Abstract
Background
The primary aim of this study was to determine the effect of 12 months of whole-body electromyostimulation (WB-EMS) exercise on appendicular muscle mass and abdominal fat mass in subjects specifically at risk for sarcopenia and abdominal obesity, but unable or unwilling to exercise conventionally.
Methods
Forty-six lean, nonsportive (<60 minutes of exercise per week), elderly women (aged 75 ± 4 years) with abdominal obesity according to International Diabetes Federation criteria were randomly assigned to either a WB-EMS group (n=23) which performed 18 minutes of intermittent, bipolar WB-EMS (85 Hz) three sessions in 14 days or an “active” control group (n=23). Whole-body and regional body composition was assessed by dual energy X-ray absorptiometry to determine appendicular muscle mass, upper leg muscle mass, abdominal fat mass, and upper leg fat mass. Maximum strength of the leg extensors was determined isometrically by force plates.
Results
After 12 months, significant intergroup differences were detected for the primary end-points of appendicular muscle mass (0.5% ± 2.0% for the WB-EMS group versus −0.8% ± 2.0% for the control group, P=0.025) and abdominal fat mass (−1.2% ± 5.9% for the WB-EMS group versus 2.4% ± 5.8% for the control group, P=0.038). Further, upper leg lean muscle mass changed favorably in the WB-EMS group (0.5% ± 2.5% versus −0.9% ± 1.9%, in the control group, P=0.033), while effects for upper leg fat mass were borderline nonsignificant (−0.8% ± 3.5% for the WB-EMS group versus 1.0% ± 2.6% for the control group, P=0.050). With respect to functional parameters, the effects for leg extensor strength were again significant, with more favorable changes in the WB-EMS group (9.1% ± 11.2% versus 1.0% ± 8.1% in the control group, P=0.010).
Conclusion
In summary, WB-EMS showed positive effects on the parameters of sarcopenia and regional fat accumulation. Further, considering the good acceptance of this technology by this nonsportive elderly cohort at risk for sarcopenia and abdominal obesity, WB-EMS may be a less off-putting alternative to impact appendicular muscle mass and abdominal fat mass, at least for subjects unwilling or unable to exercise conventionally.
Introduction
Age-related shift towards decreased muscle mass and increased (abdominal) fat mass is an important cause of frailty, loss of independence, and metabolic and cardiac disease, resulting in impaired quality of lifeCitation1 and increased mortality in the aged.Citation23 Regular exercise affects a wide range of risk factors and diseases of the aged. In this context, most trials have confirmed the positive effect of intense exercise training on muscle mass, functional capacity, and (abdominal) body fat mass in the aged.Citation4–Citation7 However, in our fundamentally sedentary society, enthusiasm for regular exercise to prevent future complaints and mortality is less prevalent. In Germany,Citation8 only 20% of women aged 65 years and older “reported” the exercise doses recommended for positively impacting body composition or bone mass.Citation9–Citation11 Even ignoring the possibility that self-reported physical activity may overestimate the real scenario,Citation12 these statistics demonstrate that the majority of elderly subjects seem either unable or unwilling to participate in regular exercise programs.Citation8 For these individuals, whole-body electromyostimulation (WB-EMS) may overcome some of the limitations of conventional types of exercise training and may be an acceptable and time-saving optionCitation13 for favorably impacting body composition and functional capacity.Citation14–Citation16 In addition to the tried and tested local application of electromyostimulation with its directly stimulating effect on the rate of skeletal muscle protein synthesis,Citation16 WB-EMS enlarges this effect by its simultaneous activation of a total area of 2.800 cm2 (16 regions, ) with different dedicated intensities/regions, and thus extends the potential of electromyostimulation. Recent WB-EMS trials in elderly cohorts not only demonstrated favorable effects on muscle mass, fat mass, and functional capacity,Citation14,Citation15 but also provided a strong body of evidence that this technology is highly acceptable to the aged user.Citation14,Citation15 However, for an aged nonsportive cohort at risk for sarcopenia and abdominal obesity, the corresponding evidence that WB-EMS favorably impacts muscle mass as the main predictor of sarcopenia and abdominal body fat as a key factor of metabolic and cardiac diseaseCitation17 has yet to be provided. Further, the long-term effects of WB-EMS with respect to feasibility parameters also remain to be established.
Thus, the primary purpose of this study was to determine the effect of 12 months of WB-EMS exercise on appendicular muscle mass and abdominal fat mass in subjects specifically at risk for sarcopenia and with abdominal obesity. Furthermore, we aimed to assess the WB-EMS-derived effect on muscle and fat mass of the upper leg region, an area with high relevance for independent livingCitation18 but specifically impacted by sarcopenia.Citation19
Our primary hypothesis was that WB-EMS training significantly increases appendicular skeletal muscle mass and decreases abdominal fat mass compared with a control group. Our secondary hypothesis was that WB-EMS training significantly increases upper leg muscle mass while upper leg fat mass decreases significantly compared with a control group.
Materials and methods
This study was based on data from the Training and ElectroStimulation Trial III (TEST-III),Citation20,Citation21 a randomized, controlled 12-month study of light-weight, nonsportive, osteopenic women aged 70 years and older that primarily focused on sarcopenia and osteoporosis. The study protocol was approved by the ethics committee of the FriedrichAlexander University Erlangen-Nürnberg, Germany (Ethik Antrag 4184) and the German Radiation Safety Agency (Z 5–22462/2–2010-027). After detailed information was provided, written informed consent was obtained from all the subjects prior to study entry. The study was conducted from November 2010 through July 2012 at the Institute of Medical Physics, Friedrich-Alexander University Erlangen-Nürnberg. The study is fully registered at www.clinicaltrials.gov (NCT 12296776).
The primary study endpoints were appendicular skeletal muscle mass and abdominal fat mass; secondary endpoints were upper leg muscle mass and upper leg fat mass; and the experimental endpoint was maximum isometric leg strength.
Participants
shows the participant flow during the different phases of the study adapted for the subanalysis performed in this article. For a more extensive description of the recruitment procedures, the reader is referred to other publications.Citation20,Citation21 Briefly, in a first step, female subjects aged 70 years and older and living in the area of Erlangen were contacted by mail that included most of the relevant eligibility criteria for the study. Four hundred and fifty-one women responded (5%), and were assessed further for eligibility by telephone interview. In order to identify a cohort with a high risk of sarcopenia, exclusion criteria focused on “leanness” and “physical inactivity”. Further, factors preventing proper application of WB-EMS or parameters that may confound the study results were addressed. Thus, subjects were excluded if they: did not meet our criteria for “lean” or “light-weight” as determined by the Broca IndexCitation22 (body weight [kg] < body height [cm] − 100); “exercised” for more than about one hour per week for the previous 5 years; reported contraindications to application of WB-EMS (ie, total hip endoprosthesis, cardiac arrhythmia); took medication and/or suffered from diseases affecting our study endpoints; or said they would be absent for more than 4 weeks during the study period.
Figure 1 Flow chart.
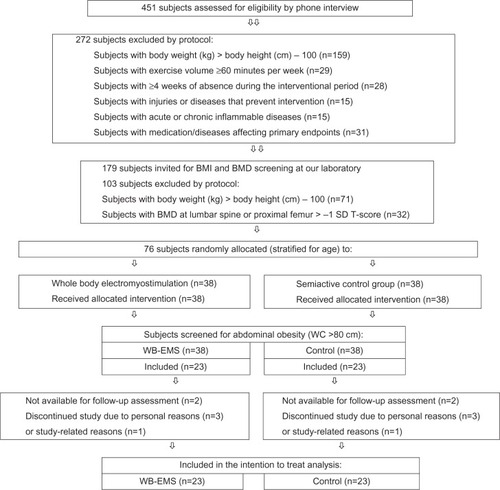
Applying these criteria, 272 subjects were excluded, and the remaining 179 women were invited to our laboratory for further examination, where a further 71 women were excluded after measuring their weight and height using calibrated devices. Finally, 32 further women were excluded because they did not meet our inclusion criteria of osteopenia at the lumbar spine or proximal femur (<−1 standard deviation t-score).
The remaining 76 eligible subjects were stratified by age (in 5-year strata) and randomly assigned to a WB-EMS group (n=38) or a control group (n=38). For the analysis discussed here, only those subjects with “central obesity” (waist circumference >80 cm for Caucasian females) at baseline as defined by the International Diabetes FederationCitation17 and the European Group for the Study of Insulin ResistanceCitation23 were retrospectively included in the intention-to-treat analysis. Applying these criteria, 46 (WB-EMS group, n=23; control group, n=23) of 78 subjects (61%) from the initial TEST-III study could finally be included. All subjects were of Caucasian race. The baseline characteristics of both groups are given in . No relevant differences between the groups were determined for these parameters.
Table 1 Baseline characteristics of the WB-EMS and control groups
Intervention
We set up two study groups, one of which carried out 54 weeks of a WB-EMS protocol, while in parallel, a control group performed an intermittent exercise program with low exercise intensity. This procedure was selected to validate the isolated effect of WB-EMS versus a motivated and “blinded” (active) control (“placebo”) group which performed gymnastics containing identical low intensity/low amplitude movements to those in the WB-EMS group. With respect to these movements, we focus on the same total exercise volume in the WB-EMS and control groups. However, in order to maintain motivation and attendance of the control subjects and to mimic recent German health care standards with their 10–12 weeks of exercise, the structure and design of the control group intervention differed from that of the WB-EMS group. Both groups exercised at the Institute of Medical Physics, which is centrally located and can be easily reached by public transport. All the sessions were supervised by certified trainers who also logged the participants’ attendance. Apart from the study intervention, subjects were asked to maintain their habitual life style.
WB-EMS intervention
Because WB-EMS is a rather novel technology, a brief introduction is given. Innovative and different from more widely known local electromyostimulation, current WB-EMS equipment enables simultaneous activation of up to 14–18 regions or 8–12 muscle groups (both upper legs, both upper arms, buttocks, abdomen, chest, lower back, upper back, latissimus dorsi, and four free options) with selectable intensity for each region. Summing up the stimulated area, up to 2.800 cm2 of area can be simultaneously activated ( and ). Strain or current intensity can be individually selected and modified during the electromyostimulation session. Our 18-minute WB-EMS program scheduled the intermitted low intensity/low amplitude movement protocol specifically evaluated for elderly subjects and carefully described in our recent pilot studies.Citation14,Citation15,Citation24 Briefly, three subjects simultaneously performed a video-guided 18-minute WB-EMS program at three electromyostimulation stations under the supervision of a certified instructor three times in 2 weeks (every Monday or Tuesday and every second Thursday or Friday) for 54 weeks with 2 weeks of holidays (). Bipolar electric current was applied with a frequency of 85 Hz, and an impulse breadth of 350 μsec intermittently with 6 seconds of electromyostimulation using a direct impulse boost to perform the slight movements and 4 seconds of rest on WB-EMS devices manufactured by miha bodytec (Gersthofen, Germany). Thus, total time under current per session ranged around 11 minutes, with approximately 7 minutes of rest between exercises. Additionally, 5–7 minutes of setup time was needed for preparation and post-processing. Due to regional and individual disparities in current sensitivity we are unable to prescribe the exact stimulation intensity in mA. To generate a sufficient but tolerable intensity of electromyostimulation, participants were asked to exercise at a rate of perceived exertion between “somewhat hard” (rate of perceived exertion 14) and “hard” (rate of perceived exertion 16). To accurately determine and record the level of rate of perceived exertion during the electromyostimulation we used a rate of perceived exertion scale ranging from 6 (very low) to 20 (maximum).Citation25 Electromyostimulation was applied during slight movements, with low amplitude performed without any additional weights in a standing position (). Amplitude, velocity, and corresponding intensity generated by the movement was set low (ie, squat: leg-flexion <35°) to prevent effects from the exercise per se.Citation14 Core exercise/movements performed were: “quarter” (leg flexion <35°) squat (6 seconds down) with arm extension/“quarter” (leg flexion <35°) deadlift (6 seconds up) with arm flexion; “quarter” squat (6 seconds down) with trunk flexion (crunches); “quarter” squat (6 seconds down) with lat pulleys/“quarter” squat (6 seconds up) with military press; “quarter” squat (6 seconds down), crunch with butterfy/“quarter” squat (6 seconds up) and reverse fly; “quarter” squat (6 seconds down) and vertical chest press/“quarter” squat (6 seconds up) and vertical rowing. The core exercises listed above were combined and slightly modified (eg, twisted crunch) to generate ten different types of slight movements/exercises; however, there was no progressive incrementing of intensity with respect to the movements during the interventional period. Thus, all in all, the WB-EMS session consisted of 10–14 dynamic exercises, structured in 1–2 sets of eight repetitions.
Current intensity was individually adapted for each region in agreement with the participants during the first sessions and after 6, 12, and 26 weeks. The corresponding setting was saved on chip cards for each muscle group/region in order to generate a fast, reliable, and valid setting in the subsequent sessions. Starting with this initial setting, instructors slightly increased the current intensity every 3–5 minutes in close cooperation with the participants in order to maintain the rate of perceived exertion between “somewhat hard” and “hard”. Thus, we generated a high relative intensity during the course of the study and therefore decided against applying a more sophisticated progression model. The rate of perceived exertion reported by the participants at the end of the session was recorded every other week.
Control group
In order to generate a “placebo condition” and to validate that WB-EMS and not slight movements were effective for impacting the study endpoints, we set up a control group that exercised in a different setting/framework. Subjects in the control group exercised for 10 weeks (one session of 60 minutes per week) interspersed by 10 weeks of rest during the 54-week interventional period. This procedure mimics the recent standards of the relevant German health care suppliers. The exercise protocol in the session consisted of a gentle warm-up (5 minutes of walking), with emphasis being placed on slight dynamic exercises identical to those performed during the WB-EMS sessions. As with the exercises/movements in the WB-EMS group protocol, no progressive increase of exercise intensity or volume was undertaken during the study period.
Testing procedures
All assessments and analyses were carried out in a blinded fashion. Baseline and follow-up tests were performed by the same assistants at the same time of the day (±60 minutes). The research assistants were not informed about or allowed to ask about participant status (WB-EMS or control group).
Anthropometry
Height was determined barefoot to the nearest 0.1 cm using a stadiometer (Holtain Ltd, Crymych Dyfed, UK). Body weight was measured to the nearest 0.1 kg on digital scales (InBody 230, Biospace, Seoul, Korea). Waist circumference was measured as the minimum circumference between the distal end of the rib cage and the top of the iliac crest along the mid axillary line. Body mass index was calculated by weight (kg)/height (m2). Normal weight, overweight, or underweight was estimated using the simple Broca Index ([body weight (kg) > body height (cm) − 100]Citation22).
Lean body mass and fat mass were assessed by dual energy X-ray absorptiometry (DXA, QDR 4500a, Discovery™ Upgrade, Hologic Inc, Bedford, MA, USA) using standard protocols.Citation26 The region of interest for abdominal body fat (abdominal fat mass) was segmented between the lower edge of the twelfth rib and the upper edge of the iliac crest. Appendicular skeletal muscle mass index (fat-free and bone-free proportion of the legs and arms [kg] as assessed by DXA/height [m2]) was calculated according to the method suggested by Baumgartner et al.Citation27 Region of interest for lean (nonosseous) and fat mass of the upper legs was segmented between the lower edge of the ischium and the lower edge of the femur.
Sarcopenia was defined as appendicular muscle mass of more than two standard deviations below the values for a “normal” healthy cohort aged approximately 30 years (ie, appendicular muscle mass <5.45 kg/m2).Citation27 In order to calculate the more recent sarcopenia scores of the European Working Group on Sarcopenia in Older People (EWGSOP),Citation28 we also determined walking speed and grip strength at baseline.
Functional testing
Usual (habitual) walking speed was tested by the 10 m walk test suggested by Lindemann et al,Citation29 which excludes the first 2.5 m of walking from the analysis. Grip strength of the dominant hand (Jamar dynamometer, Bolingbrook, IL, USA) was determined at baseline according to the method suggested by Mathiowetz et al.Citation30 The coefficient of variation for these tests was 4.5% (walk test) and 3.5% (grip strength) in our laboratory.
Maximum isometric strength during a leg press (knee flexion 85°) was determined with a force plate (MTD Systems, Neuburg vorm Wald Germany) according to the test protocol of Tusker.Citation31 For the exact positioning and procedures, the reader is referred to other publications.Citation32 The coefficient of variation for our testing scheme was 3.9% for the leg press.
A detailed validated questionnaire was usedCitation33,Citation34 to assess subject status (ie, age, diseases, medication, lifestyle), well being, pain parameters at different skeletal sites, prestudy exercise levels, and normal daily activity levels. The follow-up questionnaires also contained sections to monitor lifestyle changes, disease incidence, changes in disease severity, or sporting activity outside the prescribed training program.
The individual dietary intake was assessed by 5-day protocols completed by each study participant before and after the interventional period. Subjects were requested to weigh precisely the food they consumed. The protocols were analyzed using Prodi-4, 5/03 Expert software (Wissenschaftlicher Verlag, Freiburg, Germany).
Statistical analysis
We calculated a statistical power analysis for our primary endpoint of appendicular muscle mass based on a sample size of 23 subjects per group, a type I error of α =5%, and an exercise effect of 2.0% ± 2.5% for appendicular muscle mass. Statistical power with respect to type II error (1-β) was calculated at 0.77%. The analysis was based on an intention-to-treat strategy that included all the subjects independent of their loss to follow-up or withdrawal. Missing values (n=2 each for the WB-EMS and control groups) were imputed using the simple and conservative last observation carried forward concept. Baseline characteristics and follow-up data are reported as mean values and standard deviations. Changes between baseline and follow-up in the WB-EMS and control groups were reported as absolute (tables) and percentage changes (text). In addition, mean differences (with 95% confidence intervals) for primary, secondary, and experimental endpoints for the WB-EMS and control groups based on absolute changes are reported in and .
Table 2 Baseline and follow-up data, absolute changes, and statistical parameters of primary endpoints in the WB-EMS and control groups
Table 3 Changes in body fat and maximum isometric strength in the WB-EMS and control groups
In order to obtain normally distributed data, all study endpoints were log-transformed. Differences within groups were analyzed by paired t-tests. Analyses of variance with repeated measurements adjusted for baseline values were performed to check time-group interactions. All tests were two-tailed, and statistical significance was accepted at P<0.05. Effect sizes based on the absolute difference (± standard deviation) between baseline and follow-up in the WB-EMS group versus the control group were calculated using Cohen’s d.Citation35 Statistical Package for the Social Sciences version 21.0 software (IBM, Armonk, NY, USA) was used for all statistical procedures.
Results
Two subjects each in the WB-EMS group and control group were lost to follow-up (one case each of hip fracture and cancer in the WB-EMS group, relocation and a death in the control group). Another two subjects in the control group and three in the WB-EMS group discontinued the study for personal reasons; however, all of these subjects were fully assessed at follow-up. Of importance, two subjects listed study-related reasons for their withdrawal (one in the control group reported problems getting to classes, and one in the WB-EMS group reported discomfort, ie, regular slight muscular soreness, performing WB-EMS).
Overall, the attendance rate in the WB-EMS group (n=23) was 76% ± 19% (59.3 of 78 sessions) and 73% ± 19% (14.5 of 20 sessions) in the control group (n=23). Thus, net exercise volume averaged approximately 21 minutes per week (ie, about 13 minutes per week “under electricity”) in the WB-EMS group versus approximately 17 minutes per week in the control group. Average exercise intensity of the WB-EMS session as reported by the participants was 14.6 ± 1.2 for the WB-EMS and 10.0 ± 1.5 for the control group according to the rate of perceived exertion scale (P=0.001).
No relevant injuries were observed during the WB-EMS or control sessions. Further, no adverse effects from the interventions were reported by the participants. Lastly, changes in parameters that may have affected our primary and secondary outcomes (eg, lifestyle, dietary intake, diseases, or medication) did not vary between the two groups.
Primary endpoints
Appendicular muscle mass decreased significantly in the control group (−0.8% ± 2.0%; P=0.047) and increased slightly in the WB-EMS group (0.5% ± 2.0%; P=0.236). Differences between the WB-EMS and control groups were significant (P=0.025; effect size, d=0.69, ). Fat mass of the abdominal region of interest (abdominal fat mass) increased by 2.4% ± 5.8% (P=0.068) in the control group and decreased slightly by −1.2% ± 5.7% (P=0.294) in the WB-EMS group (abdominal fat mass, P=0.069). Differences between groups were significant (P=0.038; effect size, d=0.63, ). Change in waist circumference (−1.1 ± 2.1 cm in the WB-EMS group versus 1.0 ± 2.8 cm in the control group, P=0.007; effect size, d =0.85) confirmed the favorable effect of WB-EMS on abdominal obesity. Thus, the first hypothesis that WB-EMS training significantly increases appendicular skeletal muscle mass and decreases abdominal fat mass compared with a control group can be confirmed.
Secondary endpoints
Lean body mass of the upper legs decreased significantly in the control group (−0.9% ± 1.9%, P=0.023) and increased slightly in the WB-EMS group (0.5% ± 2.5%, P=0.346). Differences between the WB-EMS and control groups were significant (P=0.033; effect size, d=0.65, ). Fat content of the upper leg decreased by −0.8% ± 3.5% (P=0.248) in the WB-EMS group and increased (1.0% ± 2.6%, P=0.095) in the control group. Difference between the groups were borderline nonsignificant (P=0.050; effect size, d=0.60, ). Thus, the second hypothesis that WB-EMS training significantly increases upper leg muscle mass while upper leg fat mass decreases significantly compared with a control group cannot be fully confirmed.
Experimental endpoints
Maximum isometric strength of the leg extensors (9.1% ± 11.2%, P=0.002) increased significantly in the WB-EMS group (P<0.001) and increased slightly (1.0% ± 8.1%, P=0.631) in the control group (). Differences in intra-group changes between the WB-EMS and control groups were significant (P=0.010; effect size, d=0.82).
Discussion
This subanalysis of the TEST-III study is the first clinical trial to evaluate the WB-EMS-derived effect on body composition in this highly relevant cohort of elderly females with an increased risk for/or with prevalent sarcopenia and abdominal obesity.
From an epidemiologic point of view, the first interesting finding was that the majority of subjects (61%) in this cohort of elderly German females, ranging in the lowest age-adjusted body mass index quartile,Citation36,Citation37 had abdominal obesity, at least applying the definition (waist circumference >80 cm) of the International Diabetes FederationCitation17 or European Group for the Study of Insulin Resistance.Citation23 Applying the less strict World Health OrganizationCitation38 criterion of abdominal adiposity (waist circumference for women ≥88 cm) still leaves almost half (47%) of our subjects in this category. Applying the sarcopenic obesity definition of Zamboni et alCitation39 and/or Stenholm et al,Citation2 on the other hand, none of our subjects reached cutoff values for obesity,” eg, percentage body fat >38%,Citation40 >40%,Citation41 and >42.9%.Citation42
Also surprising, only three of 46 subjects were classified as sarcopenic according to the criteria of Baumgartner et al.Citation27 Applying the algorithm suggested by EWGSOP for sarcopenia,Citation28 ie, habitual gait speed ≤0.8 m/second or grip strength <20 kg and low appendicular skeletal muscle mass (ASMM) index, none among this lean, nonsportive, and osteopenic cohort of elderly women was classified as sarcopenic. This finding was unexpected given that the prevalence of sarcopenia (appendicular muscle mass <5.45 kg/m2) in the general US population was reported to be 5%–13% for subjects aged 60–70 yearsCitation27,Citation43 and approximately 30%–50% for subjects aged 80 years.Citation27,Citation44,Citation45 Obviously, independent living conflicts severely with recent cutoff values of sarcopenia, at least when including functional aspects. This idea seems consistent, and our rather low prevalence of sarcopenia has been confirmed by two current articlesCitation46,Citation47 addressing sarcopenia in 70–80-year-old home-dwelling women in Finland and the UK that assessed appendicular muscle massCitation46 or lean body massCitation46 by the DXA technique. Applying the EWGSOPCitation28 criteria, the authors reported a prevalence of 8% for the UK cohort and 0.9% for the Finnish cohort.
Summing up our results, we have clearly verified our primary hypothesis that WB-EMS training significantly impacts appendicular muscle mass and abdominal body fat mass in this highly relevant cohort of normal weight to underweight nonsportive elderly females. With respect to upper leg body composition, we observed comparable effects, although statistical significance was just missed (P=0.050) for upper leg fat mass. Thus, WB-EMS not only affected muscle parameters, which was to be expected, but also had favorable effects on abdominal body fat, which is a key factor in metabolic and cardiac disorders.Citation17,Citation48 Although total exercise volume (21 minutes per week with 13 minutes per week “under load” effectively) was quite low, the high metabolic activity during WB-EMS with its simultaneous stimulation of 2,650 cm of total area along with the subsequent adaptive response may trigger the corresponding effect. However, using indirect calorimetry we failed to demonstrate energy expenditure high enough (412 ± 61 kcal/hour) to explain the corresponding abdominal fat mass changes.Citation24 Nevertheless, due to the fact that the extra-mitochondrial fraction of energy production cannot be determined by this method,Citation49 the real effect of WB-EMS on energy expenditure and excessive post-exercise energy consumption may have been grossly underestimated. Hamada et alCitation50 noted a substantial involvement of glycolytic energy production in their thigh electromyostimulation procedure. This was paralleled by a significant rise in lactic acid during electromyostimulation compared with voluntary exercise at identical VO2 levels. In addition to these acute effects, another component for the decrease in (abdominal) fat may be the impact on resting metabolic rate observed in elderly femalesCitation14 after application of WB-EMS.
One may argue that a comparison of WB-EMS with resistance training effects on muscle or fat mass is not necessarily helpful, given that WB-EMS should be considered as an option for subjects who would not undertake resistance exercise anyway. Nevertheless, we provide here a short review in order to allow the reader to estimate the (clinical) significance of WB-induced muscle mass and fat changes compared with the gold standard therapy of “exercise training”. Unfortunately, only two relevant studies that also focus on exercise effects on appendicular muscle mass in female subjects aged 65 years and older (SEFIP study) were found. Briefly, Ades et alCitation51 were unable to generate positive effects on appendicular muscle mass after 6 months of progressive resistance exercise (three sessions per week, eight exercises for all main muscle groups, two sets with 10 repetitions at 80% repetition maximum) compared with inactive controls. On the other hand, our recent 18-month exercise trial,Citation52 which applied an intense resistance protocol using identical methods including a slightly exercising control group, showed comparable positive net effects (exercise/WB-EMS versus control) for appendicular muscle mass (TEST-III-study: 216 ± 93 g, d=0.69 versus SEFIP study: 299 ± 164 g, d=0.36). Additionally, a comparison of our lean body mass changes with data from a recent meta-analysis by Peterson et alCitation5 indicates that the effects of WB-EMS were within the range of those found with conventional resistance exercise, at least with respect to the elderly subgroup of this aging adult cohort. Interestingly, the same was also true after a comparison with high intensity resistance exercise protocols (>70% repetition maximum, as reported in several studiesCitation53–Citation55) in female subjects aged 60 years and older.
Although abdominal fat mass, as assessed by DXA, is a reliable parameter for estimating changes in abdominal obesity,Citation56 the corresponding literature is very scarce. The main reason for this lack of data is the limitation of DXA in distinguishing between intra-abdominal and subcutaneous fat. Unfortunately, therefore, only the above-mentioned SEFIP studyCitation52 was eligible for a dedicated comparison of the effects of WB-EMS versus those of conventional exercise on abdominal body fat as determined by DXA. Surprisingly, the effects of the present WB-EMS trial were comparable, ie, 368 ± 172 g (d=0.63) versus 178 ± 201 g (d=0.70), with those of the SEFIP protocol, which applied a mixed endurance/resistance protocol. Although abdominal body fat measured by DEXA is highly correlated with intraabdominal fat measurement by magnetic resonance imaging (MRI),Citation56,Citation57 we feel unable to discuss in detail to what degree these clinically relevant changes in abdominal fat relate to reductions of the more pathologic visceral fat component.Citation58 However, in a recent meta-analysis,Citation59 the authors did not determine different effects on the rate of subcutaneous/visceral fat reduction during weight loss when they compared different weight loss interventions (ie, diet, pharmaceuticals, exercise). Thus, there is no evidence that WB-EMS triggers different (more or less pronounced) effects on visceral fat compared with other interventions, including conventional types of exercise.
In addition to assessment of the effect of WB-EMS on appendicular muscle mass and abdominal fat mass, a further aim of this study was to determine the effect of WB-EMS on corresponding local muscle and fat mass of the upper leg, which is a site specifically impacted by the effects of sarcopenia,Citation19 and also highly relevant for independent living in the aged.Citation18 The underlying idea behind this approach is the mechanism of sarcopenic muscle loss with concomitant infiltration of fat and connective tissue (myosteatosis).Citation60,Citation61 We are aware that our DXA-based approach has certain limitations relevant to this issue in that our methods are unable to discriminate between subcutaneous versus intramuscular fat compartments of the upper leg. Allowing for the limitations discussed below, our results indicate favorable effects of WB-EMS with respect to the regional shift of muscle and fat mass in the upper leg.
Besides clinical effectiveness, the most relevant factor for the implementation of a given exercise method or technology is its feasibility and acceptance by subjects, which are key factors for its broad application. Adherence, dropout, and attendance rates in this WB-EMS study ranged in the upper area of exercise trials of comparable duration and number of participants.Citation62 Further, the cost of the WB-EMS equipment averaged EUR 8,000; however, taking into account the short duration of electromyostimulation and the resultant flow of users, WB-EMS is still a cost-effective way of improving body composition and corresponding functional ability.
We are aware that some features and limitations of this study may reduce the significance of its results. Two features in particular may give the reader cause for concern. One main limitation is the inclusion of an “active” control group to “blind” participants (and thus to provide a placebo scenario) and to validate that it was electromyostimulation per se and not the slight movements performed during electromyostimulation that affected our study endpoints. To achieve the latter effect, it would have been more appropriate to prescribe the identical setting (ie, 3 × 18 minutes per 2 weeks of slight exercise with WB-EMS equipment and without stimulation) continuously over 54 weeks. This procedure may be feasible and effective in animal studies, but failed in our study. Applying this strategy in a pilot studyCitation63 resulted in very unfavorable results for dropout and adherence. Further, the success of blinding was rare, with 18 of 20 subjects reporting that they believed themselves to be in the control group and were performing sham exercise. In this study, blinding was much more successful; however, strict comparability between the two groups with respect to the effect of slight movements per se was limited. In fact, due to the block periodization exercise protocol used in the control group, two main differences with potential impact on our endpoints may exist: although the total volume of “time under load” is comparable between the groups, more frequent but shorter bouts of exercise than applied in the WB-EMS session may be slightly more favorable;Citation64 and the 10-week “exercise block” finished 4 weeks before the 54-week follow-up assessment. Thus, if there were a conditioning effect, some deconditioning could occur. However, estimating the relevance of these slight, low amplitude movements per se, we detected no positive impact on muscle mass, strength, or power in a recent study of less lean women of comparable age.Citation14 However, these subjects were much more sporty, so we cannot completely rule out a positive effect in the more frail persons in this study.
Summing up this important issue:
While taking into account that the effect of slight movements per se is doubtful and also the high comparability between the two exercise groups with respect to type, intensity, and total volume of exercise, we conclude that the contribution of exercise to the effects of WB-EMS on muscle and fat parameters is quite low, should it even exist.
Although DXA is the gold standard technology for assessing body composition, MRI with its corresponding ability to discriminate between subcutaneous and intra-abdominal fat content may certainly result in more meaningful results with respect to the abdominal region. However, with respect to muscle mass and sarcopenia definition, only DXA and bioimpedance analysis-derived T-scores of appendicular muscle mass were available. Without doubt, additional MRI scans of the upper leg might have provided more insight with respect to changes in the fat and muscle compartments but, as mentioned above, the software programs currently available are (still) unable to analyze or quantify the crucial intramuscular shift of contractile, connective, and fat elements as assessed by MRI or computed tomography techniques.
It is debatable whether our eligibility criteria optimally identified the cohort of elderly subjects at risk for sarcopenia and abdominal obesity. This specifically refers to the decision to use the simple Broca Index so that subjects themselves could easily assess their eligibility before contacting the study nurse to declare their interest in joining the study. With respect to abdominal obesity, we applied the rather strict International Diabetes Federation/European Group for the Study of Insulin Resistance criteria of a >80 cm waist circumference for Caucasian women. In hindsight, this cutoff value may have been too restrictive for the aged. Indeed, there is ongoing discussion as to whether waist circumference cutoffs should be dramatically shifted upwardsCitation65 in order to generate more meaningful and health-related cutoff points for the aged population.Citation66
We are unable to prescribe the exact stimulation intensity for our WB-EMS protocol in mA. Our corresponding strategy thus focuses on prescription of a range of rates of perceived exertion validated to trigger favorable WB-EMS effects with respect to muscle mass, strength, and power.Citation67 Although subjects reported rates of perceived exertion for WB-EMS intensity within our expectations and much effort was placed on participants briefing, conditioning, and interaction, it is difficult to decide clearly whether subjects did in fact achieve the intended exercise intensity during WB-EMS training.
Conclusion
Our results clearly demonstrate that WB-EMS (at least combined with slight movements) applied for 18 minutes per session, on three sessions per 14 days over 12 months, has a beneficial impact on muscle mass and abdominal body fat, and is also safe and feasible, at least in this cohort of lean elderly females with limited interest in exercise. Although WB-EMS was unable to generate all the benefits of multipurpose exercise programs specifically designed for the multimorbid aged,Citation68 it can be regarded as an option for subjects unwilling or unable to participate in conventional exercise programs but looking to improve their muscular fitness for independent and healthy aging.
Acknowledgments
We gratefully acknowledge the support of miha-bodytec (Augsburg, Germany), which supplied the WB-EMS technology, and Rottapharm/Madaus (Cologne, Germany), which supplied the calcium and vitamin D. We further acknowledge support by Deutsche ForschungsGemeinschaft and Friedrich-Alexander-University Erlangen-Nürnberg within the funding program “Open Access Publishing”.
Disclosure
The authors report no conflicts of interest in this work.
References
- MaselMCGrahamJEReistetterTAFrailty and health related quality of life in older Mexican AmericansHealth Qual Life Outcomes200977019627598
- StenholmSHarrisTBRantanenTSarcopenic obesity: definition, cause and consequencesCurr Opin Clin Nutr Metab Care200811669370018827572
- SlentzCAAikenLBHoumardJAInactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amountJ Appl Physiol20059941613161816002776
- LathamNAndersonCBennettDProgressive resistance strength training for physical disability in older peopleCochrane Database Syst Rev20032CD00275912804434
- PetersonMDSenAGordonPMInfluence of resistance exercise on lean body mass in aging adults: a meta-analysisMed Sci Sports Exerc201143224925820543750
- StrasserBArvandiMSiebertUResistance training, visceral obesity and inflammatory response: a review of the evidenceObes Rev201213757859122385646
- WeinheimerEMSandsLPCampbellWWA systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesityNutr Rev201068737538820591106
- Robert Koch InstitutSportliche Aktivität. [Physical Exercise in Germany]Berlin, GermanyRobert Koch Institut2012 German
- [No authors listed]Physical activity guidelines for AmericansOkla Nurse200853425
- Chodzko-ZajkoWJProctorDNFiatarone SinghMAAmerican College of Sports Medicine position stand. Exercise and physical activity for older adultsMed Sci Sports Exerc20094171510153019516148
- KemmlerWvon StengelSExercise frequency, health risk factors and diseases of the elderlyArch Phys Med RehabilJune62013 [Epub ahead of print.]
- MontoyeHJKemperHCSarisWHMeasuring Physical Activity and Energy ExpenditureChampaign, ILHuman Kinetics1996
- WeineckJSportbiologie. [Exercise-Biology]Balingen, GermanySpitta Verlag2009 German
- KemmlerWSchliffkaRMayhewJLEffects of whole-body-electromyostimulation on resting metabolic rate, anthropometric and neuromuscular parameters in the elderly. The Training and Electro Stimulation Trial (TEST)J Strength Cond Res20102471880188620555279
- KemmlerWBirlaufAvon StengelSEinfluss von Ganzkörper-Elektromyostimulation auf das Metabolische Syndrom bei älteren Männern mit metabolischem Syndrom. [Effects of whole-body-electromyostimulation on body composition and cardiac risk factors in elderly men with the metabolic syndrome. The TEST-II study]Dtsch Z Sportmed2010615117123 German
- WallTBDirksMLVerdijkLBNeuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic menAm J Physiol Endocrinol Metab2012303E614E62322739107
- AlbertiKGZimmetPShawJMetabolic syndrome – a new worldwide definition. A consensus statement from the International Diabetes FederationDiabet Med200623546948016681555
- ReidKFNaumovaENCarabelloRJLower extremity muscle mass predicts functional performance in mobility-limited eldersJ Nutr Health Aging200812749349818615232
- JanssenIHeymsfieldSBWangHJSkeletal muscle mass and distribution in 468 men and women aged 18–88 yrJ Appl Physiol2000891818810904038
- KemmlerWEngelkeKVon StengelSGanzkörper-Elektromyostimulation zur Prävention der Sarkopenie bei einem älteren Risikokollektiv. Die TEST-III Studie. [Effects of whole-body-electromyostimulation on Sarcopenia in lean, elderly sedentary women. The TEST-III Study]Dtsch Z Sportmed201263121623 German
- KemmlerWVon StengelSBebenekMEffekte eines Ganzkörper-Elektromyostimulations-Trainings auf die Knochendichte eines Hochrisikokollektivs für Osteopenie. Eine randomisierte Studie mit schlanken und sportlich inaktiven Frauen. [Effects of whole-body-electromyostimulation on bone mineral density in lean, sedentary elderly women with osteopeniaThe randomized controlled TEST-III Study]Osteologie2013222121128 German
- LiebermeisterHPrognosis in obesity, what has changed?Versicherungsmedizin19954711723 German7709501
- BalkauBCharlesMAComment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR)Diabet Med199916544244310342346
- KemmlerWVon StengelSSchwarzJEffect of whole-body electromyostimulation on energy expenditure during exerciseJ Strength Cond Res201226124024522158139
- BorgGPerceived exertion as an indicator of somatic stressScand J Rehabil Med19702292985523831
- Hologic CorporationQDR Discovery – Users GuideWaltham, MAHologic Inc2008
- BaumgartnerRNKoehlerKMGallagherDEpidemiology of sarcopenia among the elderly in New MexicoAm J Epidemiol199814787557639554417
- Cruz-JentoftAJBaeyensJPBauerJMSarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older PeopleAge Ageing201039441242320392703
- LindemannUNajafBZijlstraWDistance to achieve steady state walking speed in frail elderly personsGait Posture2008271919617383185
- MathiowetzVWeberKVollandGReliability and validity of grip and pinch strength evaluationsJ Hand Surg Am1984922222266715829
- TuskerFBestimmung von Kraftparameter eingelenkiger Kraftmessungen. [Evaluation of strength parameters in single-joint strength assessments]Aachen, GermanyShaker Verlag1994
- KemmlerWVStengelSMayerSNiedermayerMHentschkeCKalenderWAEffect of whole body vibration on the neuromuscular performance of females 65 years and older: one-year results of the controlled randomized ELVIS studyZ Gerontol Geriatr2009 German
- KemmlerWLauberDWeineckJBenefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS)Arch Intern Med2004164101084109115159265
- KemmlerWWeineckJKalenderWAThe effect of habitual physical activity, non-athletic exercise, muscle strength, and VO2max on bone mineral density is rather low in early postmenopausal osteopenic womenJ Musculoskelet Neuronal Interact20044332533415615501
- CohenJStatistical Power Analysis for the Behavioral Sciences2nd edHillsdale, NJLawrence Earlbaum Associates1988
- BeneckeAVogelHÜbergewicht und Adipositas. [Overweight and Obesity]. Report No 1Berlin, GermanyRobert Koch Institut2003 Available from http://edoc.rki.de/docviews/abstract.php?id=197Accessed September 23, 2013
- DESTATISVerteilung der Bevölkerung auf Body-Mass-Index-Gruppen in ProzentDistribution of the German population with respect to Body Mass Index in percent2012 Available from http://www.gbe-bund.de/oowa921-install/servlet/oowa/aw92/dboowasys921.xwdevkit/xwd_init?gbe.isgbetol/xs_start_neu/&p_aid=i&p_aid=47297733&nummer=434&p_sprache=D&p_indsp=99999999&p_aid=6017Available from September 23, 2013
- World Health OrganizationObesity: preventing and managing the global epidemicGeneva, SwitzerlandWorld Health Organization2000 Available from: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/Accessed September 5, 2013
- ZamboniMMazzaliGFantinFSarcopenic obesity: a new category of obesity in the elderlyNutr Metab Cardiovasc Dis200818538839518395429
- BaumgartnerRNBody composition in healthy agingAnn N Y Acad Sci200090443744810865787
- DavisonKKFordESCogswellMEPercentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES IIIJ Am Geriatr Soc200250111802180912410898
- ZoicoEDi FrancescoVGuralnikJMPhysical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly womenInt J Obes Relat Metab Disord200428223424114708033
- MorleyJESarcopenia: diagnosis and treatmentJ Nutr Health Aging200812745245618615226
- MorleyJESarcopenia in the elderlyFam Pract201229Suppl 1i44i4822399555
- MorleyJEBaumgartnerRNRoubenoffRSarcopeniaJ Lab Clin Med2001137423124311283518
- PatilRUusi-RasiKPasanenMSarcopenia and osteopenia among 70–80-year-old home-dwelling Finnish women: prevalence and association with functional performanceOsteoporos Int201324378779622688541
- PatilRSyddallHSJamesonKPrevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS)Age Aging2013423378384
- PatakyZBobbioni-HarschEMakoundouVEnlarged waist circumference and cardiovascular risk factorsRev Med Suisse20095196671672674675 French19462610
- LaForgiaJWithersRTGoreCJEffects of exercise intensity and duration on the excess post-exercise oxygen consumptionJ Sports Sci200624121247126417101527
- HamadaTHayashiTKimuraTElectrical stimulation of human lower extremities enhances energy consumption, carbohydrate oxidation, and whole body glucose uptakeJ Appl Physiol200496391191614594864
- AdesPASavagePDBrochuMResistance training increases total daily energy expenditure in disabled older women with coronary heart diseaseJ Appl Physiol20059841280128515772059
- KemmlerWvon StengelSEngelkeKExercise, body composition, and functional ability: a randomized controlled trialAm J Prev Med201038327928720171529
- BinderEFYarasheskiKESteger-MayKEffects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trialJ Gerontol A Biol Sci Med Sci200560111425143116339329
- MarquesEAMotaJMachadoLMulticomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older womenCalcif Tissue Int201188211712921113584
- NelsonMEFiataroneMALayneJEAnalysis of body-composition techniques and models for detecting change in soft tissue with strength trainingAm J Clin Nutr19966356786868615349
- KamelEGMcNeillGHanTSMeasurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and womenInt J Obes Relat Metab Disord199923768669210454101
- KamelEGMcNeillGVan WijkMCChange in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurementsInt J Obes Relat Metab Disord200024560761310849583
- JensenMDIs visceral fat involved in the pathognesis of the metabolic syndrome? Human modelObesity (Silver Spring)200614Suppl 1S20S24
- ChastonTBDixonJBFactors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic reviewInt J Obes (Lond)200832461962818180786
- Kent-BraunJANgAVYoungKSkeletal muscle contractile and noncontractile components in young and older women and menJ Appl Physiol200088266266810658035
- TaaffeDRHenwoodTRNallsMAAlterations in muscle attenuation following detraining and retraining in resistance-trained older adultsGerontology200955221722319060453
- MarquesEAMotaJCarvalhoJExercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trialsAge (Dordr)20113461493151521922251
- KemmlerWTeschlerMBebenekMEffekt von Ganzkörper-Elektromyostimulation auf die übergreifende sportmotorische Leistungsfähigkeit im Handball. [Effects of whole-body electromyostimulation on general performance and skills in handball players]Dtsch Z Sportmed2011627/8271 German
- QuinnTJKloosterJRKenefickRWTwo short, daily activity bouts versus one long bout: are health and fitness improvements similar over twelve and twenty-four weeks?J Strength Cond Res200620113013516506860
- HowelDWaist circumference and abdominal obesity among older adults: patterns, prevalence and trendsPLoS One2012710e4852823119047
- HeimNSnijderMBHeymansMWOptimal cutoff values for high-risk waist circumference in older adults based on related health outcomesAm J Epidemiol2011174447948921673122
- KemmlerWvon StengelSAlternative exercise technologies to fight against sarcopenia at old age: a series of studies and reviewJ Aging Res2012201210901322500224
- KemmlerWvon StengelSEngelkeKExercise effects on bone mineral density, falls, coronary risk factors, and health care costs in older women: the randomized controlled senior fitness and prevention (SEFIP) studyArch Intern Med2010170217918520101013

