Abstract
Type 2 diabetes mellitus (T2DM) is a risk factor for cognitive dysfunction and dementia in the elderly. T2DM has been thought to be associated with vascular diseases, eventually leading to vascular dementia, but recent studies have established that T2DM is also associated with Alzheimer’s disease (AD). With the increase in the number of elderly individuals with T2DM, the number of diabetic patients with cognitive dysfunction has been increasing. T2DM may accelerate AD-associated pathologies through insulin resistance. Vascular pathologies may also be associated with cognitive dysfunction and dementia in T2DM subjects. Several other mechanisms also seem to be involved in T2DM-related cognitive dysfunction. More investigations to clarify the association of T2DM with cognitive impairment are warranted. These investigations may help to increase our understanding of AD and open a new door to the development of therapeutics. Recent pharmaceutical advancement in T2DM treatment has resulted in the availability of a wide range of antidiabetics. Some evidence has suggested that antidiabetic therapies help to prevent cognitive dysfunction. At present, however, the optimal level of blood glucose control and the best combination of medications to achieve it in terms of cognitive preservation have not been established. More investigation is warranted. Cognitive dysfunction is an emerging new complication of T2DM that requires further study.
Introduction
With the progressive aging of the population in developed countries, an increasing number of patients suffer from dementia. Dementia is a disease condition that precludes the activities of daily life and self-care behaviors, and it constitutes a great burden on the patients themselves, their careers, and society. The fundamental therapeutics of dementia have not yet been determined, although some therapies for treating symptoms are available. To establish disease-modifying therapies for dementia, a wide range of investigations to elucidate the mechanism of the development and progression of cognitive dysfunction in the elderly is needed.
Diabetes mellitus is a heterogeneous disorder that is manifested in the form of hyperglycemia and glucose intolerance due to relative insulin deficiency, impaired effectiveness of insulin action, or both. About 347 million people have diabetes worldwide, and the number is increasing.Citation1 There are two types of diabetes mellitus, type 1 diabetes (T1DM) and type 2 diabetes (T2DM). These two types are differentiated on the basis of etiology and clinical presentation. T1DM is caused by the destruction of the insulin-producing cells of the pancreas, typically due to an autoimmune reaction. In T1DM, chronic hyperglycemia is caused by insulin deficiency. On the other hand, T2DM is characterized by insulin resistance (IR) and relative insulin deficiency. IR is defined as an inadequate response by insulin target tissues, such as skeletal muscle, liver tissue, and adipose tissue to the physiological effects of circulating insulin, and is often accompanied by raised insulin levels. T2DM is often, but not always, associated with obesity, which itself can cause IR and lead to elevated blood glucose levels.
The prevalence of T2DM has been rising in many regions of the world.Citation1 Many studies have established that T2DM is a risk factor for cognitive dysfunction and dementia in the elderly. With the increase in the number of elderly individuals with T2DM, the number of diabetic patients with cognitive dysfunction has been increasing. Recent remarkable advances in pharmacological therapy have made a variety of interventions available. Patients with T2DM have benefitted from advancements in preventing and treating the classic microvascular and macrovascular complications.Citation2 Cognitive dysfunction, however, has not been targeted by the current management strategies of T2DM. Cognitive impairment and dementia in patients with T2DM creates a large burden for patients, their families, and society. In the management of T2DM, self-care behavior is very important, but this behavior is impaired by cognitive dysfunction. The future development and implementation of diabetic treatments, especially for the elderly, should take brain protection into consideration.
T2DM and cognitive dysfunction or dementia
It has been established that T2DM is associated with cognitive dysfunction. While a wide range of cognitive domains was reportedly impaired in older patients with T2DM, one of the most frequently reported cognitive functional deficiencies in T2DM is impaired psychomotor speed.Citation3
The incidences of both T2DM and dementia increase in later life, which increases the prevalence of the comorbidity of these pathologies. Moreover, recent studies have indicated that older people with T2DM have a higher risk of cognitive dysfunction or dementia.Citation4 Ample evidence has indicated that T2DM is related not only to vascular dementia (VD) but also to the clinical diagnosis of Alzheimer’s disease (AD)-type dementia.Citation5
One of the earliest findings from a large epidemiological study showing that T2DM patients had an increased risk for developing dementia came from the Rotterdam Study.Citation6 Another study demonstrated a 65% increase of the risk for developing AD in T2DM subjects.Citation7 A cohort of Japanese-Americans in HawaiiCitation8 showed a 1.8-fold higher risk for developing AD and a 2.3-fold higher risk for vascular VD, and the Hisayama study reported similar results.Citation9 The reported risks for total dementia, AD, or VD in T2DM patients are similar (). A recent comprehensive meta-analysis of population-based longitudinal studies showed that the pooled relative risk of AD in subjects with T2DM (a total of 506 subjects) was 1.46 (1.20–1.77) compared with the subjects without T2DM (36,191 subjects).Citation10 For VD, the relative risk was 2.5 (2.1–3.0), based on ten studies including 3,519 subjects with T2DM and 23,026 subjects without.Citation10
Table 1 T2DM and risk of dementia, AD, or VD
Clinical diagnosis of AD
Recently, with the advancements of biomarkers, newer clinical criteria for AD can include the positive features of AD, including abnormality in cerebrospinal fluid (CSF) markers and positive amyloid imaging by positron emission tomography (PET).Citation11 However, the diagnosis of AD has depended on older clinical criteria, mostly those from the Diagnostic and Statistical Manual of Mental DisordersCitation12 or the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) Alzheimer’s Criteria,Citation13 in published research studies. It should be noted that the definitive diagnosis of AD is only made pathologically basically postmortem according to these criteria. The clinical diagnosis of AD in most of the previously published research studies was performed at least partly through the exclusion of diseases other than AD. The coexistence of a limited number of other pathologies including ischemic lesions does not prevent the diagnosis of AD. Indeed, more than 60% of autopsy-proven cases of AD had cerebrovascular lesions.Citation14 Moreover, neuronal dysfunction and death not related to the amyloid-β peptide (Aβ) or to hyperphosphorylation of the microtubule-associated protein tau but rather to other causes may coexist in clinically diagnosed AD. Although the results of clinical and epidemiological studies largely agree with the increase in AD by comorbidity of T2DM, this does not necessarily correspond to an accelerated AD pathology in T2DM subjects. Many dementia subjects, especially older subjects, have multiple pathologies.Citation15
Underlying mechanism of cognitive dysfunction in T2DM
The precise mechanisms underlying T2DM-related cognitive dysfunction or the development of dementia, especially AD-type dementia, remain to be elucidated; however, several hypothetical mechanisms have been proposed ().
Figure 1 Mechanism of T2DM-associated cognitive dysfunction.
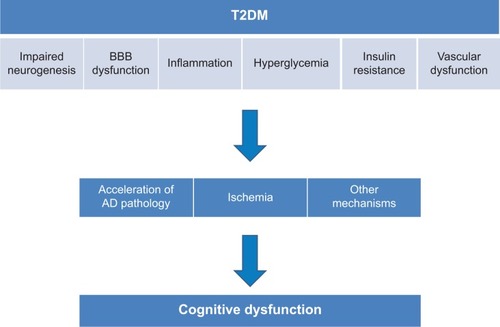
Neurogenesis
Neurogenesis in the hippocampus plays a role in learning and memory, and age-associated decline in neurogenesis has been reported.Citation16 Basic and animal experiments have indicated that a hyperglycemic environment induces the proliferation of adult neural progenitors, but is detrimental to their survival. The impaired neurogenesis in T2DM subjects may underlie an associated cognitive impairment and brain atrophy.Citation17,Citation18
Blood–brain barrier
The blood–brain barrier (BBB) consists of tight junctions between endothelial cells and astrocytic projections, which regulate paracellular and transcellular flow into the central nervous system (CNS), respectively.
Previous observations in brain tissue biopsied from AD subjects have indicated BBB breakdown in several respects.Citation19,Citation20 These include thinning of the endothelium, loss of mitochondria, and thickening of the basement membranes, the latter of which increase the accumulation of focal Aβ peptides. A break in the BBB also leads to potentially toxic substances and metabolites gaining access to the brain.Citation21,Citation22
Diabetes is associated with changes in both the barrier and transport functions of the cerebral microvessels.Citation23 Dysfunction in the BBB may be associated with cognitive impairment and/or the incidence of dementia.
Hyperglycemia
Chronically higher blood glucose levels exert a negative influence on cognition and cause structural changes in the hippocampus.Citation24 A recent longitudinal study over 6 years also reported higher glucose levels and an increased risk of the incidence of dementia in both diabetes and non-diabetes subjects.Citation25
High glucose concentration, a major pathological characteristic of diabetes, may have toxic effects on neurons in the brain through several mechanisms. Osmotic insults and oxidative stress may be involved in the mechanism, and the maintenance of chronic high glucose leads to the enhanced formation of advanced glycation end products (AGEs),Citation26 which have potentially toxic effects on neurons. Reportedly, AD patients with T2DM have increased levels of AGEs and microglial activation in the CNS compared to AD patients without T2DM. AGEs couple with free radicals and create oxidative damage, which in turn leads to neuronal injury.Citation27 In addition to their direct toxicity, AGEs also activate microglia in the CNS. A wealth of evidence has demonstrated that microglia, the resident innate immune cells in the brain, can become deleterious and damage neurons.Citation28 This process is implicated as an underlying mechanism in diverse neurodegenerative diseases, including AD. While microglial function is beneficial and mandatory for normal CNS functioning, unregulated overactivation of microglia causes damage to neurons. In diabetes, oxidative stress also increases because of reduced antioxidant capacity.Citation29 Oxidative stress has been suggested to lead to neuronal injury through mitochondrial dysfunction.Citation30
Inflammatory mechanism
Inflammation plays a role in the pathogenesis of IR and T2DM.Citation31 It has also been suggested that inflammation is associated with the pathogenesis of AD. Chronic low-grade inflammation may be a contributor to the disease process of AD.Citation32 Proinflammatory cytokines such as tumor necrosis factor alpha are known to be involved in the pathogenesis of both T2DM and AD.Citation33 The activation of glia by inflammatory cytokines damages the neurons. Therefore, inflammation may be a link between T2DM and dementia, especially AD.
IR
Insulin is a hormone that regulates blood glucose levels. It is primarily secreted by the beta cells of the pancreas and is normally released into the systemic circulation through the portal vein in response to a rise in blood glucose. It is catabolized by insulin-degrading enzyme (IDE) in the liver, kidneys, and muscles.Citation34,Citation35 Insulin working within the brain is presumably of pancreatic origin, and it has passed the BBB through a saturable transporter mechanism from the systemic circulation, although there is debate about the amount of insulin that is produced de novo within the CNS.Citation36 Insulin has multiple important functions in the brain including the control of food intake (via insulin receptors located in the olfactory bulb and thalamus) and effects on cognitive functions, including memory.Citation37,Citation38
Blood glucose abnormalities and IR may be associated with acetylcholine (ACh) synthesis. ACh transferase, which is an enzyme responsible for ACh synthesis, is expressed in insulin-receptor-positive cortical neurons, and insulin regulates ACh transferase expression. Because ACh is a critical neurotransmitter in cognitive function, it may be relevant to neurocognitive disorders in diabetics.Citation39
The critical pathological mechanism in AD is an accumulation of Aβ. Overproduction of Aβ may be one of the mechanisms by which this accumulation occurs; however, the impairment of clearance of Aβ may also play a role. The desensitization of insulin receptors, ie, IR, reduces the synthesis of several proteins, including IDE. IDE degrades Aβ as well as insulin, and reduced amounts of IDE may result in greater amyloid deposition. Basic research has indicated that insulin controls the expression of IDE in the brain, and IR in the brain may downregulate IDE expression.Citation40,Citation41
Another critical pathology in AD is the hyperphosphorylation of tau. Insulin seems to be an important determinant of tau protein phosphorylation. Impaired insulin signaling can result in the inhibition of PI3K/Akt and the activation of glycogen synthase kinase-3β. The activation of glycogen synthase kinase-3β leads to the enhanced phosphorylation of tau protein and the formation of neurofibrillary tangles.Citation42,Citation43
A recent study by Talbot et al demonstrated that IR in neurons exists in AD.Citation44 The ex vivo stimulation of insulin receptors in the brains of AD patients nicely showed that insulin signaling is greatly reduced where the phosphorylation of insulin receptor substrate 1 occurs at several serine residues, which has been known to be a feature of IR. Moreover, the levels of insulin receptor substrate 1 phosphorylation in hippocampal neurons were found to be negatively correlated with episodic and working memory. Another study investigated the critical role of the soluble Aβ oligomer in producing brain IR in AD, and successfully demonstrated the pharmacological manipulation of this pathway by a glucagon-like peptide 1 (GLP-1) agonist.Citation45 Intranasal insulin reportedly improved memory and attention in humans.Citation46 These achievements may hold promise for brain IR as a future therapeutic target in AD.
Vascular dysfunction
Excretion of even a very low amount of albumin in urine (microalbuminuria) is a good marker of cardiovascular events.Citation47 Microalbuminuria, a marker of vascular dysfunction, predicted accelerated cognitive decline in T2DM subjects.Citation48–Citation51 These findings suggest that a deficit of vascular endothelial cells can lead to impairment of the functional coordination of the vascular supply in a timely response to the demand created by nervous activity. Neural activity requires a strong increase of cerebral blood flow and an acute increase in neuronal glucose. Hemodynamic neurovascular coupling coordinates these links (neurovascular units). Dysfunction of cerebral autoregulation with increasing age along with structural and functional alterations in cerebral blood vessels due to diabetes mellitus impairs the functioning of neurovascular units. These phenomena may induce functional deficits in neurons and increase neuronal degeneration and the susceptibility to hypoxia and ischemia.Citation52 Impaired neurovascular units would also induce BBB leakage.Citation52 Recently, the hypothesis that vascular dysfunction may impair the drainage pathways of Aβ from the brain parenchyma and thus increase the deposition of Aβ has been drawing interest.Citation53 Vascular dysfunction could be associated with the progression of amyloid pathology.
Neurodegeneration, ischemic pathology, or functional alteration?
van Harten et al in their systematic review, reported consistent associations of T2DM with atrophy across many brain regions,Citation54 and more recent reports have made similar findings.Citation55–Citation61 It was reported by den Heijer et al in their review that subjects with T2DM had more hippocampal and amygdalar atrophy on magnetic resonance imaging (MRI) compared to subjects without T2DM, and they speculated that this could suggest that T2DM directly influences the development of AD-related neuropathology.Citation62 More recently, Willette et al reported that IR in an asymptomatic, late middle-aged cohort was associated with progressive atrophy in regions affected by early AD,Citation63 and similar atrophy was found in older T2DM patients by Moran et al.Citation64 A PET study demonstrated that subjects with prediabetes and early T2DM have Alzheimer-like reductions in regional cerebral glucose metabolism.Citation65 These clinical studies may suggest that T2DM induces the AD-related pathology, and the acceleration of AD pathologies leads to neurodegeneration and brain atrophy.
In the Rotterdam study, a non-diabetic population cohort study, it was reported that IR assessed by homeostasis model assessment ratio was associated with the onset of AD within 3 years.Citation66 A Japanese epidemiological study, the Hisayama study, reported that IR was associated with AD pathology.Citation67 The results of pathological studies using autopsy samples, however, have not been consistent in terms of the association of T2DM with AD pathology. One study demonstrated that diabetics show significantly less AD-associated neuropathology,Citation68 while another failed to show any relationship between diabetes and AD-associated neuropathology.Citation69 The Baltimore Longitudinal Study of Aging (BLSA) recently reported that the association between AD-related pathology and IR could not be confirmed.Citation70 Body mass index, serum insulin level, and IR level were very different between the Hisayama study and the BLSA study, which makes it difficult to interpret and compare the results of these two studies. A recently published study making use of amyloid PET imaging did not find differences in amyloid accumulation between AD subjects with or without T2DM.Citation71
If T2DM has an impact on cognition only through accelerating AD pathology, the extent of the pathology should be similar when cases of cognitive impairment with similar levels of severity are compared cross-sectionally, either pathologically or using amyloid imaging. Thus, the rate of progression would also be considered when investigating the role of T2DM in increasing the incidence of AD by using amyloid PET or CSF biomarkers.
Another issue to be addressed in future studies is the Apo E genotype. Many studies reported that the Apo E4 genotype, which is a major genetic risk factor for AD,Citation72 enhanced the association of AD with T2DM.Citation73,Citation74 Thus, considering the impact of the Apo E genotype is warranted in future pathological studies.
T2DM is an established risk factor for microvascular and macrovascular complications throughout the body, including brain stroke and small vessel disease.Citation75 Therefore, vascular damage is likely to be one of the main reasons for the cognitive impairment in T2DM subjects, including VD subjects. Recent advances in MRI technology, specifically diffusion tensor imaging, have allowed subtle changes in white matter to be revealed. Some studies have reported T2DM to be associated with microstructural abnormalities in the white matter in various pathways in the brain independent of small vessel diseases, and these abnormalities are also associated with cognitive impairment.Citation76,Citation77 The combination of vascular lesions and AD pathology is very common in T2DM subjects, and cognitive dysfunction due to vascular pathology may lower the threshold of clinical diagnosis of dementia in early AD subjects.
A neurodegenerative mechanism other than AD pathology may contribute to the cognitive dysfunction in T2DM. Oxidative stress or neuroinflammation may be associated with neuronal death or synaptic dysfunction apart from Aβ or tau pathology.
The extent of the contribution of both neurodegeneration including AD pathology and vascular changes to T2DM-associated cognitive impairment should be further explored. Moreover, functional alteration of neurovascular units and synapses that are not necessarily associated with neuronal death may also contribute. In this case, imaging studies or pathological examination by autopsy might not find the differences between T2DM and non-T2DM subjects.
The effects of blood glucose control
Several studies have suggested that higher blood glucose levels as indicated by higher glycohemoglobin (HbA1c) levels were associated with worse cognitive performance.Citation78–Citation81
A large randomized controlled trial, the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial, aimed to compare the effects of intensive versus standard glycemic control on cognitive function and brain volume. Significant differences in total brain volumes favored the intensive treatment, although the cognitive outcomes were not different.Citation82 Another randomized controlled trial demonstrated that the intervention was related to slower global cognitive decline in the intervention group. Improvements in HbA1c, but not in other clinical indices including blood pressure and lipid levels, mediated the effect of the intervention on cognitive decline.Citation83 These results suggest that blood glucose control is important for the preservation of cognitive function in elderly diabetic patients. A recent prospective study by van den Berg et al however, reported that HbA1c levels at baseline had no effect in five cognitive domains.Citation84,Citation85 Another study found an inverse relationship between HbA1c and cognitive performance.Citation86 Large prospective studies are warranted to establish the optimal blood glucose level for cognitive preservation. Moreover, with the advancement of pharmaceutical therapeutics, an increasing number of antidiabetics have become available. Each medicine has its own advantages in terms of pharmacological characteristics that may be associated with brain protection. The best combination of medicines for treating older T2DM patients should be explored. Another recent report suggested that a history of severe hypoglycemic episodes was associated with a greater risk of dementia.Citation87 The merits of pharmacological blood glucose lowering should be balanced with the risk of hypoglycemia, especially in a frail elderly population.
Future directions
The elucidation of the mechanism of T2DM-associated cognitive dysfunction and dementia may lead to the development of therapeutics for this specific condition as well as for dementia, including AD, in general. Several basic studies have suggested that IR accelerates the AD-related pathology, and clinical evidence partly supports this hypothesis; however, general agreement on this point has not been reached.Citation41,Citation43 By taking advantage of recent advancements in imaging, including amyloid imaging technology with PET,Citation84 higher field MRI with some potential for imaging small vessel diseases,Citation88 and the diffusion tensor imaging method,Citation89 it may be possible to investigate the relative contributions of AD-related pathology and ischemic changes to the increased prevalence of dementia in T2DM subjects. Furthermore, CSF biomarkers such as total tau, hyperphosphorylated tau, and the 42-amino-acid form of Aβ are now established biomarkers for ADCitation90 and can be used to identify AD in the early or mild cognitive impairment stage of the disease with high accuracy.Citation91,Citation92 Identifying AD before the development of overt dementia and closely following up with the patient would make it possible to compare both the amyloid load and ischemic lesions before and after the development of dementia. Moreover, amyloid imaging and the measuring of CSF biomarkers in non-demented older people with or without IR could verify the hypothesis that insulin plays a role in the processing and deposition of Aβ. These investigations are important considering the potential future availability of disease-modifying therapeutics such as Aβ vaccination and inhibitors of Aβ secretions.
Nasal insulin or GLP-1 analogs or other medications targeting the brain insulin system may be therapies available in the future. By the intranasal method, insulin effectively bypasses the BBB and can be delivered into the brain. Clinical trials have demonstrated that intranasal insulin has some beneficial effects on cognition in patients with mild cognitive impairment and AD.Citation93 GLP-1 analogs have been demonstrated to exert neuroprotective and aniapoptotic effects, reduce Aβ plaque accumulation, modulate long-term potentiation and synaptic plasticity, and promote the differentiation of neuronal progenitor cells. In animal models of behavior, treatment with GLP-1 receptor agonists has been demonstrated to improve measures of cognitive function, including learning and memory, as well as to reduce depressive behavior.Citation94
It is a clinically important question whether the treatment for vascular risk factors has therapeutic potential in terms of slowing the progression of cognitive dysfunction, as neurodegenerative pathologies are not yet amenable to treatment. Vascular risk factors including T2DM and hypertension are reported to be associated with the progression of lacunae and white matter lesions in the brain.Citation95 However, it remains to be investigated whether pharmaceutical interventions with antidiabetics and antihypertensives have protective effects against the progression of cognitive dysfunction and the development of dementia. If such beneficial effects do exist, the underlying mechanism of the therapeutic effects should also be explored. Such investigation may lead to the elucidation of the fundamental mechanism of the involvement of T2DM in the development of dementia.
With the ongoing increase in the size of the elderly population, T2DM-associated cognitive dysfunction and dementia are becoming increasingly larger problems. Developing a greater understanding of the relevant pathophysiology and establishing better therapeutic interventions are urgent needs.
Cognitive dysfunction in T2DM may start at a relatively young age; thus, starting the management at an early age may be important in terms of preventing not only dementia but also other complications.Citation96,Citation97
Conclusion
T2DM is associated with cognitive dysfunction and the incidence of dementia including AD. The underlying mechanism of this association should be elucidated. Such elucidation could lead to a clarification of the pathogenesis of AD and to the development of a treatment or method of prevention.Citation98 Moreover, it is urgent that the optimal level of blood glucose control and the best combination of medications for enhancing cognitive preservation be established.
Disclosure
The author reports no conflicts of interest in this work.
References
- DanaeiGFinucaneMMLuYGlobal Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose)National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participantsLancet20113789785314021705069
- Abi KhalilCRousselRMohammediKDanchinNMarreMCause-specific mortality in diabetes: recent changes in trend mortalityEur J Prev Cardiol201219337438122991697
- McCrimmonRJRyanCMFrierBMDiabetes and cognitive dysfunctionLancet201237998332291229922683129
- StewartRLiolitsaDType 2 diabetes mellitus, cognitive impairment and dementiaDiabet Med19991629311210229302
- LiLHölscherCCommon pathological processes in Alzheimer disease and type 2 diabetes: a reviewBrain Res Rev200756238440217920690
- OttAStolkRPvan HarskampFDiabetes mellitus and the risk of dementia: The Rotterdam StudyNeurology19995391937194210599761
- ArvanitakisZWilsonRSBieniasJLEvansDABennettDADiabetes mellitus and risk of Alzheimer disease and decline in cognitive functionArch Neurol200461566166615148141
- PeilaRRodriguezBLLaunerLJHonolulu-Asia Aging StudyType 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging StudyDiabetes20025141256126211916953
- OharaTDoiYNinomiyaTGlucose tolerance status and risk of dementia in the community: the Hisayama studyNeurology201177121126113421931106
- ChengGHuangCDengHWangHDiabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studiesIntern Med J201242548449122372522
- McKhannGMKnopmanDSChertkowHThe diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s diseaseAlzheimers Dement20117326326921514250
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association2000
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s DiseaseNeurology19843479399446610841
- JellingerKAPrevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseasesNeurodegener Dis201071–311211520173339
- SchneiderJAArvanitakisZBangWBennettDAMixed brain pathologies account for most dementia cases in community-dwelling older personsNeurology200769242197220417568013
- LeeSWClemensonGDGageFHNew neurons in an aged brainBehav Brain Res2012227249750722024433
- LangBTYanYDempseyRJVemugantiRImpaired neurogenesis in adult type-2 diabetic ratsBrain Res20091258253319138677
- MachidaMFujimakiSHidakaRAsashimaMKuwabaraTThe insulin regulatory network in adult hippocampus and pancreatic endocrine systemStem Cells Int2012201295973722988465
- FarrallAJWardlawJMBlood–brain barrier: ageing and microvascular disease – systematic review and meta-analysisNeurobiol Aging200930333735217869382
- KalariaRNCerebral vessels in ageing and Alzheimer’s diseasePharmacol Ther19967231932149364575
- BellRDZlokovicBVNeurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s diseaseActa Neuropathol2009118110311319319544
- IadecolaCNedergaardMGlial regulation of the cerebral microvasculatureNat Neurosci200710111369137617965657
- MooradianADCentral nervous system complications of diabetes mellitus – a perspective from the blood–brain barrierBrain Res Brain Res Rev19972332102189164671
- KertiLWitteAVWinklerAGrittnerURujescuDFlöelAHigher glucose levels associated with lower memory and reduced hippocampal microstructureNeurology201381201746175224153444
- CranePKWalkerRHubbardRAGlucose levels and risk of dementiaN Engl J Med2013369654054823924004
- YamagishiSUedaSOkudaSFood-derived advanced glycation end products (AGEs): a novel therapeutic target for various disordersCurr Pharm Des200713272832283617897026
- ValenteTGellaAFernàndez-BusquetsXUnzetaMDuranyNImmunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitusNeurobiol Dis2010371677619778613
- BlockMLZeccaLHongJSMicroglia-mediated neurotoxicity: uncovering the molecular mechanismsNat Rev Neurosci200781576917180163
- EvansJLGoldfineIDMadduxBAGrodskyGMOxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetesEndocr Rev200223559962212372842
- MoreiraPISantosMSSeiçaROliveiraCRBrain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetesJ Neurol Sci20072571–220621417316694
- BadawiAKlipAHaddadPType 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional interventionDiabetes Metab Syndr Obes2010317318621437087
- AkiyamaHBargerSBarnumSInflammation and Alzheimer’s diseaseNeurobiol Aging200021338342110858586
- ZhaoWQTownsendMInsulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s diseaseBiochim Biophys Acta20091792548249619026743
- WatsonGSCraftSThe role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatmentCNS Drugs2003171274512467491
- DavisSNGrannerDKInsulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreasHardmanJGGilmanAGLimbirdLEGoodman and Gilman’s the Pharmacological Basis of Therapeutics9th edNew YorkMcGraw-Hill199614871517
- WoodsSCSeeleyRJBaskinDGSchwartzMWInsulin and the blood–brain barrierCurr Pharm Des200391079580012678878
- HavrankovaJRothJBrownsteinMInsulin receptors are widely distributed in the central nervous system of the ratNature19782725656827829205798
- FreychetPInsulin receptors and insulin action in the nervous systemDiab Metab Res Rev2000166390392
- RiveraEJGoldinAFulmerNTavaresRWandsJRde la MonteSMInsulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholineJ Alzheimers Dis20058324726816340083
- ZhaoLTeterBMoriharaTInsulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease interventionJ Neurosci20042449111201112615590928
- HoLQinWPomplPNDiet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s diseaseFASEB J200418790290415033922
- UmegakiHPathophysiology of cognitive dysfunction in older people with type 2 diabetes: vascular changes or neurodegeneration?Age Ageing201039181019917634
- SchubertMBrazilDPBurksDJInsulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylationJ Neurosci200323187084709212904469
- TalbotKWangHYKaziHDemonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive declineJ Clin Invest201212241316133822476197
- BomfimTRForny-GermanoLSathlerLBAn anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomersJ Clin Invest201212241339135322476196
- BenedictCHallschmidMHatkeAIntranasal insulin improves memory in humansPsychoneuroendocrinology200429101326133415288712
- de ZeeuwDParvingHHHenningRHMicroalbuminuria as an early marker for cardiovascular diseaseJ Am Soc Nephrol20061782100210516825327
- de BresserJReijmerYDvan den BergEUtrecht Diabetic Encephalopathy Study GroupMicrovascular determinants of cognitive decline and brain volume change in elderly patients with type 2 diabetesDement Geriatr Cogn Disord201030538138620962529
- KawamuraTUmemuraTUmegakiHEffect of renal impairment on cognitive function during a 3-year follow-up in elderly patients with type 2 diabetes: association with microinflammationJ Diabetes Investig Epub201424
- UmegakiHIimuroSShinozakiTJapanese Elderly Diabetes Intervention Trial Study GroupRisk factors associated with cognitive decline in the elderly with type 2 diabetes: baseline data analysis of the Japanese Elderly Diabetes Intervention TrialGeriatr Gerontol Int201212Suppl 110310922435945
- UmemuraTKawamuraTUmegakiHAssociation of chronic kidney disease and cerebral small vessel disease with cognitive impairment in elderly patients with type 2 diabetesDement Geriatr Cogn Dis Extra20133121222223888167
- DalkaraTGursoy-OzdemirYYemisciMBrain microvascular pericytes in health and diseaseActa Neuropathol201112211921656168
- WellerROMasseyAKuoYMRoherAECerebral amyloid angiopathy: accumulation of A beta in interstitial fluid drainage pathways in Alzheimer’s diseaseAnn N Y Acad Sci200090311011710818495
- van HartenBde LeeuwFEWeinsteinHCScheltensPBiesselsGJBrain imaging in patients with diabetes: a systematic reviewDiabetes Care200629112539254817065699
- ManschotSMBrandsAMvan der GrondJUtrecht Diabetic Encephalopathy Study GroupBrain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetesDiabetes20065541106111316567535
- KorfESvan StraatenECde LeeuwFELADIS Study GroupDiabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS studyDiabet Med200724216617117257279
- LastDAlsopDCAbduljalilAMGlobal and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivityDiabetes Care20073051193119917290035
- GoldSMDziobekISweatVHippocampal damage and memory impairments as possible early brain complications of type 2 diabetesDiabetologia200750471171917334649
- BruehlHWolfOTSweatVTirsiARichardsonSConvitAModifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitusBrain Res2009128018619419463794
- BurnsJMDonnellyJEAndersonHSPeripheral insulin and brain structure in early Alzheimer diseaseNeurology200769111094110417846409
- BurnsJMHoneaRAVidoniEDHutflesLJBrooksWMSwerdlowRHInsulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and agingBiochim Biophys Acta20121822333333921745566
- den HeijerTVermeerSEvan DijkEJType 2 diabetes and atrophy of medial temporal lobe structures on brain MRIDiabetologia200346121604161014595538
- WilletteAAXuGJohnsonSCInsulin resistance, brain atrophy, and cognitive performance in late middle-aged adultsDiabetes Care201336244344923069842
- MoranCPhanTGChenJBrain atrophy in type 2 diabetes: regional distribution and influence on cognitionDiabetes Care201336124036404223939539
- BakerLDCrossDJMinoshimaSBelongiaDWatsonGSCraftSInsulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetesArch Neurol2011681515720837822
- SchrijversEMWittemanJCSijbrandsEJHofmanAKoudstaalPJBretelerMMInsulin metabolism and the risk of Alzheimer disease: the Rotterdam StudyNeurology201075221982198721115952
- MatsuzakiTSasakiKTanizakiYInsulin resistance is associated with the pathology of Alzheimer disease: the Hisayama studyNeurology201075976477020739649
- BeeriMSSilvermanJMDavisKLType 2 diabetes is negatively associated with Alzheimer’s disease neuropathologyJ Gerontol A Biol Sci Med Sci200560447147515933386
- ArvanitakisZSchneiderJAWilsonRSDiabetes is related to cerebral infarction but not to AD pathology in older personsNeurology200667111960196517159101
- ThambisettyMJeffrey MetterEYangAGlucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of AgingJAMA Neurol20137091167117223897112
- TomitaNFurukawaKOkamuraNBrain accumulation of amyloid β protein visualized by positron emission tomography and BF-227 in Alzheimer’s disease patients with or without diabetes mellitusGeriatr Gerontol Int201313121522122680403
- StrittmatterWJRosesADApolipoprotein E and Alzheimer diseaseProc Natl Acad Sci U S A19959211472547277761390
- DoreGAEliasMFRobbinsMAEliasPKNagyZPresence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine-Syracuse StudyDiabetologia200952122551256019693485
- IrieFFitzpatrickALLopezOLEnhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition StudyArch Neurol2008651899318195144
- LuitseMJBiesselsGJRuttenGEKappelleLJDiabetes, hyperglycaemia, and acute ischaemic strokeLancet Neurol201211326127122341034
- ReijmerYDLeemansABrundelMKappelleLJBiesselsGJUtrecht Vascular Cognitive Impairment Study GroupDisruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetesDiabetes20136262112211523349494
- ReijmerYDBrundelMde BresserJKappelleLJLeemansABiesselsGJUtrecht Vascular Cognitive Impairment Study GroupMicrostructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging studyDiabetes Care201336113714422961577
- Cukierman-YaffeTGersteinHCWilliamsonJDAction to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) InvestigatorsRelationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trialDiabetes Care200932222122619171735
- MaggiSLimongiFNoaleMILSA Study GroupDiabetes as a risk factor for cognitive decline in older patientsDement Geriatr Cogn Disord2009271243319088471
- PetersRBeckettNForetteFHYVET investigatorsIncident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trialLancet Neurol20087868368918614402
- UmegakiHKawamuraTMogiNUmemuraTKanaiASanoTGlucose control levels, ischaemic brain lesions, and hyperinsulinaemia were associated with cognitive dysfunction in diabetic elderlyAge Ageing200837445846118339615
- LaunerLJMillerMEWilliamsonJDACCORD MIND investigatorsEffects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudyLancet Neurol2011101196997721958949
- LuchsingerJAPalmasWTeresiJAImproved diabetes control in the elderly delays global cognitive declineJ Nutr Health Aging201115644544921623465
- IkonomovicMDKlunkWEAbrahamsonEEPost-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s diseaseBrain2008131Pt 61630164518339640
- van den BergEReijmerYDde BresserJKesselsRPKappelleLJBiesselsGJUtrecht Diabetic Encephalopathy Study GroupA 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitusDiabetologia2010531586519882137
- ShorrRIde RekeneireNResnickHEGlycemia and cognitive function in older adults using glucose-lowering drugsJ Nutr Health Aging200610429730116886100
- WhitmerRAKarterAJYaffeKQuesenberryCPSelbyJVHypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitusJAMA2009301151565157219366776
- NovakVAbduljalilAMNovakPRobitaillePMHigh-resolution ultrahigh-field MRI of strokeMagn Reson Imaging200523453954815919599
- KodlCTFrancDTRaoJPDiffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive functionDiabetes200857113083308918694971
- ZetterbergLOWahlundKBlennowCerebrospinal fluid markers for prediction of Alzheimer’s diseaseNeurosci Lett20033521676914615052
- HanssonHZetterbergPBuchhaveELondosEBlennowKMinthonLAssociation between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up studyLancet Neurol20065322823416488378
- EwersMBuergerKTeipelSJMulticenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCINeurology200769242205221218071141
- SchiöthHBCraftSBrooksSJFreyWHBenedictCBrain insulin signaling and Alzheimer’s disease: current evidence and future directionsMol Neurobiol201246141022205300
- McIntyreRSPowellAMKaidanovich-BeilinOThe neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disordersBehav Brain Res201323716417123000536
- GouwAAvan der FlierWMFazekasFLADIS Study GroupProgression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability studyStroke20083951414142018323505
- van EerselMEJoostenHGansevoortRTDullaartRPSlaetsJPIzaksGJThe interaction of age and type 2 diabetes on executive function and memory in persons aged 35 years or olderPLoS One2013812e8299124367577
- JoostenHvan EerselMEGansevoortRTBiloHJSlaetsJPIzaksGJCardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjectsStroke20134461543154923640826
- TangJPeiYZhouGWhen aging-onset diabetes is coming across with Alzheimer disease: comparable pathogenesis and therapyExp Gerontol201348874475023648584
