180-day mortality.
Introduction
Frailty, characterized as a state of vulnerability to inadequate resolution of homeostasis following stressors, represents the most challenging manifestation of population aging.Citation1 The characteristics of frailty includes shrinking, weakness, exhaustion, slowness, and low physical activity level. Frailty could validly predict the risks of falls, hospitalizations, disability, and death among geriatric patients.Citation2 The previous literature reported that frailty, assessed using comprehensive geriatric assessment, was a significant predictive factor for poor outcomes in the emergency department (ED).Citation3
Rapid and feasible frailty assessments were recommended in the context of ED population with higher acuity illness.Citation4 The Clinical Frailty Scale (CFS) served as a validated tool for frailty assessment, providing predictive insights with respect to mortality and the requirement for institutional care.Citation5,Citation6 The CFS was affirmed as a quick and appropriate assessment tool used in the ED, with substantial inter-rater reliability.Citation7,Citation8 It has been claimed than the CFS is a precise predictor of short-term and long-term mortality among ED population.Citation9–11 However, the capacity of the CFS to forecast clinical outcomes was found to be constrained in other studies.Citation12,Citation13
The impact of CFS on mortality remains uncertain, and there is also limited research concerning the correlation between the CFS and 90-day mortality within the ED setting. Moreover, the Taiwan Triage and Acuity Scale (TTAS) is a validated computerized five-level triage system currently utilized in Taiwan.Citation14,Citation15 Therefore, our research sought to validate the CFS as a tool for stratifying mortality risk and to compare its performance with the TTAS system among the ED population.
Methods
Study Design and Setting
This prospective observational cohort study was executed in the ED observation room of the Taipei Veterans General Hospital, Taiwan. This hospital is a medical center that annually received nearly 83,000 ED visits. The study protocol underwent review and received approval from the Ethics Committee of our hospital, with the approval number 2021–01-017BC. Between March 8, 2021, and November 30, 2021, individuals who were first-time admitted to the 57-bed ED observation room during the period for either illness surveillance or while waiting for hospitalization were screened.
Selection of Participants
All individuals aged 65 years and older who visited the observation room during daytime hours with stable clinical status and a willingness to provide written informed consent were included. Patients exhibiting unconsciousness or uncooperative status, those who declined to participate in this investigation, and individuals discharged prior to the research visit were excluded from the study.
Data Collection
Demographic information, encompassing age, gender, education level, marital status, living floor, living arrangement, and triage acuity, was gathered for analysis. The TTAS categorizes ED patients based on their acuity in a descending order, encompassing level 1 for resuscitation, level 2 for emergent cases, level 3 for urgent conditions, level 4 for less urgent situations, and level 5 for non-urgent cases. Enrolled participants were categorized into two groups, specifically level 1 and level 2–4, based on their TTAS level. Frailty was assessed using the CFS, with the updated 9-point version being the predominant choice in recent studies and utilized in this investigation.Citation5,Citation16,Citation17 Enrolled participants in the ED observation room were assessed and additionally categorized into three groups according to their CFS scores: individuals with scores ranging from 1 to 3 (representing “very fit” to “managing well”), those with scores from 4 to 6 (indicating “very mild frailty” to “moderate frailty”), and patients with scores from 7 to 9 (reflecting “severe frailty” to “terminally ill”).Citation16,Citation18
Outcomes
The primary outcome was 90-day mortality. The secondary outcomes included 1) hospitalization; 2) ICU admission; 3) 30-day mortality; and 4) 180-day mortality. The outcomes, stratified by the CFS score and TTAS level, were retrieved 6 months after the ED visit.
Patient and Public Involvement
Participants or the public were not involved in the design, conduct, reporting, or dissemination plans of this study.
Data Analysis
Normality tests were conducted on the continuous variable. Continuous variables were reported as medians with their respective interquartile ranges (IQRs), while categorical data are presented as frequency and corresponding percentages. Mann–Whitney U-test or Kruskal–Wallis test was employed to compare continuous variables across different groups, and Chi-square test was utilized to compare categorical variables between the groups.
Univariate and multivariate logistic regression analyses were performed to detect the association between the CFS scores and outcomes, and the results were presented as odds ratio (OR) and 95% confidence intervals (CIs). Survival analysis was undertaken using Kaplan–Meier curves and Log rank tests. Using Receiver Operating Characteristic (ROC) curve analysis, Model 1, which incorporated age and gender to predict clinical outcomes, was evaluated. In Model 2, TTAS levels were added to age and gender as covariates and were subsequently analyzed. Similarly, the predictive performance of Model 3, which includes the CFS score in addition to the variables from Model 2, was also assessed using ROC curve analysis. All analyses were performed using IBM SPSS software (version 21.0; Armonk, NY). A two-sided p value less than 0.05 was considered statistically significant in all analyses.
This study adhered to the STROBE guideline in both the execution of the research and the subsequent reporting.
Results
Cohort Characteristics
A total of 948 individuals were screened in the ED observation room during the study period. Of them, 663 patients were age 65 years or older. A total of 190 participants were excluded because of unconscious or uncooperative status (n = 114), refusal (n = 55), and discharge before research visiting (n = 21) (Figure S1). Finally, 473 geriatric participants were enrolled for analysis. presents the baseline characteristics of included subjects. Of the included individuals, the mean age was 82.1 years (range 65 to 105 years), with 60.5% being males, and 36.4% being single, widowed, or divorced, while 8.2% lived in long-term care institutions. Most people (65.1%) were triaged as level 3 by TTAS. Among the enrolled patients, 141 (29.8%) were classified as CFS score 1–3, 162 (34.2%) as CFS score 4–6, and 170 (35.9%) as CFS score 7–9. The prevalence of frailty, as determined by the CFS, was 70.2%.
Table 1 Patient Characteristics
Clinical Profiles of Study Population
Clinical profiles and outcomes of older ED population stratified by TTAS level are presented in . The triage results indicated that 32 participants (6.8%) were categorized as level 1, while 441 individuals (93.2%) fell into the level 2–4 category. The two groups did not exhibit any disparities in age or gender distribution. lists the clinical profiles and prognosis of study population, categorized based on their CFS scores. The group with CFS scores of 7–9 had a significantly older age (p value < 0.001) and a higher percentage of TTAS level 1 compared to the other groups (p value = 0.004).
Table 2 Clinical Profiles and Outcomes of Older ED Patients Stratified by TTAS Level
Table 3 Clinical Profiles and Outcomes of Older ED Patients Stratified by CFS Score
Primary Outcomes
The overall 90-day mortality rate was 10.6%. The TTAS level 1 group demonstrated a 90-day mortality rate of 18.8%, which was not significantly different from the 10.0% observed in the level 2–4 group (p = 0.119). After subgrouping by the CFS score, the group with CFS scores of 7–9 exhibited the highest 90-day mortality rate at 15.9%, followed by the group with CFS scores of 4–6 at 8.0%, and the group with CFS scores of 1–3 at 7.1% (p = 0.018).
Secondary Outcomes
In the overall groups, the hospitalization rate was 63.6%, with ICU admission at 3.4%, 30-day mortality at 5.5%, and 180-day mortality at 12.7%. The TTAS level 1 group disclosed a significantly higher hospitalization rate at 84.4%, in contrast to the TTAS level 2–4 group, which had a rate of 62.1% (p = 0.012). However, no significant differences were observed in terms of ICU admission, 30-day mortality, and 180-day mortality between the different TTAS level groups. Peoples with higher CFS score had worse prognosis, including significantly more hospitalization (p = 0.003) and higher 180-day mortality rate (p < 0.001). There were no significant differences observed in terms of ICU admission and 30-day mortality among the various CFS score groups.
Logistic Regression Analysis
describes the logistic regression analysis for factors associated with 90-day mortality. The TTAS level and CFS score were the significant factors in the unadjusted model (p value = 0.046 and 0.020 respectively). The adjusted odd ratios of TTAS level and CFS score for 90-day mortality were 1.24 (95% CI 1.06–1.47) and 0.79 (95% CI 0.52–1.20), respectively, after being adjusted for age, gender and TTAS level. The CFS score was also the significant factor for hospitalization (adjusted OR 1.18 with 95% CI 1.06–1.31) and 180-day mortality (adjusted OR 1.30 with 95% CI 1.12–1.52) after controlling for age, gender and TTAS level (Tables S1 and S2).
Table 4 Logistic Regression Analysis of Factors Associated with 90-Day Mortality Among Older ED Patients
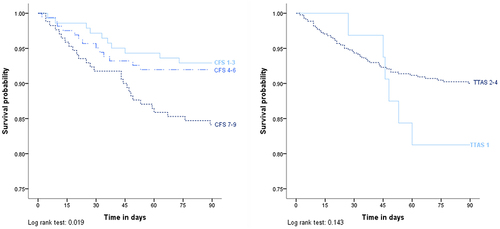
Survival Analysis
Survival curves of 90-day mortality stratified by CFS score and TTAS level are presented in . In the survival analysis, the CFS score 7–9 group had lowest 90-day survival probability compared with other groups in the Kaplan–Meier curves, with p value 0.019 in Log rank test. The Kaplan–Meier method and Log rank test demonstrated no significant effect of TTAS level on mortality. The Figure S2 also describes the impact of CFS score on 180-day mortality (Log rank test, p value = 0.001).
ROC Curves
Figure S3 shows the receiver operating characteristic (ROC) curves of three adjusted logistic regression models for prediction of hospitalization, 90-day mortality, and 180-day mortality. The ROC curves disclosed an increasing trend in the area under the ROC curve (AUROC) as TTAS level and CFS score were added to age and gender for prognosis prediction. Compared to Model 1, adding TTAS level in Model 2 increased the AUROC for predicting hospitalization from 0.54 (95% CI 0.49 to 0.59) to 0.56 (95% CI 0.51 to 0.60), for predicting 90-day mortality from 0.50 (95% CI 0.41 to 0.58) to 0.54 (95% CI 0.45 to 0.63), and for predicting 180-day mortality from 0.53 (95% CI 0.45 to 0.62) to 0.57 (95% CI 0.49 to 0.65). Moreover, incorporation of the CFS score into covariates in Model 3 further increased the AUROC values to 0.60 (95% CI 0.55 to 0.64) in predicting hospitalization, to 0.61 (95% CI 0.53 to 0.69) in predicting 90-day mortality, and to 0.64 (95% CI 0.57 to 0.72) in predicting 180-day mortality, respectively.
Discussion
In this prospective observational cohort study involving the use of the Clinical Frailty Scale, 70.2% of the patients were identified as frail, and the prognostic impact of frailty assessed using this instrument was validated among older individuals in an emergency department. Participants with higher CFS scores experienced significantly higher acuity level and poorer outcomes, including higher rates of hospitalization, 90-day mortality, and 180-day mortality. Compared to TTAS, the predictive ability of the CFS score for hospitalization and mortality were disclosed after covariate adjustment. Incorporating the CFS into the TTAS-based model increased the AUROC for prognosis prediction.
This research reported a frailty prevalence rate of 70.2% among older ED population using the CFS with a threshold score of 4 or higher. The prevalence of frailty assessment using this tool was reported as 69.4% by Shang et al, which closely aligns with our findings.Citation13 Another two studies reported a prevalence of 33.6% to 36.8%, where frailty was defined as a CFS score of 5 or higher.Citation9,Citation19
In our study, the hospitalization rate was 63.6%, and the ICU admission rate was 3.4%, both of which are comparable to rates reported in previous published studies (hospitalization rates ranging from 50.3% to 74.7% and ICU admission rates ranging from 1.7% to 8.9%).Citation9,Citation19–21 Our investigation exhibited a 30-day mortality rate of 5.5% and a 90-day mortality rate of 10.6%. Previous literature has reported analogous clinical outcomes in the older ED population, with 30-day mortality rates ranging from 5.3% to 7.3% and a 90-day mortality rate of 9.2%.Citation9,Citation19,Citation22,Citation23 Our research revealed a 180-day mortality rate of 12.7%. In contrast, Pulok et al reported a higher 6-month mortality rate of 34%, but it is worth noting that their study included a higher proportion of high-acuity participants (their 38% compared to our 32%).Citation24
This investigation was conducted among a cohort of relatively hemodynamically stable individuals within an ED observation room. Another study evaluating the efficacy of a multicomponent frailty intervention was also executed in an ED observation unit, mirroring the setting of our research.Citation25 In published literature, high acuity was linked to a substantial risk of mortality even among individuals with lower levels of frailty.Citation24 Hence, the focus of our study was on validating the CFS among these relatively stable older ED patients. In this cohort, TTAS level 1 accounted for 6.8%, while level 2 accounted for 25.2%, resulting in a total of 32% initially triaged as requiring resuscitation or as emergent cases. This percentage of high acuity triage in our study was higher than in previous literature (Ng et al, 21.1%) for the general ED population and similar to other studies (Ng et al, 20.1%; Chien et al, 34.7%) for the older ED population in Taiwan.Citation14,Citation20,Citation26
This study stated a limited predictive ability of illness acuity, assessed by the TTAS system, on clinical prognosis in these older ED population. The predictive capacity of the TTAS system for in-hospital mortality was effective, but it decreased with increasing age.Citation26 The current models of emergency care, which are focused on specific diseases and episodic treatment, are insufficient in addressing the complex care needs of older individuals living with frailty.Citation27 Older ED population have unique patterns of service utilization and care requirements, rendering them susceptible to under-triage.Citation28 Nevertheless, when it comes to predicting mortality, whether it be short-term or long-term, the CFS also demonstrated superior performance compared to the acuity assessment conducted using the Emergency Severity Index among the older ED population.Citation9,Citation10 Using the TTAS acuity level as the sole determinant for diverting non-urgent patients away from the ED is considered inadequate, and it is suggested to incorporate frailty as a modifier in TTAS to more accurately reflect the illness severity and requirements of ED interventions and following medical care.Citation29 Moreover, Pulok et al also proposed that combining an acuity measure with a frailty measure provides more informative results than either measure alone.Citation24 Moreover, the risk of under-triage among older ED population could be reduced by integrating the CFS into the TTAS system.Citation20
In our study, the prognostic capability of the CFS score for 90-day and 180-day mortality was described after adjusting for covariates, as compared to TTAS. Following validation based on comprehensive geriatric assessment, the CFS was found to be more accurate than other tools, suggesting its consideration as a concise screening tool for assessing frailty in the ED.Citation30 However, there is a paucity of research examining the association between the CFS and 90-day mortality within the context of an emergency department. Compared to well patients with low acuity, the odds of 180-day mortality were approximately 14.5 times higher (95% CI: 8.3–29.1) for severely frail patients with high acuity.Citation24 The investigation, accomplished in the UK with data from over 26,000 individuals with recorded CFS scores, proved that CFS assessed at ED triage could be a significant predictor of long-term mortality.Citation21
Higher frailty levels were associated with an increased risk of hospitalization, as found in our investigation. Our previously published research also stated that instrumental activities of daily living (IADL), close to the judgement components for a CFS score of 5, were independent predictors of admission during an ED visit.Citation3 Previous research has confirmed that older patients are prone to sustaining femur fractures due to low-energy injuries. Furthermore, the invasive soft tissue dissection required during open surgical repair may provoke a sterile inflammatory response, potentially exacerbating their frailty.Citation31 Older individuals living with frailty have reduced resilience to stressors from environment, trauma, or disease, which can lead to a cycle preventing them from returning to their baseline state.Citation2 The frailty was also associated with increased likelihood of developing septic shock among ED individuals with suspected infection, which may lead to more need for medical service.Citation32 Kaeppeli et al also recognized the CFS as a validated predictive factor of hospitalization, regardless of age, gender, and clinical condition.Citation9 In addition, the prospective research in Switzerland disclosed a strong association between the CFS and admission or other adverse outcomes among older ED patients during the COVID pandemic.Citation19 The CFS score was the independent predictive factor in terms of hospitalization.Citation33 Furthermore, the frail hospitalized individuals also experienced worse geriatric and hospital outcomes, as investigated in previous studies.Citation34,Citation35
This study demonstrated that the inclusion of the CFS in the TTAS-based model resulted in an enhanced AUROC for prognostic prediction. However, the predictive ability of CFS on mortality varied, with AUROC values ranging from 0.56 to 0.82 in different investigations.Citation5,Citation10–12,Citation20,Citation23 Relying solely on the CFS for prognosis prediction is insufficient. A comprehensive assessment considering clinical severity, comorbidities, and frailty may prove effective in predicting the risk of mortality among the older people at the time of their ED presentation.Citation36,Citation37 Consequently, there are numerous other prognostic factors associated with the mortality rate of ED patients that require validation and inclusion in a prediction model.
There is a conspicuous requirement for studies that examine the influence of CFS assessment in ED environment even the utilization of the CFS in ED research has experienced a substantial increase.Citation16,Citation17 Integrating frailty status and acuity severity during ED service could be important and synergistic predictors of mortality.Citation20,Citation23 The addition of the CFS into ED patient triage and care may assist in risk stratification, disposition decisions, and the improvement of patient-centered outcomes.Citation25,Citation38 However, further well-designed randomized clinical studies are needed to establish the benefits.
Our study had several limitations. Firstly, it was a single-center study. However, there is still limited research on the association between the CFS and mortality in an Asian ED context, and our investigation reinforces the significance and feasibility of utilizing this suitable frailty assessment instrument in the ED. Further multi-center studies with external validation should be considered. Secondly, this investigation specifically targeted a relatively hemodynamically stable older population in the ED observation room, excluding some participants with unstable vital signs or critical illness, which may limit generalizability of our finding in this study. Thirdly, this study focused on assessing mortality and service-related outcomes, but it did not include an analysis of other patient-centered measures like functional status, patient satisfaction, or quality of life. Fourthly, this research encompassed older ED individuals without a specific focus on any specific disease. The use of the CFS in hospitalized or ED patients has been investigated in research across various disease categories, such as acute cholecystitis, surgery, and hypothermia.Citation18,Citation19,Citation22,Citation39 Lastly, TTAS level 1 did not show significantly worse mortality, which might be due to the small sample size and the limited number of people in this category.
Conclusions
This prospective cohort study reported a frailty prevalence of 70.2% and demonstrated the significant impact of frailty assessed by the Clinical Frailty Scale on hospitalization, 90-day mortality, and 180-day mortality among the older ED population. The Clinical Frailty Scale score was an important predictor of clinical outcomes after adjusting age, gender, and acuity level. Further well-designed ED investigations, targeting specific disease and emphasizing patient-centered functional outcomes, could be considered in future.
Ethics Approval
This study adhered to the principles outlined in the Declaration of Helsinki. The study was reviewed and approved by the Institutional Review Board of Taipei Veterans General Hospital, with the approval number 2021-01-017BC.
Informed Consent Statement
All subjects signed informed consent forms.
Author Contributions
All authors made significant contributions to the work reported. J-WL, P-YL and H-HH conceived and designed the study. J-WL, P-YL and T-YW undertook recruitment of participating patients and conducted data collection. J-WL, Y-JC and H-HH conducted statistical analysis. DH-TY and H-HH validated the results and provided statistical advice on the design. J-WL and H-HH drafted the manuscript, and all authors participated in critically reviewing the article. All authors gave final approval of the version to be published, agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We extend our sincere gratitude to the Department of Emergency Medicine at Taipei Veterans General Hospital, Taiwan, for their generous support and permission to conduct this study.
Data Sharing Statement
The authors confirmed that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Additional information
Funding
References
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–156. doi:10.1093/gerona/56.3.M146
- Huang HH, Chang JC, Tseng CC, et al. Comprehensive geriatric assessment in the emergency department for the prediction of readmission among older patients: a 3-month follow-up study. Arch Gerontol Geriatr. 2021;92:104255. doi:10.1016/j.archger.2020.104255
- Boreskie KF, Hay JL, Boreskie PE, Arora RC, Duhamel TA. Frailty-aware care: giving value to frailty assessment across different healthcare settings. BMC Geriatr. 2022;22(1):13. doi:10.1186/s12877-021-02722-9
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi:10.1503/cmaj.050051
- Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: an observational study. Eur J Intern Med. 2016;35:24–34. doi:10.1016/j.ejim.2016.08.033
- Elliott A, Phelps K, Regen E, Conroy SP. Identifying frailty in the emergency department-feasibility study. Age Ageing. 2017;46(5):840–845. doi:10.1093/ageing/afx089
- Hörlin E, Munir Ehrlington S, Henricson J, John RT, Wilhelms D. Inter-rater reliability of the clinical frailty scale by staff members in a Swedish emergency department setting. Acad Emerg Med. 2022;29(12):1431–1437. doi:10.1111/acem.14603
- Kaeppeli T, Rueegg M, Dreher-Hummel T, et al. Validation of the clinical frailty scale for prediction of thirty-day mortality in the emergency department. Ann Emerg Med. 2020;76(3):291–300. doi:10.1016/j.annemergmed.2020.03.028
- Rueegg M, Nissen SK, Brabrand M, et al. The clinical frailty scale predicts 1-year mortality in emergency department patients aged 65 years and older. Acad Emerg Med. 2022;29(5):572–580. doi:10.1111/acem.14460
- Lee JH, Park YS, Kim MJ, et al. Clinical Frailty Scale as a predictor of short-term mortality: a systematic review and meta-analysis of studies on diagnostic test accuracy. Acad Emerg Med. 2022;29(11):1347–1356. doi:10.1111/acem.14493
- Shrier W, Dewar C, Parrella P, Hunt D, Hodgson LE. Agreement and predictive value of the Rockwood clinical frailty scale at emergency department triage. Emerg Med J. 2021;38(12):868–873. doi:10.1136/emermed-2019-208633
- Shang N, Liu H, Wang N, Guo S, Ma L. Comparison of three frailty screening instruments for prediction of adverse outcomes among older adults in the emergency department. Geriatr Gerontol Int. 2022;22(10):851–856. doi:10.1111/ggi.14469
- Ng CJ, Yen ZS, Tsai JC, et al. Validation of the Taiwan triage and acuity scale: a new computerised five-level triage system. Emerg Med J. 2011;28(12):1026–1031.
- Sung SF, Huang YC, Ong CT, Chen W. Validity of a computerised five-level emergency triage system for patients with acute ischaemic stroke. Emerg Med J. 2013;30(6):454–458. doi:10.1136/emermed-2012-201423
- Fehlmann CA, Nickel CH, Cino E, Al-Najjar Z, Langlois N, Eagles D. Frailty assessment in emergency medicine using the Clinical Frailty Scale: a scoping review. Intern Emerg Med. 2022;17(8):2407–2418. doi:10.1007/s11739-022-03042-5
- Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20(1):393. doi:10.1186/s12877-020-01801-7
- Covino M, Salini S, Russo A, et al. Frailty assessment in the emergency department for patients ≥80 years undergoing urgent major surgical procedures. J Am Med Dir Assoc. 2022;23(4):581–588. doi:10.1016/j.jamda.2021.12.039
- Simon NR, Jauslin AS, Rueegg M, et al. Association of frailty with adverse outcomes in patients with suspected COVID-19 infection. J Clin Med. 2021;10(11):2472. doi:10.3390/jcm10112472
- Ng CJ, Chien LT, Huang CH, et al. Integrating the clinical frailty scale with emergency department triage systems for elder patients: a prospective study. Am J Emerg Med. 2023;66:16–21. doi:10.1016/j.ajem.2023.01.002
- Elliott A, Taub N, Banerjee J, et al. Does the clinical frailty scale at triage predict outcomes from emergency care for older people? Ann Emerg Med. 2021;77(6):620–627. doi:10.1016/j.annemergmed.2020.09.006
- Alakare J, Kemp K, Strandberg T, Castrén M, Tolonen J, Harjola VP. Low body temperature and mortality in older patients with frailty in the emergency department. Aging Clin Exp Res. 2022;34(6):1453–1457. doi:10.1007/s40520-022-02098-9
- Kabell Nissen S, Rueegg M, Carpenter CR, et al. Prognosis for older people at presentation to emergency department based on frailty and aggregated vital signs. J Am Geriatr Soc. 2022;71:1250–1258. doi:10.1111/jgs.18170
- Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020;49(6):1071–1079. doi:10.1093/ageing/afaa089
- Chong E, Zhu B, Ng SHX, et al. Emergency department interventions for frailty (EDIFY): improving functional outcomes in older persons at the emergency department through a multicomponent frailty intervention. Age Ageing. 2022;51(2). doi:10.1093/ageing/afab251
- Chien CY, Chaou CH, Yeh CC, Hsu KH, Gao SY, Ng CJ. Using mobility status as a frailty indicator to improve the accuracy of a computerised five-level triage system among older patients in the emergency department. BMC Emerg Med. 2022;22(1):86. doi:10.1186/s12873-022-00646-0
- Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39(3):238–247. doi:10.1067/mem.2002.121523
- Grossmann FF, Zumbrunn T, Frauchiger A, Delport K, Bingisser R, Nickel CH. At risk of undertriage? Testing the performance and accuracy of the emergency severity index in older emergency department patients. Ann Emerg Med. 2012;60(3):317–325.e313. doi:10.1016/j.annemergmed.2011.12.013
- Ng CJ, Liao PJ, Chang YC, Kuan JT, Chen JC, Hsu KH. Predictive factors for hospitalization of nonurgent patients in the emergency department. Medicine (Baltimore). 2016;95(26):e4053. doi:10.1097/MD.0000000000004053
- O’Caoimh R, McGauran J, O’Donovan MR, et al. Frailty screening in the emergency department: comparing the variable indicative of placement risk, clinical frailty scale and PRISMA-7. Int J Environ Res Public Health. 2022;20(1):290. doi:10.3390/ijerph20010290
- Moldovan F. Sterile inflammatory response and surgery-related trauma in elderly patients with subtrochanteric fractures. Biomedicines. 2024;12(2):354. doi:10.3390/biomedicines12020354
- Fernando SM, Guo KH, Lukasik M, et al. Frailty and associated prognosis among older emergency department patients with suspected infection: a prospective, observational cohort study. CJEM. 2020;22(5):687–691. doi:10.1017/cem.2020.377
- O’Shaughnessy Í, Romero-Ortuno R, Edge L, et al. Home FIRsT: interdisciplinary geriatric assessment and disposition outcomes in the Emergency Department. Eur J Intern Med. 2021;85:50–55. doi:10.1016/j.ejim.2020.11.015
- Jung HW, Baek JY, Kwon YH, et al. At-point clinical frailty scale as a universal risk tool for older inpatients in acute hospital: a cohort study. Front Med Lausanne. 2022;9:929555. doi:10.3389/fmed.2022.929555
- Chen CL, Chen CM, Wang CY, et al. Frailty is associated with an increased risk of major adverse outcomes in elderly patients following surgical treatment of hip fracture. Sci Rep. 2019;9(1):19135. doi:10.1038/s41598-019-55459-2
- Covino M, Russo A, Salini S, et al. Frailty assessment in the emergency department for risk stratification of COVID-19 patients aged ≥80 years. J Am Med Dir Assoc. 2021;22(9):1845–1852.e1841. doi:10.1016/j.jamda.2021.07.005
- Huang HH, Lin PY, Chen TY, et al. Geriatric syndromes predict mortality of people aged 75+ years in the observation room of emergency department: towards function-centric emergency medicine. Arch Gerontol Geriatr. 2022;100:104662. doi:10.1016/j.archger.2022.104662
- Giroux M, Sirois MJ, Boucher V, et al. Frailty assessment to help predict patients at risk of delirium when consulting the emergency department. J Emerg Med. 2018;55(2):157–164. doi:10.1016/j.jemermed.2018.02.032
- Rosa F, Covino M, Russo A, et al. Frailty assessment as independent prognostic factor for patients ≥65 years undergoing urgent cholecystectomy for acute cholecystitis. Dig Liver Dis. 2023;55(4):505–512. doi:10.1016/j.dld.2022.10.012