Abstract
Introduction
The aim of this study is to report our 6-year single-center experience with L5–S1 axial lumbar interbody fusion (AxiaLIF).
Methods
A total of 131 patients with symptomatic degenerative disc disease refractory to nonsurgical treatment were treated with AxiaLIF at L5–S1, and were followed for a minimum of 1 year (mean: 21 months). Main outcomes included back and leg pain severity, Oswestry Disability Index score, working status, analgesic medication use, patient satisfaction, and complications. Computed tomography was used to determine postoperative fusion status.
Results
No intraoperative complications, including vascular, neural, urologic, or bowel injuries, were reported. Back and leg pain severity decreased by 51% and 42%, respectively, during the follow-up period (both P < 0.001). Back function scores improved 50% compared to baseline. Clinical success, defined as improvement ≥30%, was 67% for back pain severity, 65% for leg pain severity, and 71% for back function. The employment rate increased from 47% before surgery to 64% at final follow-up (P < 0.001). Less than one in four patients regularly used analgesic medications postsurgery. Patient satisfaction with the AxiaLIF procedure was 83%. The fusion rate was 87.8% at final follow-up. During follow-up, 17 (13.0%) patients underwent 18 reoperations on the lumbar spine, including pedicle screw fixation (n = 10), total disc replacement of an uninvolved level (n = 3), facet screw fixation (n = 3), facet screw removal (n = 1), and interbody fusion at L4–L5 (n = 1). Eight (6.1%) reoperations were at the index level.
Conclusion
Single-level AxiaLIF is a safe and effective means to achieve lumbosacral fusion in patients with symptomatic degenerative disc disease.
Introduction
Degeneration of the lumbar intervertebral discs is common with advancing age,Citation1,Citation2 and it represents the primary cause of chronic low back pain in adults.Citation3 Nonsurgical treatments such as physical therapy, analgesics, and activity modification are the first-line therapies for low back pain secondary to degenerative disc disease (DDD); however, patient prognosis with nonsurgical treatments is poor when symptoms persist for 6 months or more.Citation4–Citation7 Lumbar interbody fusion is performed to improve lumbar stability and decrease painful motion at the offending motion segment when conservative treatments fail. Traditional surgical access corridors for interbody fusion include posterior, transforaminal, anterior, and extreme lateral – each of which is associated with a distinct risk profile that is largely related to iatrogenic injury of the anatomic structures that must be traversed to gain access to the index interspace. Minimally invasive spine surgery has been widely adopted since smaller access portals and less tissue disruption offer distinct patient advantages including less blood loss, shorter hospitalization, and fewer complications compared to open surgery.Citation8 However, minimally invasive spine surgery is technically demanding and utilizes the same access points and trajectories as open surgery. Consequently, the same anatomic structures remain at risk, albeit lower, for iatrogenic injury.Citation9
The presacral corridor is a largely aneural and avascular space beginning at the midline of S1–S2 and extending to the inferior endplate of S1, with a trajectory between the parietal fascia and the visceral fascia. Axial lumbar interbody fusion (AxiaLIF) is a minimally invasive technique that exploits this anatomic “safe zone” to achieve lumbosacral interbody access without the risk of jeopardizing critical neurovascular or musculoligamentous structures.
Studies with AxiaLIF have collectively reported clinically important reductions in pain and improvements in back function, with low complication rates.Citation10–Citation18 The largest study with AxiaLIF to date reported average improvements of 63% in back pain severity and 54% in back function with a 94% fusion rate and no major complications in 156 patients followed for 2 years.Citation13 We performed a retrospective evaluation of patients treated with AxiaLIF at our institution over a 6-year period that had a minimum of 1-year clinical and radiographic outcome data.
Methods
Patients
A total of 131 patients with radiographically confirmed DDD who underwent L5–S1 interbody fusion via the axial presacral approach and had a minimum of 1 year follow-up were retrospectively included in this report. Surgeries were performed by a single surgeon at the Neurosurgical Centre in Zwolle or Bergman Clinics in Naarden and Nedspine in Ede, the Netherlands between March 2006 and January 2012. All patients underwent a minimum of 6 months (mean: 5 years) of unsuccessful nonsurgical management before the AxiaLIF procedure.
Pretreatment evaluations
Each patient underwent a complete physical and neurologic examination and completed a detailed medical and medication history. The diagnosis of DDD was confirmed with magnetic resonance imaging and X-rays using anteroposterior and lateral views, as well as dynamic flexion–extension images. Additionally, provocative discography and subsequent anesthetization of the disc was routinely performed. Midsagittal T1- and T2-weighted magnetic resonance imaging of the sacrum and coccyx ruled out vascular anomalies, tumor, or surgical scarring that would preclude safe access through the presacral space. Patients with previous pelvic surgery were not considered for this procedure. No patient presented with spondylolisthesis, lumbar spinal stenosis, herniated nucleus pulposus, or failed back syndrome.
AxiaLIF system
Patients underwent L5–S1 interbody fusion with the AxiaLIF system via a minimally invasive presacral approach (). The AxiaLIF system is indicated for patients requiring lumbar interbody fusion to treat DDD, pseudarthrosis, spinal stenosis, or spondylolisthesis (grade 1 or grade 2). Contraindications to this procedure include severe scoliosis, severe spondylolisthesis (grade 3 or grade 4), tumor, osteoporosis, or trauma. AxiaLIF received CE Mark clearance in March 2005.
Surgical procedure
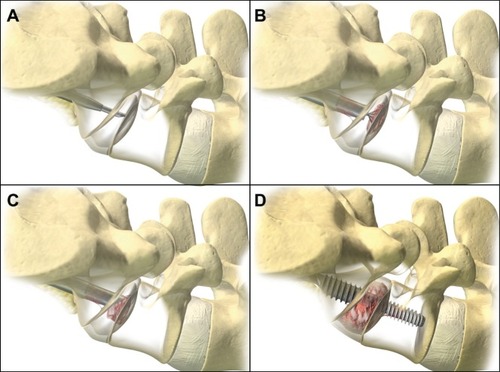
Procedural steps have been discussed in detail elsewhere.Citation19 Patients followed a bowel preparation protocol the night before surgery. At surgery, the patient was placed prone and, under fluoroscopic guidance, a 2 cm incision was made on either side of the paracoccygeal notch to gain entry to the presacral corridor. Blunt finger dissection was used to displace the rectum away from the sacrum and a blunt guide pin was then advanced through the presacral space and docked on the inferior endplate of the sacrum. A series of dilators were advanced over the guide pin and a working cannula was anchored with K wires to the sacrum. A cannulated drill created a bony channel in the sacrum and provided access to the L5–S1 disc. Nitinol cutters were used to morselize the disc material, completely preserving the annulus, and to denude and abrade the endplates to promote fusion. Bone graft substitute (Actifuse™ 75, Baxter International, Inc., Deerfield, IL, USA; Allomatrix® 26, Wright Medical Technology, Inc, Arlington, TN, USA; DBX® 23, Synthes, Inc., West Chester, PA, USA; Tutoplast® 4, Tutogen Medical, Inc, West Paterson, NJ, USA; NANOSTIM™ 3, Medtronic, Inc, Minneapolis, MN, USA) was inserted into the disc space. The guide pin was then advanced to the inferior L5 endplate and a twist drill was used to remove bone. The AxiaLIF rod was advanced through the sacrum and 0.5 cm to 1 cm into the L5 vertebral body. Advancement of the screw increases distraction across the disc space, leading to disc height restoration and opening of the L5–S1 neuroforamen (). We began using percutaneous facet screw fixation with all AxiaLIF cases beginning in mid-2008. No other supplemental fixation devices were utilized in this series.
Figure 2 Presacral access with the AxiaLIF system.
Abbreviation: AxiaLIF, axial lumbar interbody fusion.
Main outcomes and follow-up
Clinical and radiographic outcomes were collected during regularly scheduled office visits at pretreatment, 6 weeks, and annually (±3 months) thereafter. All patients were followed at least through the 1-year follow-up visit (mean: 21 ± 8 months). Fusion mass was assessed by independent radiologists with thin-slice (1–2 mm), high-resolution computed tomography (CT) scan in coronal and sagittal planes at the 1-year follow-up visit. Fusion status was assessed on a 4-point grading scale; solid fusion was defined as radiographic evidence of bridging bone between L5 and S1. Back and leg pain severity were each assessed by the patient with a 0 to 100 numeric scale. Back function was evaluated with the Oswestry Disability Index (ODI).Citation20 Clinical success was defined as a ≥30% improvement in pain scoresCitation21,Citation22 and ODI,Citation21,Citation23 respectively. Working status, pain medication usage, and patient satisfaction were determined at each follow-up visit. Reoperations and complications were tracked throughout the follow-up period.
Statistical analyses
Data were analyzed using Predictive Analytics Software (version 18; IBM Corporation, Armonk, N Y, USA). Continuous data were reported as the mean ± standard deviation, and categorical data were reported as frequencies and percentages. Longitudinal changes in back and leg pain severity, as well as ODI score were assessed using repeated measures analysis of variance. McNemar’s test was used to analyze changes in employment status before and after treatment. Predictors of solid fusion were determined by univariate logistic regression. Statistical significance was set at P < 0.05.
Results
Baseline patient characteristics
Patients were predominantly middle-age (mean: 41 years; range: 20–58 years) and female (67%). Patients typically presented with long-standing back pain with a mean severity score of 79 ± 12, moderate back dysfunction (ODI 46% ± 16%), and a mean index disc height of 52% ± 18% relative to L4–L5. Leg pain of nonradicular origin was reported in 84% of patients. Eight (6.1%) patients had undergone previous lumbar disc surgery ().
Table 1 Baseline patient characteristics
Procedural details
The AxiaLIF procedure was successfully completed in all patients. Implant lengths used in this series were 40 mm (n = 43), 45 mm (n - 55), and 50 mm (n = 30), with the 55 mm and 60 mm devices rarely used (n = 3). Posterior facet screw fixation was used in 61 (47%) patients. Mean procedural time was 37 ± 7 minutes, blood loss was minimal (<100 mL in all cases), and median hospitalization was 2 days (range: 0 to 14 days).
Pain severity and back function
Back pain severity decreased 51% during the follow-up period (P < 0.001), from 79 ± 12 at the pretreatment visit to 39 ± 30 at final follow-up. Leg pain similarly decreased during follow-up, from 45 ± 30 to 26 ± 29, representing a 42% average improvement (P < 0.001). Clinical success was achieved in 67% of patients for back pain severity and in 65% for leg pain severity. ODI scores improved 50% during follow-up, from 46% ± 16% to 23% ± 20% (P < 0.001). ODI clinical success was 71%.
Work status, medication use, and patient satisfaction
Before treatment with AxiaLIF, 47% of patients were employed with 24% working in a full-time capacity. Employment rates at final follow-up were significantly higher (P < 0.001), with a 64% overall employment rate and 44% of patients employed full-time. Nearly six in ten patients were able to completely discontinue analgesic medications and 18% reported only occasional (1–3/week) use. Patient satisfaction with the AxiaLIF procedure was excellent, as 83% of patients reported that they would absolutely or probably have the same surgery again.
Fusion status
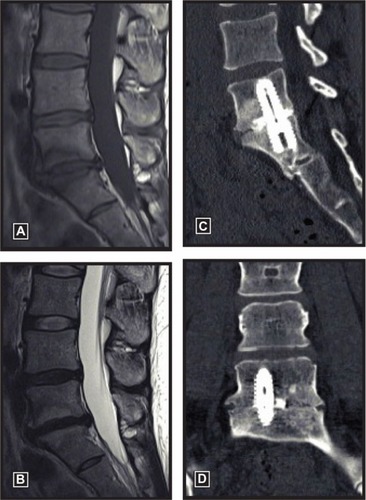
The fusion rate at final follow-up was 87.8% (n = 115), while 9.2% (n = 12) were graded as indeterminate (no clear signs of bony bridging, but no signs of loosening), and 3.1% (n = 4) showed frank pseudarthrosis. Female sex was a strong predictor of solid fusion (odds ratio: 5.7, 95% confidence interval: 1.8 to 17.7, P < 0.01). Fusion rates were 94% (83 of 88) in females and 74% (32 of 43) in males. No other baseline variable loaded into the logistic regression model at P < 0.10 (). A typical example of a solid fusion construct following AxiaLIF is presented in .
Table 2 Univariate baseline predictors of solid fusion at final postoperative follow-up
Complications
No intraoperative complications, including vascular, neural, urologic, or bowel injuries, were reported. During follow-up, 17 (13.0%) patients underwent 18 reoperations on the lumbar spine, including pedicle screw fixation (n = 10), total disc replacement of an uninvolved level (n = 3), facet screw fixation (n = 3), facet screw removal (n = 1), and interbody fusion at L4–L5 (n = 1). Eight (6.1%) reoperations were at the index level. Broken facet screws were identified in three asymptomatic patients who required no treatment.
Discussion
This report presents the largest known single-surgeon experience with AxiaLIF and demonstrates that this minimally invasive surgery is a safe and effective means to achieve L5–S1 fusion in patients with symptomatic DDD. Patient outcomes in the current series are comparable to those reported in other AxiaLIF studies (). The fusion rate in the current series was 88%. Although others have reported higher fusion rates with AxiaLIF, many of these studies assessed fusion status exclusively with radiographs or with a combination of CT and radiographs. Discordance in fusion rates assessed with CT and radiographs is well known, and is illustrated by the study by Bohinski et alCitation15 who reported a fusion rate of 100% with AxiaLIF using postoperative radiographs, but only 88% using CT. After accounting for differences in imaging modality, the fusion rates reported in our series are comparable to other studies of AxiaLIF.
Table 3 Comparison of studies with axial lumbar interbody fusion for degenerative disc disease
Although the presacral corridor to the sacrum is generally considered an anatomic safe zone, access through this region can be safely exploited only if the surgeon is intimately familiar with the relevant pelvic anatomy.Citation24 The surgeon relies entirely on fluoroscopic guidance since the operative field cannot be directly visualized. Complication risks can be minimized by proper patient selection, use of imaging studies to prospectively identify the proper access trajectory, preoperative bowel preparation techniques, perioperative antibiotic administration, and meticulous operative technique. Bowel perforation is a rare and largely preventable complication with the AxiaLIF. Gundanna et alCitation16 reported a 0.6% bowel injury rate in patients treated with AxiaLIF, with 42% of these perforations due to frank surgeon error or deviations from recommended procedural steps. In the current series of 131 patients, no bowel perforations were detected. We attribute our success to several strategies that have been adopted during our 6-year experience with the AxiaLIF technology. The anticipated trajectory is predetermined based on a detailed review of imaging studies, with special emphasis on determining the thickness of the perirectal fat pad, locating the interface between the rectum and sacrum, identifying potential aberrant vasculature, and estimating the probability of achieving proper trajectory. Mechanical bowel cleansing the night before surgery is mandatory since this enhances rectal pliability and reduces sepsis risk in case of a bowel perforation. A Foley catheter may be inserted in the rectum, which helps to delineate the rectum-sacrum interface. Blunt finger dissection taking care to displace the rectum away from the sacrum further reduces risk of bowel puncture.
An analysis of the postmarket surveillance experience in over 9,000 patients treated with AxiaLIF, including over 8,000 L5–S1 cases, reported an overall 1.3% complication rate.Citation16 The AxiaLIF technique spares the posterior musculature, ligaments, and neural elements, as well as major organs and vessels typically encountered during anterior approaches. The advantages of this access route were realized in the current series since no operative complications have been identified over our 6-year experience. Ultimately, 13% of patients required a reoperation, with 6% occurring at the index level, over the 21-month mean follow-up period. This reoperation rate compares favorably to a recent meta-analysis that reported a 13% reoperation rate, including 9% at the index level, in patients followed for at least 1 year after lumbar fusion surgery for DDD.Citation25 Additionally, the lack of intraoperative complications with the AxiaLIF system compares favorably with the typical 10%–15% complication rate reported in Food and Drug Administration-regulated trials with open lumbar fusion for DDD.Citation26–Citation31 Lastly, serious adverse events such as nerve injury (0%–2.0%), vascular injury (1.5%–8.8%), and infection (0%–1.3%) were reported in these trials,Citation26–Citation31 whereas no serious adverse events were reported in the current series.
Our study is limited by the retrospective nature of the analysis. Additionally, all patients underwent fusion at L5–S1 and, therefore, no conclusions can be drawn regarding the effectiveness or safety of two-level AxiaLIF from this report. Lastly, mean patient follow-up was 21 months. Although this represents one of the longest follow-up reports following AxiaLIF surgery, long-term clinical and radiographic outcomes are unknown. However, it is reasonable to assume that the clinical course of a patient with an accomplished fusion will not be different than after fusions obtained by other techniques.
Overall, single-level AxiaLIF is a safe and effective means to achieve lumbosacral fusion in patients with symptomatic DDD.
Acknowledgments
The authors thank Mr Randy Asher for graphical assistance.
Disclosure
DJZ, LEM, and JEB are consultants to TranS1, Inc. The authors report no other conflicts of interest in this work.
References
- NiuGYangJWangRDangSWuEXGuoYMR imaging assessment of lumbar intervertebral disk degeneration and age-related changes: apparent diffusion coefficient versus T2 quantitationAJNR Am J Neuroradiol20113291617162321799044
- HicksGEMoroneNWeinerDKDegenerative lumbar disc and facet disease in older adults: prevalence and clinical correlatesSpine (Phila Pa 1976)200934121301130619455005
- DePalmaMJKetchumJMSaulloTWhat is the source of chronic low back pain and does age play a role?Pain Med201112222423321266006
- ChouRAtlasSJStanosSPRosenquistRWNonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guidelineSpine (Phila Pa 1976)200934101078109319363456
- CareyTSGarrettJMJackmanAMBeyond the good prognosis. Examination of an inception cohort of patients with chronic low back painSpine (Phila Pa 1976)200225111512010647169
- Vo n KorffMStudying the natural history of back painSpine Spine (Phila Pa 1976)199419Suppl 182041S2046S
- SmithSEDardenBVRhyneALWoodKEOutcome of unoperated discogram-positive low back painSpine (Phila Pa 1976)1995201819972000 discussion 2000–20018578375
- MobbsRJSivabalanPLiJMinimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologiesJ Clin Neurosci201219682983522459184
- PayerM“Minimally invasive” lumbar spine surgery: a critical reviewActa Neurochir (Wien)201115371455145921533888
- CraggACarlACastenedaFDickmanCGutermanLOliveiraCNew percutaneous access method for minimally invasive anterior lumbosacral surgeryJ Spinal Disord Tech2004171212814734972
- StipplerMTurkaMGersztenPCOutcomes after percutaneous TranS1 AxiaLIF® L5–S1 interbody fusion for intractable lower back painThe Internet Journal of Spine Surgery200951112
- ToblerWDFerraraLAThe presacral retroperitoneal approach for axial lumbar interbody fusion: a prospective study of clinical outcomes, complications and fusion rates at a follow-up of two years in 26 patientsJ Bone Joint Surg Br201193795596021705570
- ToblerWDGersztenPCBradleyWDRaleyTJNascaRJBlockJEMinimally invasive axial presacral L5–S1 interbody fusion: two-year clinical and radiographic outcomesSpine (Phila Pa 1976)20113620E1296E130121494201
- GersztenPCToblerWDNascaRJRetrospective analysis of L5–S1 axial lumbar interbody fusion (AxiaLIF): a comparison with and without the use of recombinant human bone morphogenetic protein-2Spine J201111111027103222122835
- BohinskiRJJainVVToblerWDPresacral retroperitoneal approach to axial lumbar interbody fusion: a new, minimally invasive technique at L5–S1: clinical outcomes, complications, and fusion rates in 50 patients at 1-year follow-upSAS J2010425462
- GundannaMIMillerLEBlockJEComplications with axial presacral lumbar interbody fusion: a 5-year postmarketing surveillance experienceSAS J2011539094
- LindleyEMMcCulloughMABurgerELBrownCWPatelVVComplications of axial lumbar interbody fusionJ Neurosurg Spine201115327327921599448
- PatilSSLindleyEMPatelVVBurgerELClinical and radiological outcomes of axial lumbar interbody fusionOrthopedics2010331288321162514
- MarottaNCosarMPimentaLKhooLTA novel minimally invasive presacral approach and instrumentation technique for anterior L5–S1 intervertebral discectomy and fusion: technical description and case presentationsNeurosurg Focus2006201E916459999
- FairbankJCPynsentPBThe Oswestry Disability IndexSpine (Phila Pa 1976)2000252229402952 discussion 295211074683
- OsteloRWDeyoRAStratfordPInterpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important changeSpine (Phila Pa 1976)2008331909418165753
- HäggOFritzellPNordwallASwedish Lumbar Spine Study GroupThe clinical importance of changes in outcome scores after treatment for chronic low back painEur Spine J2003121122012592542
- ZiglerJDelamarterRSpivakJMResults of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc diseaseSpine2007321111551162 discussion 116317495770
- YuanPSDayTFAlbertTJAnatomy of the percutaneous presacral space for a novel fusion techniqueJ Spinal Disord Tech200619423724116778656
- PhillipsFMSlosarPJYoussefJAAnderssonGPapatheofanisFLumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic reviewSpine (Phila Pa 1976)2013387E409E42223334400
- US Food and Drug AdministrationSummary of Safety and Effectiveness: BAK Interbody Fusion System with InstrumentationRockville, MDFood and Drug Administration1996 Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/p950002.pdfAccessed June 3, 2013
- US Food and Drug AdministrationSummary of Safety and Effectiveness: Ray Threaded Fusion Cage (TFC) with InstrumentationRockville, MDFood and Drug Administration1996http://www.accessdata.fda.gov/cdrh_docs/pdf/P950019a.pdfAccessed June 3, 2013
- US Food and Drug AdministrationSummary of Safety and Effectiveness: INTER FIX Threaded Fusion DeviceRockville, MDFood and Drug Administration1999http://www.accessdata.fda.gov/cdrh_docs/pdf/P970015b.pdfAccessed June 3, 2013
- US Food and Drug AdministrationSummary of Safety and Effectiveness: InFUSE Bone Graft/LT-CAGE Lumbar Tapered Fusion DeviceRockville, MDFood and Drug Administration2002http://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdfAccessed June 3, 2013
- US Food and Drug AdministrationSummary of Safety and Effectiveness: PRODISC-L Total Disc ReplacementRockville, MDFood and Drug Administration2006http://www.accessdata.fda.gov/cdrh_docs/pdf5/P050010b.pdfAccessed June 3, 2013
- US Food and Drug AdministrationSummary of Safety and Effectiveness: CHARITE Artificial DiscRockville, MDFood and Drug Administration2004http://www.accessdata.fda.gov/cdrh_docs/pdf4/P040006b.pdfAccessed June 3, 2013
- AxiaLIF [patient literature]Raleigh, NCBaxano Surgical, Inc2013