Abstract
There are many age-associated changes in the respiratory and pulmonary immune system. These changes include decreases in the volume of the thoracic cavity, reduced lung volumes, and alterations in the muscles that aid respiration. Muscle function on a cellular level in the aging population is less efficient. The elderly population has less pulmonary reserve, and cough strength is decreased in the elderly population due to anatomic changes and muscle atrophy. Clearance of particles from the lung through the mucociliary elevator is decreased and associated with ciliary dysfunction. Many complex changes in immunity with aging contribute to increased susceptibility to infections including a less robust immune response from both the innate and adaptive immune systems. Considering all of these age-related changes to the lungs, pulmonary disease has significant consequences for the aging population. Chronic lower respiratory tract disease is the third leading cause of death in people aged 65 years and older. With a large and growing aging population, it is critical to understand how the body changes with age and how this impacts the entire respiratory system. Understanding the aging process in the lung is necessary in order to provide optimal care to our aging population. This review focuses on the nonpathologic aging process in the lung, including structural changes, changes in muscle function, and pulmonary immunologic function, with special consideration of obstructive lung disease in the elderly.
Keywords:
Introduction
Pulmonary disease has significant consequences for the aging population. Chronic lower respiratory tract disease, defined as asthma, emphysema, chronic bronchitis, bronchiectasis, and chronic obstructive pulmonary disease (COPD), is the third leading cause of death in people aged 65 years and older.Citation1 According to 2010 census data, 13% of the US population, or 40.3 million people, are older than age 65, which is higher than any previous census. Additionally, the population is aging at an increasingly faster rate each year. Between 2000 and 2010, the population age 65 years and over increased by 15.1% compared to the total US population which only increased by 9.7%.Citation2 With such a large and rapidly growing aging population it is critical to understand how the body changes with age and how this impacts the entire respiratory system. Understanding the aging process in the lungs is necessary in order to provide optimal care to our aging population.
Structural and functional changes with age
The structure of the thoracic cavity, which houses and protects the lungs, is vital for optimal lung function. Changes to the spine, muscles, and ribs over time with aging impact normal lung function. As people normally age, narrowing of the intervertebral disk spaces causes kyphosis or curvature of the spine.Citation3 This curvature decreases the space between the ribs and creates a smaller chest cavity.Citation4 While a small amount of anterior curvature or kyphosis of the thoracic spine is normal, an angle greater than 40°, which is the 95th percentile of normal, is defined as hyperkyphosis.Citation5,Citation6 After age 40, the kyphosis angle begins to increase more rapidly in women than men, from a mean of 43° in women aged 55–60 years, to a mean of 52° in women 76–80 years of age.Citation5 The prevalence and incidence of hyperkyphosis is reported in older adults varying from approximately 20%–40% among both men and women.Citation5,Citation6 In a study of 55 nonsmoking women with variable degrees of thoracic kyphosis Lombardi et alCitation7 found that with increasing vertebral angle there was a significant decline in the fraction of exhaled volume in 1 second (FEV1) and vital capacity (VC) during spirometry testing.Citation7 This effect was most significant once the kyphotic angle was over 55°. Culham et al hypothesized that this effect is not from a decrease in thoracic cavity size alone, but due to the rib space narrowing, which decreases the length of the intercostal muscles.Citation8 The angle of the muscle fibers in relation to the ribs may also affect the efficiency and decrease the movement of the lower ribs during inspiration.Citation8 These changes are structural and based on the origin and insertion of the muscles.
In addition to structural changes, there are changes in intrinsic function of the muscles with age. Overall muscle function in the body decreases by 2% annually as we age.Citation9,Citation10 Aging is associated with reduced inspiratory and expiratory respiratory muscle strength.Citation11 Respiratory muscle decline can lead to an inability to ventilate in the face of increasing demands, such as that seen in respiratory disease. There is also evidence that at the cellular level, the muscles of elderly individuals have less mitochondrial adenosine triphosphate reserves to sustain a sudden increase in metabolic demand.Citation12 If an elderly person becomes ill with pneumonia, and therefore has increased metabolic demands for oxygen in the setting of decreased respiratory muscle strength, decreased cellular energy reserve, and decreased overall muscle function, he or she may not be able to meet those demands. This leads to an increased risk of respiratory failure in older individuals.Citation13
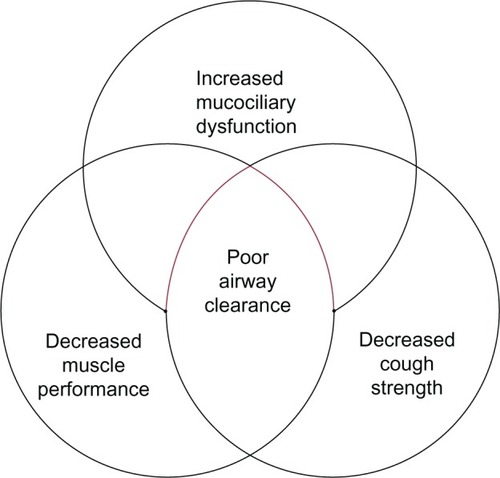
With aging there is a decreased ability to clear mucus from the lungs. Two mechanisms primarily contribute to this decline: 1) reduced cough strength and 2) alterations in the body’s ability to clear particles in the airways. First, cough plays a vital role in clearing mucus from the airways. Coughing is a maneuver that requires generation of a high forced expiratory flow. During a cough maneuver, inspiratory muscles contract to allow the lungs to take in a large tidal volume necessary to augment a sustained high expiratory flow.Citation14 Next, the expiratory muscles contract to build high positive intrapleural and intraairway pressures for the development of peak expiratory flow rates.Citation15 Finally, when the glottis is opened, the cough occurs, and the mucus is expelled from the airway into the mouth. Any decrease in the strength of the respiratory muscles will greatly impact an individual’s ability to generate the force required for an effective cough.Citation16 Aging is associated with both inspiratory and expiratory respiratory muscle strength reduction.Citation11 Polkey et al showed a 13% decrease in transdiaphragmatic pressure gradients, a surrogate for diaphragm strength, in older subjects (ages 67–81) as compared to younger subjects (ages 21–40).Citation17 Tolep et al compared the maximum transdiaphragmatic pressure (Pdimax) obtained during voluntary maximal inspiratory efforts in nine young (19–28 years) and ten elderly (65–75 years) subjects and found that the average Pdimax of the elderly subjects (128 ± 9 cm H2O) was significantly lower than the average Pdimax of the younger subjects (171 ± 8 cm H2O).Citation18 More specifically, there is age-related atrophy of muscle fibers, termed sarcopenia, which may also explain the reduced respiratory strength in the elderly. The decrease in muscle fiber strength can be as high as 20% by age 70.Citation18–Citation21 There are complex changes involving the mitochondria, muscle fiber disorganization, age-related muscle fiber transitions, and metabolic shifts in the aging muscle that can also explain the reduction in muscle strength.Citation22
The mucociliary elevator refers to the action of ciliated cells along the upper and lower airway to beat in synchrony, trapping and clearing mucus and foreign particles out of the lungs.Citation23 The upper airway nasal mucociliary cells work to remove large particles before they enter the smaller airways, and the lower airway mucociliary cells remove fine particles from the airway over a longer period of time.Citation24 There are alterations in both the clearance of large and small particles with aging. De Oliveira-Maul et al used the clearance of saccharin that was inhaled through the nares of healthy subjects to measure large airway nasal mucociliary function. They demonstrated that in people over age 40, there was a delayed nasal mucociliary clearance time of saccharin compared to subjects under 40 years of age.Citation25 Using radiolabeled particles that can travel past the upper airway and enter into the smaller airways in healthy nonsmoking subjects, Svartengren et al evaluated clearance of the labeled particles in different age groups ranging from age 19–81 years. They found that age alone was negatively associated with airway clearance of radiolabeled particles at 1, 2, 7, 14, and 21 days.Citation26 The association between age and decreased clearance by the mucociliary elevator may be due to beat frequency of the cilia. The studies of beat frequency in cilia are confounded by the presence of cigarette smoking, which has a large impact on beat frequency. Age has not been a statistically significant predictor of decreased beat frequency.Citation27 The impact of structure and functional changes created by the aging process is summed up in .
Aging and inflamm-aging
In addition to these age-related structural changes in the lung, advanced age contributes to systemic immune dysfunction. Of particular interest is the basal activation of the innate immune system in aged individuals in the absence of an immunologic threat.Citation28 This phenomenon, referred to as “inflamm-aging”, is marked by elevated levels of tissue and circulating proinflammatory cytokines in aged subjects.Citation28 Specifically, increased levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) have been observed in aging rodent and human studies.Citation29–Citation32 Theoretically, heightened levels of these cytokines in the absence of an immunologic threat or infectious target may be a contributory factor to reduced elasticity and destruction of the delicate lung parenchyma with advanced age. Related to inflamm-aging is the blunted immune response, known as “immunosenescence”, following a pathogenic threat or tissue injury.Citation28 Multiple studies have established reduced levels of mediators such as TNF-α, IL-6, interferon-γ, nitric oxide, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1α after different types of antigenic stimulation in aged animals.Citation33–Citation38 This basal level of inflammation, for example, elevated levels of IL-6, has been suggested to contribute to this subsequent immunosenescence following an immune challenge.Citation28,Citation39 Using a model of IL-6 knockout mice, Gomez et al demonstrated restoration of cytokine production of IL-1β, IL-12, and TNF-α following lipopolysaccharide (LPS) challenge in aged IL-6 knockout animals to levels comparable to young wild-type.Citation40 These data suggest that the basal elevation of circulating IL-6 observed in aged wild-type animals prior to stimulation may contribute to the inability to upregulate cytokine production in the presence of an infectious threat as represented by LPS.Citation40 In addition to these cytokine alterations with aging, more recent data demonstrate a role for microRNAs in inflamm-aging and cellular senescence.Citation41 Specifically, microRNA 146a has been associated with a “senescent associated proinflammatory status” in the setting of vascular remodeling.Citation42 Together, these data support the relationship between inflamm-aging and immunosenescence, suggesting that a disruption in the balance of pro- and anti-inflammatory mediators results in a baseline proinflammatory environment with advanced age that subsequently dampens an appropriate innate and adaptive immune response ().
Figure 2 Increasing age leads to elevated basal levels of inflammation (inflamm-aging) and increased immunosenescence, which are associated with changes in both innate and adaptive immune responses, contributing to the heightened morbidity and mortality seen in the elderly.
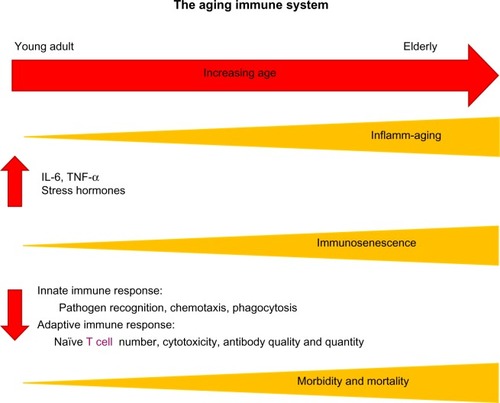
In addition to this reduced activation, there are data that support a shift in the temporal response to injury with aging, such that this initial immunosenescence over time results in a protracted immune response and chronic inflammation.Citation43,Citation44 These studies will be discussed in depth later in the context of pulmonary inflammation with aging; however, it is important to consider that this imbalance of immune mediators, delayed immune activation, and protracted course of inflammation may result in increased morbidity and mortality in aging individuals following infection, environmental exposures, or systemic injuryCitation45–Citation48
Age and pulmonary immunity
The lung has immunologic defenses that are both complex and resourceful, utilizing both an innate and adaptive immune response to inhaled antigens. Innate immunity is the critical first line of defense for the lungs. Adaptive immunity (acquired immunity) is antigen-specific and is required to ward off encapsulated bacteria, viruses, and intracellular pathogens. This form of immunity relies on immunologic memory and lymphocyte production of antibodies to nonself threats. Some important changes in the innate immunologic response occur with aging. Toll like receptors (TLRs) are key molecules in recognition and initiation of the innate immune response. In the context of aging, there are conflicting data regarding the impact of age on murine and human expression of TLRs or downstream signaling mediators.Citation38,Citation49–Citation51 While some of these murine studies on monocytes and macrophages report reduced expression of one or several TLRs,Citation34,Citation49 another report demonstrates alterations in downstream TLR signaling involving p36.Citation51 Reduction in p38 signaling is supported by studies in human monocytes from aged subjects, where dampened p38 signaling was associated with diminished phosphorylation of p38.Citation37 Additionally, these authors observed a reduction in TLR1 but no changes in TLR2 expression. While the data are divergent on how aging impacts TLR expression, the data do suggest that alteration in the TLR pathways plays a role in an age-related aberrant initiation of the innate immune response and may contribute to an inability in rapidly recognizing and eradicating a pathogen. In studies of cigarette smoke exposure, elevated expression and nuclear translocation of nuclear factor-kβ murine neutrophil chemokines, CXCL1 and CXCL2, were observed in aged mice.Citation52 This was accompanied by a protracted neutrophilia in the lung parenchyma.Citation52 Moreover, following exposure to environmental toxins such as diesel exhaust, the increased pulmonary neutrophilia in lung parenchyma led to congestion and delayed clearance in aged animals as compared to young mice.Citation53 These data suggest that inhaled pollutants cause a prolonged, aberrant pulmonary immune response, which may translate into increased tissue damage, playing a part in environmental, age-related pathology like COPD. Pulmonary infection with Francisella tularensis in aged rodents demonstrated delayed production of neutrophil chemokines in conjunction with an attenuated neutrophil recruitment at early times points,Citation43 supporting the concept of an aberrant initial immune response with age. At later time points, inoculation of LPS into the respiratory tract of aged mice was associated with subsequent heightened levels of chemokines CXCL1 and CXCL2, IL-1β, and lingering pulmonary neutrophilia at 72 hours in aged animals as compared to young.Citation44 Considering the delicate lung alveolar architecture and the highly hydrolytic enzymatic degranulation products of activated neutrophils, this may contribute to excessive tissue damage and reduced lung function over time.
In addition to age-related perturbations in recruitment following inhalational injury or infectious insult, McLachlan et al examined cytotoxic activity of monocytes in older patients compared to younger patients in response to LPS exposure, and found that older patients display less reactive oxygen species (ROS).Citation54 Cytotoxicity generated by the production of ROS and reactive nitrogen intermediates (RNI) is a key function of M1, or proinflammatory macrophages, responsible for activating the Type 1 helper T cells (Th1) pathway. Alteration in macrophage polarization marked by reduced ROS and RNI is reported with advanced age.Citation55 Supporting the study by McLachlan et al, alveolar macrophages from aged rats had reduced basal and LPS-activated levels of ROS and RNI.Citation56 Our lab and others have also demonstrated that aging is associated with lower levels of inducible nitric oxide synthase (iNOS), an enzyme that regulates production of ROS and RNI under control of the interferon-γ receptor.Citation57,Citation58 In conjunction with these changes in macrophage phenotype and cytotoxicity, others found that neutrophils from older individuals (≥85 years) produced less superoxide.Citation54,Citation59 These changes in reactivity have implications for compromising host defenses with age.
In addition to changes in innate system functioning with age, there are changes seen in adaptive immunity with age. In order to activate B- and T cells, dendritic cells (DCs) must migrate from sites of tissue injury and infection to local lymph nodes. Several studies demonstrated DCs from aged mice show poor migration and homing. DC migration in response to chemokine ligand-21, a key DC chemokine that is localized in lymph nodes and binds chemokine receptor type 7 and presents on DC cell membranes, was reduced in aged mice as compared to young.Citation60 Interestingly, following respiratory infection in the lungs of aged mice with either mouse hepatitis virus-1, respiratory syncytial virus, influenza A virus, or severe acute respiratory syndrome coronavirus, elevated expression of prostaglandin D2 correlated with reduced homing of lung DCs to regional lymph nodes and T cell activation.Citation61 Functional antagonism of prostaglandin D2 resulted in upregulation of chemokine receptor type 7, the critical receptor for DC migration, and improved DC homing to draining lymph nodes, subsequently improving T cell activation and survival after viral infection.Citation61
There are many adaptive immune functions that are inefficient with age. The thymus is primarily responsible for producing naïve T cells and is replaced by fatty tissue by age 60 years.Citation62 This leads to an increase in memory T cells relative to naïve T cells.Citation63 Both naïve CD4+ and CD8+ T cells are reduced in aged animals and humans relative to their memory T cells counterparts.Citation64–Citation66 In regard to CD8+ T cells, it has been suggested that repeated or latent cytomegalovirus infection may results in expansion of CD8+ memory cells, again diminishing the naïve CD8+ T cell pool.Citation67 Moreover, the proliferative aptitude of CD4+ T cells from aged donors appears to be reduced following T cell receptor engagement with high-dose anti-CD3 antibody in comparison to young controls,Citation68 suggesting a weaker novel pathogen-specific immune response. Meyer et al looked at the ratio of CD4+ to CD8+ T cells in bronchoalveolar lavage fluid in young versus old normal volunteers and found an increase in CD4+/CD8+ ratio as a function of age, suggesting there are fewer naïve cells available to be converted to memory cells in the face of a novel infection.Citation69,Citation70 Recently, forkhead box N1, a transcription factor known to play a role in embryonic thymus development, has been shown to play a critical role in preventing thymic involution and preservation of naïve T cell subsets with aging.Citation71 Overexpression of forkhead box N1 was demonstrated to increase early thymic progenitors, decrease splenic CD4+ memory T cells, and increase splenic naïve CD4+ and CD8+ T cells.Citation71 These data suggest a possible potential target to increase the number of naïve T cells in aged individuals, increasing the ability of the elderly to respond to novel antigens. The antibody-secreting capacity of B cells is reduced with age, perhaps leading to a less robust immunologic response.Citation72
Aging and respiratory disease development
The prevalence of COPD is two to three times higher in people over age 60.Citation73,Citation74 It is projected that from 1990 to 2020, COPD will move from the sixth to the third leading cause of death worldwide.Citation75 The Rotterdam study found that of healthy 55-year-olds without COPD, one in six women and one in four men will develop COPD later in life, with the risk for developing COPD over the coming 40 years being 24% and 16%, respectively.Citation76 Cigarette smoking is the greatest risk factor for developing COPD in genetically susceptible individuals. COPD is characterized by airway and lung inflammation, mucociliary dysfunction, alveolar destruction, and airway fibrosis.Citation77 The increased burden of COPD seen in the elderly population may be due to age-associated changes in the structure and function of the lung, increasing the pathogenetic susceptibility to COPD. These changes, described in elderly lifelong nonsmokers, are characterized by airspace dilatation resulting from loss of supporting tissue without alveolar wall destruction, similar to changes seen with COPD.Citation77 The Global initiative for chronic Obstructive Lung Disease (GOLD) criteria, accepted by the American Thoracic Society and the European Respiratory Society, is the standard for the diagnosis and classification of COPD, and is assessed by measuring the ratio of FEV1 to the forced vital capacity (FVC).Citation78 FEV1 peaks between ages 20–36 years, and then begins to decline as we age.Citation79 The annual rate of decline after the age of 25 is 20 mL per year and further declines to a loss of 38 mL per year after age 65.Citation79 FVC begins to decline later in life than FEV1 and at a slower pace. Because of the unparalleled rate of decline, use of the FEV1/FVC ratio alone to diagnose COPD will over represent a COPD diagnosis when no such pathology may exist.Citation80 To complicate this matter, Ohar et al found that COPD is underdiagnosed in the United States, arguing that this is due to underutilization of spirometry as a screening test for COPD.Citation81 Therefore, it is recommended to practitioners that a combination of spirometry and symptoms typical of COPD be utilized in the diagnosis of COPD in the elderly.
COPD is often associated with multiple comorbidities which can effect overall severity of disease. These include osteoporosis, mental illness, malnutrition, risk of cardiovascular disease, and skeletal muscle dysfunction.Citation82 Low body mass index is commonly seen in patients with COPD and has been shown to be inversely related to mortality in COPD.Citation83,Citation84 Anxiety and depression are prevalent comorbidities and have also been shown to be related to negative outcomes in COPD. It is estimated that 40% of individuals with COPD have depression, compared to a prevalence of 15% in the general population.Citation85 Cognitive impairments are common and associated with COPD in the elderly. It is estimated that anywhere between 42%–70% of aged persons with COPD have concomitant dementia or neurocognitive impairment.Citation86–Citation88 This may be related to hypoxemia and hypercapnia associated with COPD.Citation89 It may also be due to the common occurrence of cardiovascular disease in elderly with COPD.Citation90 These conditions certainly impact the ability to tolerate and comply with prescribed COPD therapies.
Inhaled bronchodilator therapy is the mainstay of treatment for the management of COPD. Treatment options are varied and include metered dose inhaler, dry powder inhaler, or nebulized formulations. There are many factors which may impact effective treatment use in the elderly COPD population including arthritis, weakness, poor manual dexterity, cognitive impairments, and visual limitations.Citation91 Careful consideration regarding treatment recommendations must be made in the aging COPD population.
Summary
There are many age-associated changes in the respiratory and pulmonary system. The size of the thoracic cavity decreases, limiting lung volumes and altering the muscles that aid in respiration. Muscle function on a cellular level is less efficient and has decreased reserve. Cough strength is reduced in the elderly population due to anatomic changes and muscle atrophy. Clearance of particles from the lung through the mucociliary elevator is negatively impacted and associated with ciliary dysfunction. There are many complex changes in immunity with aging that increase susceptibility to infections, including a less robust immune response from both the innate and adaptive immune systems. Finally, COPD has the highest prevalence in the elderly and deserves special consideration in regard to treatment in this fragile population. Additional research is needed to improve our understanding of the determinants of lung aging and the effects on lung immunity.
Disclosure
The authors report no conflicts of interest in this work.
References
- MiniñoAMDeath in the United States, 2011NCHS Data Brief20131151823742756
- WernerCACensus 2010 Brief C2010BR-09: The Older Population: 2010Washington, DCUS Department of Commerce2011 Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdfAccessed May 28, 2013
- BartynskiWSHellerMTGrahovacSZRothfusWEKurs-LaskyMSevere thoracic kyphosis in the older patient in the absence of vertebral fracture: association of extreme curve with ageAJNR Am J Neuroradiol20052682077208516155162
- SharmaGGoodwinJEffect of aging on respiratory system physiology and immunologyClin Interv Aging20061325326018046878
- EnsrudKEBlackDMHarrisFEttingerBCummingsSRCorrelates of kyphosis in older women. The Fracture Intervention Trial Research GroupJ Am Geriatr Soc19974566826879180660
- FonGTPittMJThiesACJrThoracic kyphosis: range in normal subjectsAJR Am J Roentgenol198013459799836768276
- LombardiIJrOliveiraLMMayerAFJardimJRNatourJEvaluation of pulmonary function and quality of life in women with osteoporosisOsteoporosis Int2005161012471253
- CulhamEGJimenezHAKingCEThoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosisSpine19941911125012558073317
- AroraNSRochesterDFEffect of body weight and muscularity on human diaphragm muscle mass, thickness, and areaJ Appl Physiol198252164707061279
- BrownMHasserEMComplexity of age-related change in skeletal muscleJ Gerontol A Biol Sci Med Sci1996512B117B1238612095
- FreitasFSIbiapinaCCAlvimCGBrittoRRParreiraVFRelationship between cough strength and functional level in elderlyRev Bras Fisioter201014647047621340240
- DeslerCHansenTLFrederiksenJBMarckerMLSinghKKJuel RasmussenLIs there a link between mitochondrial reserve respiratory capacity and aging?J Aging Res20122012 Article ID 192503
- SevranskyJEHaponikEFRespiratory failure in elderly patientsClin Geriatr Med200319120522412735123
- McCoolFDGlobal physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelinesChest2006129Suppl 148S53S16428691
- HeglandKWTrocheMSDavenportPWCough expired volume and airflow rates during sequential induced coughFront Physiol2013416723847546
- KimJDavenportPSapienzaCEffect of expiratory muscle strength training on elderly cough functionArch Gerontol Geriatr200948336136618457885
- PolkeyMIHarrisMLHughesPDThe contractile properties of the elderly human diaphragmAm J Respir Crit Care Med19971555156015649154857
- TolepKHigginsNMuzaSCrinerGKelsenSGComparison of diaphragm strength between healthy adult elderly and young menAm J Respir Crit Care Med199515226776827633725
- ChenHIKuoCSRelationship between respiratory muscle function and age, sex, and other factorsJ Appl Physiol19896629439482708222
- BerryJKVitaloCALarsonJLPatelMKimMJRespiratory muscle strength in older adultsNurs Res19964531541598637796
- FaulknerJABrooksSVZerbaESkeletal muscle weakness and fatigue in old age: underlying mechanismsAnnu Rev Gerontol Geriatr1990101471662102709
- MobasheriAMendesAFPhysiology and pathophysiology of musculoskeletal aging: current research trends and future prioritiesFront Physiol201347323576994
- WannerAClinical aspects of mucociliary transportAm Rev Respir Dis1977116173125327882
- ChilversMAO’CallaghanCLocal mucociliary defence mechanismsPaediatr Respir Rev200011273416263440
- de Oliveira-MaulJPde CarvalhoHBMiyuki GotoDAging, diabetes, and hypertension are associated with decreased nasal mucociliary clearanceChest201314341091109723100111
- SvartengrenMFalkRPhilipsonKLong-term clearance from small airways decreases with ageEur Respir J200526460961516204590
- AgiusAMSmallmanLAPahorALAge, smoking and nasal ciliary beat frequencyClin Otolaryngol Allied Sci19982332272309669071
- PandaAArjonaASapeyEHuman innate immunosenescence: causes and consequences for immunity in old ageTrends Immunol200930732533319541535
- KovacsEJGrabowskiKADuffnerLAPlackettTPGregoryMSSurvival and cell mediated immunity after burn injury in aged miceJ Am Aging Assoc20022513923604885
- GomezCRNomelliniVBailaHOshimaKKovacsEJComparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus i.p. LPS administrationShock200931217818418636046
- GómezCRAcuña-CastilloCNishimuraSSerum from aged F344 rats conditions the activation of young macrophagesMech Ageing Dev2006127325726316343598
- ErshlerWBKellerETAge-associated increased interleukin-6 gene expression, late-life diseases, and frailtyAnnu Rev Med20005124527010774463
- RenZGayRThomasAEffect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 miceJ Med Microbiol200958Pt 121559156719729455
- MurcianoCYáñezAO’ConnorJEGozalboDGilMLInfluence of aging on murine neutrophil and macrophage function against Candida albicansFEMS Immunol Med Microbiol200853221422118445021
- Shaik-DasthagirisahebYBKantarciAGibsonFCImmune response of macrophages from young and aged mice to the oral pathogenic bacterium Porphyromonas gingivalisImmun Ageing201071521114831
- MurcianoCVillamónEYáñezAO’ConnorJEGozalboDGilMLImpaired immune response to Candida albicans in aged miceJ Med Microbiol200655Pt 121649165617108267
- van DuinDMohantySThomasVAge-associated defect in human TLR-1/2 functionJ Immunol2007178297097517202359
- ChelvarajanRLLiuYPopaDMolecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophagesJ Leukoc Biol20067961314132716603589
- GomezCRGoralJRamirezLKopfMKovacsEJAberrant acute-phase response in aged interleukin-6 knockout miceShock200625658158516721265
- GomezCRKaravitisJPalmerJLInterleukin-6 contributes to age-related alteration of cytokine production by macrophagesMediators Inflamm2010201047513920671912
- OlivieriFRippoMRMonsurroVMicroRNAs linking inflamm-aging, cellular senescence and cancerAgeing Res Rev Epub5172013
- OlivieriFLazzariniRRecchioniRMiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodellingAge (Dordr)20133541157117222692818
- MaresCAOjedaSSLiQMorrisEGCoalsonJJTealeJMAged mice display an altered pulmonary host response to Francisella tularensis live vaccine strain (LVS) infectionsExp Gerontol2010452919619825409
- ItoYBetsuyakuTNasuharaYNishimuraMLipopolysaccharide-induced neutrophilic inflammation in the lungs differs with ageExp Lung Res200733737538417849263
- BruunsgaardHAndersen-RanbergKHjelmborgJvPedersenBKJeuneBElevated levels of tumor necrosis factor alpha and mortality in centenariansAm J Med2003115427828312967692
- CarusoCLioDCavalloneLFranceschiCAging, longevity, inflammation, and cancerAnn N Y Acad Sci2004102811315915584
- TurrentineFEWangHSimpsonVBJonesRSSurgical risk factors, morbidity, and mortality in elderly patientsJ Am Coll Surg2006203686587717116555
- ButcherSKKillampalliVChahalHKaya AlparELordJMEffect of age on susceptibility to post-traumatic infection in the elderlyBiochem Soc Trans200331244945112653659
- RenshawMRockwellJEnglemanCGewirtzAKatzJSambharaSCutting edge: impaired Toll-like receptor expression and function in agingJ Immunol200216994697470112391175
- ChelvarajanRLCollinsSMVan WilligenJMBondadaSThe unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage functionJ Leukoc Biol200577450351215629885
- BoehmerEDMeehanMJCutroBTKovacsEJAging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathwayMech Ageing Dev2005126121305131316154177
- MoriyamaCBetsuyakuTItoYAging enhances susceptibility to cigarette smoke-induced inflammation through bronchiolar chemokinesAm J Respir Cell Mol Biol201042330431119491340
- SunilVRPatelKJMainelisGPulmonary effects of inhaled diesel exhaust in aged miceToxicol Appl Pharmacol2009241328329319729031
- McLachlanJASerkinCDMorreyKMBakoucheOAntitumoral properties of aged human monocytesJ Immunol199515428328437814887
- DaceDSApteRSEffect of senescence on macrophage polarization and angiogenesisRejuvenation Res200811117718518279031
- TasatDRMancusoRO’ConnorSMolinariBAge-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophagesAging cell20032315916412882408
- KissinETomasiMMcCartney-FrancisNGibbsCLSmithPDAge-related decline in murine macrophage production of nitric oxideJ Infect Dis19971754100410079086170
- DingAHwangSSchwabREffect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolismJ Immunol19941535214621527519641
- PolignanoATortorellaCVeneziaAJirilloEAntonaciSAge-associated changes of neutrophil responsiveness in a human healthy elderly populationCytobios1994803221451537774287
- Grolleau-JuliusAHarningEKAbernathyLMYungRLImpaired dendritic cell function in aging leads to defective antitumor immunityCancer Res200868156341634918676859
- ZhaoJZhaoJLeggeKPerlmanSAge-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in miceJ Clin Invest2011121124921493022105170
- GruverALHudsonLLSempowskiGDImmunosenescence of ageingJ Pathol2007211214415617200946
- TimmJAThomanMLMaturation of CD4+ lymphocytes in the aged microenvironment results in a memory-enriched populationJ Immunol199916227117179916690
- PosnettDNSinhaRKabakSRussoCClonal populations of T-cells in normal elderly humans: the T-cell equivalent to “benign monoclonal gammapathy”J Exp Med199417926096188294871
- HongMSDanJMChoiJYKangIAge-associated changes in the frequency of naïve, memory and effector CD8+ T-cellsMech Ageing Dev2004125961561815491679
- ProvincialiMMoresiRDonniniALisaRMReference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderlyGerontology200955331432119190395
- KhanNShariffNCobboldMCytomegalovirus seropositivity drives the CD8 T-cell repertoire toward greater clonality in healthy elderly individualsJ Immunol200216941984199212165524
- MirzaNPollockKHoelzingerDBDominguezALLustgartenJComparative kinetic analyses of gene profiles of naive CD4+ and CD8+ T-cells from young and old animals reveal novel age-related alterationsAging Cell201110585386721711441
- FranceschiCBonafèMValensinSHuman immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological spaceVaccine200018161717172010689155
- MeyerKCErshlerWRosenthalNSLuXGPetersonKImmune dysregulation in the aging human lungAm J Respir Crit Care Med19961533107210798630547
- ZookECKrishackPAZhangSOverexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T-cellsBlood2011118225723573121908422
- SongHPricePWCernyJAge-related changes in antibody repertoire: contribution from T-cellsImmunol Rev199716055629476665
- BuistASMcBurnieMAVollmerWMBOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523
- FukuchiYNishimuraMIchinoseMCOPD in Japan: the Nippon COPD Epidemiology studyRespirology20049445846515612956
- MurrayCJLopezADAlternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease StudyLancet19973499064149815049167458
- van DurmeYMVerhammeKMStijnenTPrevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam studyChest2009135236837719201711
- SharmaGHananiaNAShimYMThe aging immune system and its relationship to the development of chronic obstructive pulmonary diseaseProc Am Thorac Soc20096757358019934352
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- BrandstetterRDKazemiHAging and the respiratory systemMed Clin North Am19836724194316827880
- DyerCThe interaction of ageing and lung diseaseChron Respir Dis201291636722308556
- OharJASadeghnejadAMeyersDADonohueJFBleeckerERDo symptoms predict COPD in smokers?Chest201013761345135320363841
- AgustiASorianoJBCOPD as a systemic diseaseCOPD20085213313818415812
- PauwelsRABuistASCalverleyPMJenkinsCRHurdSSGOLD Scientific CommitteeGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med200116351256127611316667
- LandboCPrescottELangePVestboJAlmdalTPPrognostic value of nutritional status in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199916061856186110588597
- NorwoodRJA review of etiologies of depression in COPDInt J Chron Obstruct Pulmon Dis20072448549118268923
- PettyTLBlissPLAmbulatory oxygen therapy, exercise, and survival with advanced chronic obstructive pulmonary disease (the Nocturnal Oxygen Therapy Trial revisited)Respir Care2000452204211 discussion 211–21310771792
- GrantIHeatonRKMcSweenyAJAdamsKMTimmsRMNeuropsychologic findings in hypoxemic chronic obstructive pulmonary diseaseArch Intern Med19821428147014767103628
- HungWWWisniveskyJPSiuALRossJSCognitive decline among patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180213413719423714
- KirkilGTugTOzelEBulutSTekatasAMuzMHThe evaluation of cognitive functions with P300 test for chronic obstructive pulmonary disease patients in attack and stable periodClin Neurol Neurosurg2007109755356017532116
- FillitHNashDTRundekTZuckermanACardiovascular risk factors and dementiaAm J Geriatr Pharmacother20086210011818675769
- ArmitageJMWilliamsSJInhaler technique in the elderlyAge Ageing19881742752783177088