Abstract
The Lifestyle Interventions and Independence for Elders (LIFE) Study is a Phase III randomized controlled clinical trial (Clinicaltrials.gov identifier: NCT1072500) that will provide definitive evidence regarding the effect of physical activity (PA) on major mobility disability in older adults (70–89 years old) who have compromised physical function. This paper describes the methods employed in the delivery of the LIFE Study PA intervention, providing insight into how we promoted adherence and monitored the fidelity of treatment. Data are presented on participants’ motives and self-perceptions at the onset of the trial along with accelerometry data on patterns of PA during exercise training. Prior to the onset of training, 31.4% of participants noted slight conflict with being able to meet the demands of the program and 6.4% indicated that the degree of conflict would be moderate. Accelerometry data collected during PA training revealed that the average intensity – 1,555 counts/minute for men and 1,237 counts/minute for women – was well below the cutoff point used to classify exercise as being of moderate intensity or higher for adults. Also, a sizable subgroup required one or more rest stops. These data illustrate that it is not feasible to have a single exercise prescription for older adults with compromised function. Moreover, the concept of what constitutes “moderate” exercise or an appropriate volume of work is dictated by the physical capacities of each individual and the level of comfort/stability in actually executing a specific prescription.
Introduction
The 2010 US census revealed that the population of adults aged 65+ experienced an annual net increase of just over 800,000 people; in that same year, the group of older adults aged 75–84 was 17-times larger than in 1900 and the age group 85+ was 45 times larger.Citation1 Although prolongation of life remains an important public health goal, of even greater significance is that extended life should involve preservation of the capacity to live independently and to function well.Citation2 Because compromised mobility poses a risk for the loss of independenceCitation3 and compromises quality of life,Citation4 an important challenge for public health is to identify interventions that might prevent major mobility disability in at-risk aging populations.Citation5
The Lifestyle Interventions and Independence for Elders (LIFE) Study is a Phase III multicenter randomized controlled trialCitation6 funded by a cooperative agreement with the National Institute of Aging.Citation7 It has been designed to compare the effects of a supervised moderate-intensity physical activity (PA) program with a successful aging health-education program on the incidence of major mobility disability – the inability to walk 400 m – in sedentary older persons, 70–89 years of age, with objectively measured functional limitations. Participants will be followed for an average of 2.7 years.
This paper provides an in-depth presentation of the methods employed in the delivery of the LIFE Study PA intervention. Descriptive data are presented on participants’ motives and self-perceptions related to the adoption of a physically active lifestyle along with accelerometry data describing participants’ patterns of activity during center-based exercise sessions.
Methods: the LIFE Study PA intervention
Participants
The LIFE Study eligibility criteria targeted older persons, aged 70–89, who were: sedentary; at high risk for mobility disability; able to walk 400 meters (m) in ≤15 minutes without sitting, using a walker, or needing the help of another person; and able to safely participate in the intervention. Individuals with a Short Physical Performance Battery scoreCitation8 ≤7 were preferentially enrolled (45% of the sample) to enrich the sample with individuals at high risk for major mobility disability. A total of 1,635 participants were randomized at eight field centers (see “Acknowledgments” section), with 818 being randomized to the PA intervention. Further details on inclusion and exclusion can be found in the study design paper.Citation7
Pre-training semi-structured interview
Once participants had been randomized to the PA intervention, they were scheduled for a one-on-one semi-structured interview/information session that took ~1 hour to complete. Described in detail following, these sessions overviewed the specific structure of the intervention, involved a 5-minute walk with participants that permitted a quick assessment of gait during activity as well as a guided mastery experience, and were used to gather information related to participants’ involvement in the LIFE Study PA program.Citation7
Participants were first asked an open-ended question concerning what motivated them to join the LIFE Study and, specifically, what benefits they hoped to achieve. They were then asked to describe their prior experiences with exercise and whether there was anything that could get in the way of their participation such as taking care of a spouse or other family member, health issues, or current physical symptoms. At this point in the interaction, the interventionist provided an overview of the structure of the center- and home-based components of the PA program. Following this, participants were asked to rate the positive or negative influence that their neighborhoods, family/friends, and physicians would have on them being physically active. Each factor was rated on a seven-point scale ranging from +3 to −3, with 0 the neutral point (neither positive nor negative). After this information was collected, a self-efficacy item was asked: “At this point in time, how confident are you that you will be able to do what we are asking you to do?” A 0–10 scale was used that ranged from 0 (not at all confident) to 10 (extremely confident). Finally, cognizant of participants’ functional status and health, the interviewer discussed long-term goals that they might achieve resulting from their involvement in the LIFE Study. They were then asked: “Considering everything in your life at the present time, how much do you value these goals on a scale from 0 (not at all) to 10 (extremely so)?”
The PA training program
The PA intervention includes aerobic, strength, balance, and flexibility training; each session is preceded by a brief warm up (5 minutes) and followed by a brief cool down (5 minutes). The aerobic component involves up to 40 minutes of walking, dependent on participants’ physical symptoms and health status. The strength-training component is of 10 minutes duration and focuses on the lower extremity muscle groups by using variable-weight ankle weights or the person’s own body weight. Similarly, the balance component is of 10 minutes duration. The session ends with a 5-minute flexibility routine. The specific exercises used for strength, balance, and flexibility can be requested from the LIFE Study public website (see www.thelifestudy.org). Participants attend center-based small-group sessions led by trained interventionists two times each week with home-based activity prescribed as frequently as tolerated. The strength and balance components of training are performed at the two center-based sessions held each week with one additional session prescribed for completion at home. Further details on the training program can be found in the LIFE Study design paper.Citation7
The LIFE PA intervention: a conceptual model in action
Maintaining the fidelity of the LIFE Study PA intervention and problem-solving behavioral issues that arise during the course of the study is the responsibility of two senior behavioral scientists who are members of the Lifestyle Resource Core (LRC) in conjunction with behavioral scientists at each site. These individuals are directly responsible to and work with the LIFE Study Intervention Committee. The two LRC investigators each supervise four sites, holding monthly phone calls with those sites under their supervision. In addition, each site is responsible for holding weekly staff meetings in conjunction with their behavioral scientist and other staff members to review intervention-related reports that are generated by the coordinating center and to discuss problem participants. The LRC calls are used to distribute news items, review adherence across the sites, assist in problem-solving challenges that may arise, and, most importantly, share strategies that are proving useful in managing individual participants.
Promotion of the adoption and maintenance of a physically active lifestyle within the LIFE Study is best characterized as a dynamic process of self-regulation; one that is dependent on the individual, the center-based group environment in which the PA behavior is shaped, and the larger environmental context of each participant’s life.Citation9 Although the active role played by interventionists is largely limited to interactions with participants both as individuals and members of these center-based small-group sessions, there are three ways in which the LIFE Study PA intervention considers the larger environmental context of participants’ lives. First, for a sizable group of individuals, arrangements are made for transportation to center-based sessions. The methods used by sites vary widely but include public transportation, taxi services, and private study-supported vans. Second, home-based exercise is an important component of the intervention. If neighborhood environments are unsafe, local resources are explored as options for performing PA outside the home. Third, to maximize the success of home-based PA, highly specific PA plans are mapped out with each participant; participants then complete weekly self-monitoring logs and submit them to the staff at center-based visits. When the logs are returned, interventionists review data with participants, modify goals as needed, and then enter the data into a web-based tracking system.
Central to the design and conduct of the LIFE Study PA intervention are two constructs from social cognitive theory: self-efficacy beliefs and outcome expectations.Citation10 Specifically, participants are likely to adhere to prescribed behaviors if they feel confident in their ability to do so (self-efficacy) and if they experience valued, tangible benefits for their efforts (outcome expectancies). Relevant to the primary study outcome in the LIFE Study are recent data of ours showing that the enhancement of self-efficacy beliefs specific to physical functioning is important to subsequent improvements observed in 400 m walk time.Citation11 A feature of the center-based portion of the intervention is that it facilitates the development of favorable social cognitions related to PA. We have found that group-mediated interventions are particularly effective for promoting behavior change among older adults for several reasons.Citation12,Citation13 The social environment of the group is valued independently of the behavior being promoted and serves to enhance regular attendance. Participants are often motivated by the persistence of more disabled peer group members, and benefit from witnessing individuals who reinitiate PA after significant health events. As shown following, the group also becomes a means of promoting important self-regulatory behaviors and a venue for instilling pride in accomplishments.
Interventionists, in collaboration with each participant, foster self-efficacy beliefs by establishing tangible but challenging goals that are modified when faced with unpredictable life events such as a change in health status. A web-based tracking system is used to dynamically monitor participants’ progress toward these goals and to provide routine feedback on their accomplishments. Sites often post publicly individual milestones achieved, such as membership in a 100-mile club, and participants are routinely asked to reflect on how their improved fitness has led to valued outcomes in day-to-day life. Participants have the opportunity to share these outcomes with other group members during center-based sessions. One strategy to reinforce these accomplishments is to visually display the outcomes as leaves on a tree of life for continued reflection. Further, the small-group environment is a platform for teaching about and actively employing important self-regulatory skills including goal setting, self-monitoring, social reinforcement, and social problem solving.Citation14
Although the LIFE Study strongly endorses conscious, social cognitive influences on the adoption and maintenance of a physically active lifestyle, we also appreciate that these beliefs have been found very unstable within the context of health-related behaviorsCitation15 and can be quickly altered by bodily states such as fatigue, acute illness, pain, and other health events.Citation16 When participants miss four or more center-based PA sessions consecutively, due either to a self-reported health event or when instructed to do so by their primary care physician, they are placed on “extended medical leave.” Once participants are classified as being on extended medical leave, the interventionists note their change of status on a web-based tracking system. Regaining “active” status and restarting the PA program requires review and approval by a health professional, who may be the participant’s physician, the site medical safety officer, and/or the site study physician. During periods of extended leave, participants are contacted every 2 weeks to obtain a status update, provide support, and assist them in making plans to move to an active status when appropriate. Interventionists are encouraged to use cognitive techniques that have evolved from research on Stages of Change, such as “consciousness raising,” to ready participants for a restart of their PA program.Citation17
Two other ways in which the LIFE Study PA intervention makes use of the group, a powerful agent of change for use with older adults to enhance motivation and to further reinforce self-efficacy beliefs and outcome expectations,Citation12,Citation13 is through the use of two to three site-specific mini-campaigns each year and study-wide campaigns. The mini-campaigns are developed by interventionists at each site and vetted by the LRC. provides examples of three mini-campaigns and describes the study-wide campaign held during the second year of the LIFE Study. These campaigns bring novelty to the PA program, provide social enrichment, add enjoyment to the PA setting, reinforce the use of self-management skills, and create valued outcomes that, for the most part, are not anticipated by participants. Typically, these events result in short-term increases in performance (10%–25%) over pre-campaign levels and often result in participants realizing that they are just a little more capable than they thought.
Table 1 Description of mini-campaigns
Table 2 LIFE campaign: Stride for LIFE
The web-based tracking system
A unique feature of the LIFE Study has been the development of an intervention website and a web-based intervention tracking system that provides access to data and information central to the integrity and conduct of the LIFE Study PA intervention. This system is based on an interactive web-user interface built with Adobe® ColdFusion®, Version 10 (Adobe Systems, San Jose, CA, USA) with a Microsoft SQL Server 2008 (Microsoft Corporation, Redmond, WA, USA) database. SAS 9.3 and SAS/IntrNet® (SAS Institute Inc, Cary, NC, USA) are used to program dynamic reporting features. The website and tracking system have two broad goals: to create a platform for monitoring treatment fidelity and to provide clinic staff with a tool to assist in the delivery and management of the intervention with participants.
Maintaining treatment fidelity is the responsibility of the LRC in conjunction with the LIFE Study Intervention Committee. The value of the tracking system in facilitating this responsibility cannot be overstated. The system enables members of the LRC and the chairs of the Intervention Committee to dynamically monitor attendance and minutes walked at center-based sessions by study participants as well as access to self-reported minutes walked at home. Reports provide both the mean (±90% confidence interval) and medians (Q1 and Q3) for each variable and generate results by individual clinic site and aggregated across sites. Other valuable reporting features of the tracking system are the capability to generate a dynamic list of participants who are currently on extended medical leave and create lists of participants whose attendance falls below 50% and those who are at or above 80%. These features facilitate communication among the site interventionists, the LRC, and the Intervention Committee and allow for efficient problem solving and participant management.
Achievement of the goal of monitoring treatment fidelity would not be possible without timely data entry on the part of the interventionists; thus, a complementary and second major goal of the web-based tracking system was to provide a tool for clinic sites to be able to track individual and group data for use in monitoring and managing PA behavior. Specifically, the tracking system provides interventionists at each clinic site with the capacity to generate data and graphic displays of individual participants’ PA behavior over time so that they can provide regular feedback to participants on their progress toward meeting established goals. Group-level reports can also be generated for weekly staff meetings. An additional feature of the website is that it provides online access to participants’ baseline medical and functional profiles to enhance staff–participant interactions and to allow for the tailoring of the PA intervention. Finally, the website serves as a platform from which intervention-related materials/videos can be posted and the sharing of information between sites can be facilitated.
Level of PA performed in center-based walking sessions
We used accelerometry in a subsample of LIFE Study PA participants to determine the amount of walking activity that was being performed at center-based sessions. A stratified random list of potential participants from the PA arm at each of four clinic sites was generated by the coordinating center based on three age groupings (70–75, 76–80, and 80+ years old) and sex. Interventionists at each site selected participants from within each stratum on this list (with the goal of having approximately an equal number in each subgroup) until they had collected data on 35 participants for a total target number of 140. Participants wore an ActiGraph GT3X (ActiGraph, LLC, Pensacola, FL, USA) accelerometer on two separate occasions during center-based walking exercise within the maintenance phase of the intervention. Two recordings were made so that we could report on the variability of activity within this older adult population. The repeated assessments were separated by an interval of at least 1 week but not more than 2 weeks.
The accelerometer, which is worn on the right hip, produces output that is digitized by a twelve-bit analog-to-digital convertor at a rate of 30 Hz. Once digitized, the signal passes through a digital filter limiting the frequency range from 0.25 to 2.5 Hz. Each sample was 1 second in duration and was aggregated over 60 seconds to create a data file in counts per minute. Only the vertical axis is used for processing procedures. Participants wore the monitor for the entire walking session without any additional monitoring from the staff. The device was shaken vigorously both at the beginning and completion of the exercise session so that start and stop times were clearly marked. Participants were instructed to exercise as usual and to include rest stops if needed, conversations with peers, and so forth. A “rest bout” was defined as any consecutive string of minutes during which the activity count was <100.
Means of counts/minutes and total minutes of activity were calculated by sex for activity and rest periods. For continuous variables, Wilcoxon two-sample tests were used to compare minutes/counts of activity between groups defined by sex. Differences in the number of rest and activity bouts between men and women were compared using Chi-square tests. Analyses were performed in SAS v 9.3.
Results
Descriptive data on participants in the PA intervention are presented in . These data reveal that the cohort of older adults is largely White, comes from a diverse educational background, and has multiple comorbidities. A detailed description of recruitment and baseline characteristics can be found in a paper by Marsh et al.Citation18
Table 3 LIFE Study baseline characteristics
Data from pre-training interview
The most frequent motive, given by 38.0% of participants in the PA intervention, for joining the LIFE Study had to do with adopting an active lifestyle that would improve their fitness or general health. Another 26.6% said that they were concerned about and hoped to regain or preserve their independence, while 14.0% were interested in improving a chronic health condition or the symptoms associated with these conditions, most commonly back and joint pain. A further 10.7% hoped to lose weight and 3.6% wanted to enhance the quality of their lives. The remaining 7.1% cited a variety of motives including the hope of reducing boredom, looking to satisfy the desire of significant others who recommended the program to them, pro-social interests, and the enjoyment that they experienced from being part of research.
When asked if there was anything that might get in the way of their participation in the program, 22.8% said yes: 5.2% mentioned caregiving, 6.0% health issues, 5.3% pain, with the remaining reasons falling into an “other” category that included activities such as vacations, sporadic traveling, and volunteer work. After being fully informed about the details of the intervention, participants were asked about inter-goal conflict – the degree to which other values or priorities in their lives might interfere with participation in the program: 60% reported no anticipated conflict, 31.4% said that conflict would be slight, 6.4% said moderate, and only 0.9% indicated that the degree of conflict would be likely to be severe.
summarizes participants’ perceptions of whether the neighborhood environment, family and friends, and doctors would be likely to have a positive (+) or negative (−) influence on their participation in the LIFE Study PA program. Ratings for all three factors were skewed in a positive direction, with family and friends being rated the highest (89% of the ratings ranging between +1 and +3), as compared with either the neighborhood environment (80.5%, P < 0.01) or doctor, (76%, P < 0.01). However, it is instructive to note that 14.2% of participants rated the neighborhood environment between −1 and −3, whereas the aggregate negative rating for family and friends was 1% and <1% for doctors.
Table 4 Barriers/facilitators to self-regulation (%)
Finally, although 82.1% of the participants gave a self-efficacy rating between 8 and 10 (on a 0–10 scale), 16.7% fell between 5 and 7, with 0.4% giving a rating of 3 or 4 (). In this same table, we provide data on how much participants valued the long-range goals in the LIFE Study, considering these goals in light of their overall life experience. Of the participants, 84.1% gave ratings between 8 and 10, and 9.3% fell between 5 and 7.
Accelerometry data from center-based training
Column 3 in provides the descriptive statistics for the accelerometry subsample (n = 140) of LIFE Study participants and permits a comparison with those not in the accelerometry study (column 2). A cursory examination of the P-values reveals that those in the substudy had similar characteristics to those not selected – it is important to remember that this random subset of participants, unlike the entire study cohort, was selected based upon sex and age groupings.
provides a detailed examination of patterns observed for rest and activity during center-based exercise stratified by sex. First, note that 68.8% of men and 51.3% of women never stopped to rest (P = 0.04, Chi-square test); that is, they performed their exercise in a single bout. Most participants who did stop to rest had one, two, or three rest intervals lasting on average 1.2 minutes for men and 1.6 minutes for women (P = 0.07, Wilcoxon test). On average, total time resting was the same for men and women (mean of 2.5 minutes for each group, P = 0.10, Wilcoxon test). Second, the average bout of exercise was 30.9 minutes for men and 26.3 minutes for women (P = 0.07, Wilcoxon test), yet men and women spent an identical total time exercising (mean of 36.9 minutes, P = 0.87, Wilcoxon test). Finally, the average intensity of exercise expressed as counts/minute was slightly higher for men than women (1,555 vs 1,237, P = 0.04, Wilcoxon test).
Table 5 Patterns of activity during center-based exercise by sex
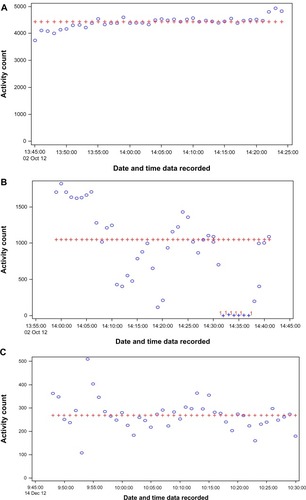
To illustrate the heterogeneity of the absolute intensities observed during training bouts both within and between participants, provides accelerometry recordings from center-based exercise for three different participants. As described in the notes that accompany this Figure, the participant in is exercising well above the standard adult cutoff point for moderate-intensity exercise and has a very consistent pace across the entire exercise bout. The participants in have median activity counts well below the adult cutoff point for moderate PA, with the individual in being the most compromised. Note that the person in exhibits a high degree of variability in intensity and had to take a rest stop. Such patterns are common for participants with joint pain or various chronic health conditions.
Figure 1 Panels (A–C) represent three representative accelerometry recordings from center-based exercise training.
Discussion
The LIFE Study involves a relatively diverse group of older adults from both a demographic and biometric perspective. Of particular interest is that, despite the advanced age of the cohort, over 40% are obese and there is considerable variability in mobility; that is, time to complete a self-paced 400 m walk. It is well known that obesity is a risk factor for physical disabilityCitation19 among older adults; some data suggest that obesity may compromise the functional benefits that older adults achieve via exercise training.Citation20
Semi-structured interviews conducted prior to initiating the intervention revealed that older adults who elect to participate in clinical trials involving PA do so with a variety of motives in mind. While roughly 65% were interested in enhancing their fitness and health or were concerned about losing their independence, 14.0% wanted to improve a chronic health condition. The remaining participants (~20%) hoped to lose weight, enhance the quality of their lives, reduce boredom, were looking to please a significant other, had pro-social interests, or simply enjoyed being a part of research.
This diversity in motivation helps to explain why promoting adherence to PA behavior in older adults presents a challenge. In a number of instances, anticipated outcomes on the part of the participants are unrealistic or are unlikely to be sufficient to sustain the demands required of the intervention; for example, in the absence of being coupled with caloric restriction, exercise in older adults causes little change in weight.Citation21 Also, prior to even beginning the intervention, almost 40% of participants indicated that there would be some level of conflict between fulfilling the requirements of the intervention and other demands in their lives – albeit that most perceived the conflict to be slight. This conundrum underscores the importance of the web-based tracking system to dynamically monitor participants’ progress toward study goals, along with the importance of training interventionists on self-regulatory skills, the conceptual model underlying the delivery of the intervention, and the behavioral strategies described earlier in this paper.
It was encouraging to find that most participants felt that their families, friends, and physicians were supportive of their involvement in the LIFE Study, yet it is noteworthy that 14.2% rated neighborhood environments as a barrier to home-based PA. Just prior to the end of the interview, when asked to rate their confidence in being able to meet the demands of the LIFE Study PA intervention, most participants were highly confident in their ability to do so, while 16.7% gave moderate ratings of between 5 and 7. Generally, participants perceived a high level of value associated with the long-range goals of the LIFE Study when compared with other commitments in their lives, with 90% giving a rating of 8 or higher on a eleven-point scale ranging from zero to ten. However, it should be kept in mind that physical symptoms such as pain and fatigue as well as acute and chronic illness are common in aging and that positive health-related beliefs can be eroded almost instantaneously by adverse interoceptive input.Citation16
The accelerometry data collected during the walking component of the exercise sessions indicate that the average counts/minute for the stimulus phase of exercise (1,555 counts/minute for men and 1,237 counts/minute for women) was well below the published cutoff point that is used to classify exercise as being of moderate intensity or higher for adults – 2,690 counts/minute.Citation22 Even more compelling is the large standard deviations associated with these average counts achieved during bouts of exercise for men (970.8 counts/minute) and for women (761.5 counts/minute). Although 68.5% of men and 51.3% of women never stopped to rest, a sizable subgroup required one or more rest stops. Whereas the total number of minutes of exercise achieved by men and women was 36.9 minutes, again variability was high, with a standard deviation of 11.8 minutes for men and 11.6 minutes for women. In combination with the example accelerometry tracings provided in , these data illustrate that it is not feasible to have a single exercise prescription for older adults with compromised function; rather, it is absolutely essential to tailor the intervention. Moreover, the concept of what constitutes “moderate” exercise or an appropriate volume of work is dictated by the physical capacities of each individual and the level of comfort/stability in actually executing a specific prescription. These data coupled with information gathered during the initial interviews underscore the importance of collaboration between participants and interventionists.Citation9 Our results also have implications for evaluating adherence in an exercise trial such as the LIFE Study, for making decisions on what constitutes valid data for participants, and in establishing national exercise guidelines for older adults. PA programs and interventionists must take into account the unique needs of different populations and must possess the flexibility and expertise to tailor protocols and prescriptions to ensure long-term participation and sustainability.
Conclusion
The LIFE Study is a landmark Phase III randomized controlled clinical trial that will provide definitive evidence regarding the effect of exercise on major mobility disability – failure to complete a self-paced 400 m walk in ≤15 minutes – in older adults, aged 70–89 years who have compromised physical function. This paper has provided a detailed account of the LIFE Study PA intervention, providing insight into participants’ motivations and expectations, the goals and implementation of the exercise prescription, the conceptual model employed to guide the promotion of target behaviors, procedures implemented to secure treatment fidelity, and data relevant to the actual walking behavior of participants during structured exercise. This information is critical to fully comprehend the content of the PA program in the LIFE Study, an important element of external validity.Citation23 It provides a valuable foundation for subsequent exercise trials in older adults and will be important to those whose goal is to design dissemination research on this important topic.
* Research investigators for the LIFE Study
Administrative Coordinating Center, University of Florida, Gainesville, FL, USA
Marco Pahor, MD – Principal Investigator of the LIFE Study; Jack M Guralnik, MD, PhD – Co-Principal Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD, USA); Stephen D Anton, PhD; Thomas W Buford, PhD; Christiaan Leeuwenburgh, PhD; Susan G Nayfield, MD, MSc; Todd M Manini, PhD; Connie Caudle; Lauren Crump, MPH; Latonia Holmes; Jocelyn Lee, PhD; Ronald Lester, PhD, MBA; and Ching-ju Lu, MPH.
This research is partially supported by the University of Florida Claude D Pepper Older Americans Independence Center (1 P30 AG028740).
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston-Salem, NC, USA
Michael E Miller, PhD – Data Management, Analysis and Quality Control Center (DMAQC) Principal Investigator; Mark A Espeland, PhD – DMAQC Co-Principal Investigator; Walter T Ambrosius, PhD; William Applegate, MD; Don Babcock, BSEE, PE; Daniel P Beavers, PhD, MS; Robert P Byington, PhD, MPH, FAHA; Delilah Cook, CCRP; Curt D Furberg, MD, PhD; Candace Goode; Jason Griffin, BS; Lea N Harvin, BS; Leora Henkin, MPH, Med; John Hepler, MA; Fang-Chi Hsu, PhD; Kathy Joyce; Laura Lovato, MS; Wesley Roberson, BSBA; Julia Robertson, BS; Julia Rushing, BSPH, MStat; Scott Rushing, BS; Cynthia L Stowe, MPM; Michael P Walkup, MS; Don Hire, BS; W Jack Rejeski, PhD; Jeffrey A Katula, PhD, MA; Peter H Brubaker, PhD; Shannon L Mihalko, PhD; Janine M Jennings, PhD; Kathy Lane, BA; John Luopa; Sherri Moore; Hoa V Teuschler, BA.
National Institutes of Health, Bethesda, MD, USA
Evan C Hadley, MD (National Institute on Aging); Sergei Romashkan, MD, PhD (National Institute on Aging); Denise E Bonds, MD, MPH (National Heart, Lung and Blood Institute); and Kushang V Patel, PhD (National Institute on Aging).
Field centers
Northwestern University, Chicago, IL, USA
Mary M McDermott, MD – Field Center Principal Investigator; Bonnie Spring, PhD – Field Center Co-Investigator; Joshua Hauser, MD – Field Center Co-Investigator; Diana Kerwin, MD – Field Center Co-Investigator; Kathryn Domanchuk, BS; Rex Graff, MS; and Alvito Rego, MA.
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Timothy S Church, MD, PhD, MPH – Field Center Principal Investigator; Steven N Blair, PED (University of South Carolina); Valerie H Myers, PhD; Ron Monce, PA-C; Nathan E Britt, NP; Melissa Nauta Harris, BS; Ami Parks McGucken, MPA, BS; Ruben Rodarte, MBA, MS, BS; Heidi K Millet, MPA, BS; Catrine Tudor-Locke, PhD, FACSM; Ben P Butitta, BS; Sheletta G Donatto, MS, RD, LDN, CDE; and Shannon H Cocreham, BS.
Stanford University, Palo Alto, CA, USA
Abby C King, PhD – Field Center Principal Investigator; Cynthia M Castro, PhD; William L Haskell, PhD; Randall S Stafford, MD, PhD; Veronica Yank, MD; Leslie A Pruitt, PhD; Kathy Berra, MSN, NP-C, FAAN; Carol Bell, NP; Rosita M Thiessen; Kate P Youngman, MA; Selene B Virgen, BAS; Eric Maldonado, BA; Kristina N Tarin, MS, CSCS; Heather Klaftenegger, BS; Carolyn A Prosak, RD; Ines Campero, BA; Dulce M Garcia, BS; José Soto, BA; Linda Chio, BA; and David Hoskins, MS.
Tufts University, Boston, MA, USA
Roger A Fielding, PhD – Field Center Principal Investigator; Miriam E Nelson, PhD; Sara C Folta, PhD; Edward M Phillips, MD; Christine K Liu, MD; Erica C Cifarelli, MS; Kieran F Reid, MSc, MPH; Paige C Lacasse, BS; Dylan R Kirn, BS; Evan P Pasha, BS; Karen R Ruais, NP; Won S Kim, BS; Julie M Krol, MS; Vince E Beard, BS; Eleni X Tsiroyannis, BS; and Cynthia Hau, BS.
Dr Fielding’s contribution is partially supported by the US Department of Agriculture, under agreement no 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Dept of Agriculture.
This research is also supported by the Boston Claude D Pepper Older Americans Independence Center (1 P30 AG031679).
University of Florida, Gainesville, Fl, USA
Todd M Manini, PhD – Field Center Principal Investigator; Marco Pahor, MD – Field Center Co-Principal Investigator; Stephen D Anton, PhD; Thomas W Buford, PhD; Michael Marsiske, PhD; Susan G Nayfield, MD, MSc; Bhanuprasad D Sandesara, MD; Mieniecia L Black, MS; William L Burk, MS; Brian M Hoover, BS; Jeffrey D Knaggs, BS; William C Marena, MT, CCRC; Irina Korytov, MD; Stephanie D Curtis, BS; Megan S Lorow, BS; Chaitalee S Goswami; Melissa A Lewis; Michelle Kamen BS; Jill N Bitz; Brian K Stanton, BS; Tamika T Hicks, BS; Charles W Gay, DC; Chonglun Xie, MD (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Holly L Morris, MSN, RN, CCRC (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Floris F Singletary, MS, CCC-SLP (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Jackie Causer, BSH, RN (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Susan Yonce, ARNP (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Katie A Radcliff, MA (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Mallorey Picone Smith, BS (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Jennifer S Scott, BS (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Melissa M Rodriguez BS (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Margo S Fitch, PT (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); Mendy C Dunn, BSN (Assessment) (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA); and Jessica Q Schllesinger, BS (Brooks Center for Rehabilitation Studies, Jacksonville, FL, USA).
This research is partially supported by the University of Florida Claude D Pepper Older Americans Independence Center (1 P30 AG028740).
University of Pittsburgh, Pittsburgh, PA, USA
Anne B Newman, MD, MPH – Field Center Principal Investigator; Stephanie A Studenski, MD, MPH – Field Center Co-Principal Investigator; Bret H Goodpaster, PhD; Oscar Lopez, MD; Nancy W Glynn, PhD; Neelesh K Nadkarni, MD, PhD; Diane G Ives, MPH; Mark A Newman, PhD; George Grove, MS; Kathy Williams, RN, BSEd, MHSA; Janet T Bonk, MPH, RN; Jennifer Rush, MPH; Piera Kost, BA; Pamela Vincent, CMA; Allison Gerger, BS; Jamie R Romeo, BS; and Lauren C Monheim, BS.
The Pittsburgh Field Center is partially supported by the Pittsburgh Claude D Pepper Older Americans Independence Center (P30 AG024827).
Wake Forest University, Winston-Salem, NC, USA
Stephen B Kritchevsky, PhD – Field Center Principal Investigator; Anthony P Marsh, PhD – Field Center Co-Principal Investigator; Tina E Brinkley, PhD; Jamehl S Demons, MD; Kaycee M Sink, MD, MAS; Kimberly Kennedy, BA, CCRC; Rachel Shertzer-Skinner, MA, CCRC; Abbie Wrights, MS; Rose Fries, RN, CCRC; and Deborah Barr, MA, RHEd, CHES.
The Wake Forest University Field Center is, in part, supported by the Claude D Pepper Older Americans Independence Center (1 P30 AG21332).
Yale University, New Haven, CT, USA
Thomas M Gill, MD – Field Center Principal Investigator; Robert S Axtell, PhD, FACSM – Field Center Co-Principal Investigator (Southern Connecticut State University, Exercise Science Department); Susan S Kashaf, MD, MPH (Veterans Administration Connecticut Healthcare System); Nathalie de Rekeneire, MD, MS; Joanne M McGloin, MDiv, MS, MBA; Karen C Wu, RN; Lynne P Iannone, MS, CCRP; Raeleen Mautner, PhD; Denise M Shepard, RN, MBA; Barbara Fennelly, MA, RN; Theresa Sweeney Barnett, MS, APRN; Sean N Halpin, MA; Matthew J Brennan, MA; Julie A Bugaj, MS; Maria A Zenoni, MS; Bridget M Mignosa, AS; Sharon M Huie-White, MPH; and Janice Zocher.
Dr Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24 AG021507) from the National Institute on Aging.
The Yale Field Center is partially supported by the Claude D Pepper Older Americans Independence Center (P30 AG021342).
Cognition Coordinating Center, Wake Forest University, Winston-Salem, NC, USA
Jeff Williamson, MD, MHS – Center Principal Investigator; Kaycee M Sink, MD, MAS – Center Co-Principal Investigator; Hugh C Hendrie, MB, ChB, DSc (Indiana University); Stephen R Rapp, PhD; Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University); Nancy Woolard; Mark Espeland, PhD; and Janine Jennings, PhD.
Electrocardiogram Reading Center, University of Florida, Gainesville, FL, USA
Carl J Pepine MD, MACC; Mario Ariet, PhD; Eileen Handberg, PhD, ARNP; Daniel Deluca, BS; James Hill, MD, MS, FACC; and Anita Szady, MD.
Spirometry Reading Center, Yale University, New Haven, CT, USA
Geoffrey L Chupp, MD; Gail M Flynn, RCP, CRFT; Thomas M Gill, MD; John L Hankinson, PhD (Hankinson Consulting, Inc); and Carlos A Vaz Fragoso, MD.
Dr Fragoso is the recipient of a Career Development Award from the Department of Veterans Affairs.
Cost Effectiveness Analysis Center
Erik J Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System) and Robert M Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health).
Acknowledgments
The LIFE Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement no UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute (3U01AG022376-05A2S). It is sponsored in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health.
Disclosure
The authors declare no conflicts of interest in this work.
References
- Administration on Aging (AoA)Profile of older Americans [web page on the Internet]Washington DCAoA2011 Available from: http://www.aoa.gov/Aging_Statistics/Profile/2011/Index.aspxAccessed May 13, 2013
- KatzSBranchLGBransonMHPapsideroJABeckJCGreerDSActive life expectancyN Engl J Med198330920121812246633571
- FriedLPBandeen-RocheKChavesPHJohnsonBAPreclinical mobility disability predicts incident mobility disability in older womenJ Gerontol A Biol Sci Med Sci2000551M43M5210719772
- RejeskiWJFochtBCMessierSPMorganTPahorMPenninxBObese, older adults with knee osteoarthritis: weight loss, exercise, and quality of lifeHealth Psychol200221541942612211508
- LIFE Study InvestigatorsPahorMBlairSNEffects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) studyJ Gerontol A Biol Sci Med Sci200661111157116517167156
- University of FloridaThe LIFE Study – Lifestyle Interventions and Independence for EldersClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2010 [updated March 29, 2013]. Available from: http://clinicaltrials.gov/ct2/show/NCT1072500. NLM identifier: NCT1072500Accessed July 24, 2013
- FieldingRARejeskiWJBlairSLIFE Research GroupThe Lifestyle Interventions and Independence for Elders Study: design and methodsJ Gerontol A Biol Sci Med Sci201166111226123721825283
- GuralnikJMSimonsickEMFerrucciLA short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admissionJ Gerontol199449M85M948126356
- RejeskiWJBrawleyLRJungMESelf-management in geriatric medicineHalterJOuslanderJGTinettiMEStudenskiSHighKPAsthanaSHazzard’s Principles of Geriatric Medicine and Gerontology6th edNew York, NYMcGraw-Hill2008
- BanduraASocial Foundations of Thought and Action: A Social Cognitive TheoryEnglewood Cliffs, NJPrentice-Hall1986
- BrawleyLRejeskiWJGauksternJEAmbrosiusWTSocial cognitive changes following weight loss and physical activity interventions in obese, older adults in poor cardiovascular healthAnn Behav Med201244335336422773225
- RejeskiWJBrawleyLRAmbrosiusWOlder adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestylesHealth Psychol200322441442312940398
- RejeskiWJBrubakerPHGoffDCJrTranslating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular healthArch Intern Med20111711088088621263080
- D’ZurillaTJNezuAMProblem-solving Therapy: A Social Competence Approach to Clinical Intervention2nd edNew York, NYSpringer1999
- NordgrenLFvan der PligtJvan HarreveldFThe instability of health cognitions: visceral states influence self-efficacy and related health beliefsHealth Psychol200827672272719025267
- RejeskiWJGauvinLThe embodied and relational nature of the mind: implications for clinical interventions in aging individuals and populationsClin Interv Aging20138565766523776330
- MarcusBHBanspachSWLefebvreRCRossiJSCarletonRAAbramsDBUsing the stages of change model to increase the adoption of physical activity among community participantsAm J Health Promot19926642442910146803
- MarshAPLovatoLCGlynnNWLifestyle Interventions and Independence for Elders Study: Recruitment and Baseline CharacteristicsJ Gerontol A Bioll Sci Med Sci Epub5282013
- RejeskiWJMarshAPChmeloERejeskiJJObesity, intentional weight loss and physical disability in older adultsObes Rev201011967168519922431
- ManiniTMNewmanABFieldingRLIFE Research GroupEffects of exercise on mobility in obese and nonobese older adultsObesity (Silver Spring)20101861168117519834467
- MessierSPLoeserRFMillerGDExercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion TrialArthritis Rheum20045051501151015146420
- SasakiJEJohnDFreedsonPSValidation and comparison of ActiGraph activity monitorsJ Sci Med Sport201114541141621616714
- BrachtGHGlassGVThe external validity of experimentsAm Educ Res J196854437474