Abstract
Objective
Acetylcholinesterase inhibitors (AChEIs) may reduce the oxidative stress in brain of Alzheimer’s disease (AD) patients. Forkhead box O1 (FOXO1) protein has been reported as the link between oxidative stress and AD. We evaluated a potential association between FOXO1 gene locus and the response to AChEI treatment in patients with sporadic AD.
Methods
In this prospective study, 109 Caucasian AD patients were treated with standard doses of donepezil, galantamine, or rivastigmine for 6 months. Functional and cognitive status were evaluated at baseline and after treatment. Response to therapy was defined according to the National Institute for Health and Clinical Excellence criteria. Genotype analyses, including the APOE polymorphism, were made in blinded fashion.
Results
A significantly higher frequency of FOXO1 rs7981045 G/G genotype was observed in nonresponders compared with responders (17.14% versus 2.70%, P=0.010). Age, sex, and APOE-adjusted logistic regression analysis confirmed that patients with the G/G genotype had a significantly higher risk of poor response to AChEI treatment (odds ratio =10.310; 95% confidence interval, 1.510–70.362). Haplotype analysis revealed significant differences in haplotype frequency distribution between these groups.
Conclusion
FOXO1 may influence the clinical response to AChEIs in AD patients.
Introduction
The global increases in population size and life expectancy have led sporadic Alzheimer’s disease (AD) to become a main health problem. Sporadic AD occurs in later life with a prevalence of 20% after 75 years increasing to 30% after 85 years. About 5% of people aged 65 years or older have AD, and with about 3 to 4 million people affected and about 350,000 new cases per year, AD is the most frequent cause of dementia in Western countries. Accordingly, it has been estimated that AD prevalence will nearly double every 20 years, rising to 65.7 million in 2030 and 115.4 million in 2050.Citation1–Citation3
Despite the progress of modern pharmacology in developing drugs against AD, for most of these drugs, positive clinical outcomes are missing.Citation4,Citation5 Thus, acetylcholinesterase inhibitors (AChEIs), and in particular donepezil, rivastigmine, and galantamine still remain the main drugs currently used for the symptomatic treatment of AD.Citation6,Citation7
Recent studies reported strong evidence toward a key role of functional variants in gene encoding drug-metabolizing enzymes on the efficacy of AChEIs, such as donepezil.Citation8–Citation10 However, unrelated drug-metabolism pathways that conversely underlie the pathogenesis of AD may contribute to the response to AChEIs. Oxidative stress, recently related to the pathogenesis of AD, may be one of these pathways. An overproduction of reactive oxygen species may activate microglia and astrocytes, inducing neurotoxicity through deposition of amyloid β peptides.Citation11,Citation12 Recent data suggested that AChEIs may act directly on this process, enhancing antioxidant activity and attenuating oxidative stress.Citation13
Many genes have been described for their role in the physiological response to oxidative stress. In particular, proteins of the forkhead box family, class O (FoxO) has been suggested as a link among pathophysiology of AD and the response to oxidative stress. Recent data strongly suggested that FoxO protein 1 (FOXO1), the most important protein of the FoxO family, plays this role.Citation14 FOXO1 is encoded by the FOXO1 gene at locus 13q14.1. We also analyzed all samples for APOE genotype, which is being used clinically to provide additional information regarding patients with dementia and indicates whether there is an increased risk of AD, although not specifically diagnostic of AD.
The aim of the present study was to investigate the role of the FOXO1 gene as a potential background factor influencing the response to AChEIs in older patients with AD.
Materials and methods
Patient recruitment
This was a prospective cohort study fulfilling the Declaration of Helsinki,Citation15 the guidelines for Good Clinical Practice,Citation16 the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines,Citation17 and the National Institute for Health and Clinical Excellence (NICE) requirements.Citation18 Approval of the study for experiments using human subjects was obtained from the local ethics committees on human experimentation. Written informed consent for research was obtained from each patient or from a relative or legal guardian in the case of critically disabled demented patients, prior to participation in the study. All patients included in this study were Caucasians, with most individuals from families that have lived in Central and Southern Italy for at least two generations. All patients included in this study were selected from patients consecutively attending from July 1, 2009, through July 31, 2011, the Geriatric Unit of the Istituto di Ricovero e Cura a Carattere Scientifico Casa Sollievo della Sofferenza in San Giovanni Rotondo, Italy.
Inclusion/exclusion criteria
Inclusion criteria were 1) age ≥65 years; 2) diagnosis of mild or moderate AD; 3) informed consent for research. Exclusion criteria were 1) diagnosis of vascular dementia (VaD), mixed dementia, or mild cognitive impairment; 2) presence of serious comorbidity, tumors, other diseases, or physiological status (ascertained blood infections, vitamin B12 deficiency, anemia, disorders of the thyroid, kidneys, or liver) that could be causally related to cognitive impairment; 3) history of alcohol or drug abuse; 4) head trauma; 5) current or previous use of psychoactive substances; or 6) diabetes mellitus.
Data collection
Baseline demographic and clinical characteristics were collected by a structured interview, clinical evaluation, and review of records from patients’ general practitioners. All patients included in the study were initially treated for 1 month with an AChEI, that is, donepezil 5 mg/daily; or rivastigmine 1.5 mg ×2/daily (pill) or 4.6 mg/daily (transdermal patch); or galantamine 8 mg/daily. Thereafter, patients who had followed the treatment with a satisfactory or good compliance without clinically relevant drug-related adverse events increased the dosage of donepezil to 10 mg/daily; rivastigmine to 3 mg ×2/daily (pill) or to 9.5 mg/daily (transdermal patch) for the following 5 months; or galantamine to 16 mg/daily for a further 1 month, which was increased to 24 mg/daily for the following 4 months. Patients who needed a coadministration of memantine were excluded from the study. At 6-month follow-up, the clinical assessment, including the evaluation of cognitive and functional status, compliance, and drug-related adverse events, was repeated.
Cognitive-functional evaluation and diagnosis of AD
In all patients, cognitive status was screened by means of the mini-mental state examination (MMSE),Citation19 the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog),Citation20 and the Clinical Dementia Rating scale (CDR).Citation21,Citation22 Dementia (CDR 1+) was confirmed and diagnosed by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DMS-IV), whereas diagnosis of questionable dementia (QD) was made according to a CDR value of 0.5+.Citation23 Diagnosis of mild cognitive impairment was made according to the Petersen criteriaCitation24 in subjects with CDR 0.5+ and an MMSE value from 24 to 27. Diagnosis of QD or MCI caused the exclusion from the study. Diagnosis of possible/probable AD was made according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association Work Group (NINCDS-ADRDA).Citation25 Diagnosis of possible/probable vascular dementia (VaD) was made according to the criteria of the National Institute of Neurological Disorders and Stroke – Association Internationale pour la Recherche et l’Enseignement en Neurosciences Work Group (NINDS-AIREN).Citation26 Differential diagnosis between AD and VaD was also based on the Hachinski Ischemic Score to address unclear AD/VaD diagnoses.Citation27 In particular, scores ≤4 were considered as probable AD, scores ≥7 were included in the VaD group that was excluded from the study. Scores between 5 and 6 were diagnosed as mixed dementia and were also excluded from the study. Diagnosis of AD or VaD was always supported by neuroimaging evidences (computed tomography scan and/or nuclear magnetic resonance). In particular, the presence of multiple cortical/subcortical infarcts or an infarct in a strategic area such as the thalamus or temporal lobe and/or lesions of the white matter indicated probable VaD. The absence of the above-mentioned cerebrovascular lesions indicated AD. Functional status was evaluated using the Activities of Daily Living (ADL) indexCitation28 and the Instrumental Activities of Daily Living (IADL) scale.Citation29
Responder/nonresponder assessment criteria
According to the NICE requirements,Citation18 a responder to AChEI treatment was defined as a patient who showed improvement or no deterioration in cognition, as evaluated by means of ADAS-cog and MMSE, and an improvement in functional status, as evaluated by means of ADL or IADL indexes.
Genetic analysis
Genomic deoxyribonucleic acid (DNA) was purified from fresh/frozen blood samples following a salting-out method.Citation30 On the basis of their level of heterozygosity (>5% in Caucasians),Citation31 we selected the three single-nucleotide polymorphisms (SNPs) rs2721069 (41,143,720 bases from pter) (C102,015→T), rs4943794 (41,173,408 bases from pter) (T72,327→G), and rs7981045 (41,209,236 bases from pter) (C36,499→T) spanning a 65 kb block at the FOXO1 locus (13q14.1). All SNPs were investigated in a blinded fashion by means of the allele discrimination assay. The analysis was made using TaqMan technology on an ABI PRISM 7700 Sequence Detector system (Life Technologies Corporation, Carlsbad, CA, USA) with assay c_15926664_10 (rs2721069), c_30366093_20 (rs4943794), and c_30886685_10 (rs7981045) according to manufacturer’s instructions. The APOE genotypes were determined as previously described.Citation32
Statistical analyses
Patients’ baseline characteristics and FOXO1 genotypes were reported as frequencies and percentage, mean ± standard deviation (SD), and comparisons between groups were performed with Pearson’s chi-square test and Mann–Whitney U-test for categorical and continuous variables, respectively. Differences between clinical evaluation at baseline and after 6 months of follow-up were assessed using a Student’s t-test for paired samples. Age, sex, APOE, and AChEI administration were evaluated as potential predictors of responsiveness to AChEI treatment at the 6-month follow-up. Results were reported as odds ratios (ORs) along with their 95% of confidence intervals (CIs). Furthermore, univariate and multivariate logistic regression models were assessed in order to test the association between the genotypes and the responsiveness to AChEIs under free, dominant, recessive, and additive genetic models of inheritance. The Hardy-Weinberg equilibrium (HWE) was verified for all the investigated polymorphisms using the exact test proposed by Wigginton et al.Citation33 The HaploView 4.2 genetic software packageCitation34 was used to estimate values of linkage disequilibrium (LD) coefficient r2 as well as to estimate haplotype frequency and to compare it among the study groups. A P-value of <0.05 was considered for statistical significance. All statistical analyses were performed using SAS Release 9.1 (SAS Institute, Cary, NC, USA).
Results
Characteristics of patients at baseline according to sex are summarized in . No difference was observed in age distribution between men and women. Conversely, the educational level was higher in men (6.10±4.49 years versus 3.60±2.22 years; P<0.001). Men also had a higher MMSE score than women (17.57±4.41 versus 15.14±3.64; P=0.003). No differences were observed in mean values of ADL and IADL scores. Men had a higher ADAS-cog score than women (40.39±10.27 versus 35.27±8.47; P=0.003). A significant difference was also observed in mean value of CDR score, which was lower in men than in women (1.51±0.65 versus 1.76±0.57; P=0.041). Donepezil was administered less frequently in men than in women (35.14% versus 43.06%; P<0.001). Conversely, rivastigmine was administered more frequently in men (62.16% versus 55.56%; P<0.001). Accordingly, pill administration was more frequent in men than in women (24.32% versus 19.44%; P=0.002). Similarly, transdermal patch administration was more frequent in men than in women (37.84% versus 36.11%; P<0.001, respectively). No difference in frequency of galantamine treatment was observed (P=0.999). APOE genotype and estimated allele frequencies according to sex were evaluated. No significant differences were observed in the distribution of the APOE genotypes and alleles between males and females.
Table 1 Characteristics of patients at baseline according to sex
The clinical evaluation of responder/nonresponder phenotype to AChEI treatment at the 6-month follow-up is summarized in . At follow-up, 68% of patients were responders, and 32% were nonresponders to AChEI treatment. As compared with baseline, according to the NICE criteria,Citation18 responder patients had higher mean MMSE (18.61±4.86 versus 15.78±4.14; P<0.001), ADL (4.42±2.66 versus 3.89±2.00; P=0.001), IADL (2.40±2.68 versus 2.09±2.58; P=0.003), and ADAS-cog (43.36±11.33 versus 36.77±9.64) scores at follow-up. No difference in CDR scores were observed. Conversely, nonresponder patients had lower mean MMSE (12.73±3.90 versus 16.35±3.93; P<0.001), IADL (0.47±0.61 versus 1.18±1.33; P=0.016) and ADAS-cog (29.68±9.10 versus 38.10±9.15; P<0.001) scores at follow-up, whereas no difference in mean ADL score was observed. In these patients, a higher CDR score (2.06±0.77 versus 1.74±0.56; P=0.005) at follow-up was also observed. No differences in age and sex distribution were observed between responder and nonresponder patients.
Table 2 Clinical evaluation of responders/nonresponders to AChEI treatment at the 6-month follow-up
Genotype distribution at FOXO1 locus according to the response to AChEI treatment is summarized in . Two multivariate analyses were performed: the first one included MMSE score at baseline, AChEI treatment, and age and sex as confounding factors; whereas the second one also included APOE genotypes. No associations were observed for rs2721069 and rs4943794. Conversely, a significant association was observed for rs7981045. Genotype G/G was overrepresented in nonresponder as compared with responder patients (17.14% versus 2.70%; P=0.010). This association was present also when age and sex or age, sex, and APOE genotypes were considered as confounding factors (P=0.017 in both cases). Thus, the G/G genotype may be at risk for a poor response to AChEI treatment in the crude analysis (OR =9.429, 95% CI 1.706–52.108) as well as in the adjusted analysis (OR =9.194, 95% CI 1.481–57.086 and OR =10.308, 95% CI 1.510–70.362, respectively).
Table 3 Genotype distribution at FOXO1 locus according to the response to AChEI treatment and results from univariate and multivariate logistic regressions
An evaluation of the relationships between FOXO1 genotypes and the response to AChEI treatment assuming different genetic models of inheritance is summarized in . Assuming a dominant, recessive, or additive model of inheritance, in both univariate and multivariate analyses, the SNPs rs2721069 and rs4943794 did not show significant associations. Conversely, for the SNP rs7981045, assuming a dominant model of inheritance, a significant association was observed when the analysis was adjusted for age and sex (P=0.042) or age, sex, and APOE genotypes (P=0.038). Thus, rs7981045 may be at risk for a poor response to AChEIs in this model when the analysis was adjusted for age and sex (OR =2.398, 95% CI 1.033–5.566) or when the analysis was adjusted for age, sex, and APOE genotypes (OR =2.452, 95% CI 1.055–6.122). Assuming a recessive model of inheritance a significant association was observed in both crude (P=0.018) and adjusted analyses (P=0.027 and P=0.028, respectively), suggesting that rs7981045 may be at risk for a poor response to AChEI in the univariate analysis (OR =7.448, 95% CI 1.420–39.069) as well as in the multivariate analysis considering age and sex (OR =7.675, 95% CI 1.256–48.885) or age, sex, and APOE (OR =8.511, 95% CI 1.265–57.258) as confounding factors. Similarly, assuming an additive model of inheritance, this association was confirmed in both univariate (P=0.011) and multivariate analyses (P=0.012 and P=0.011, respectively), suggesting that rs7981045 may be at risk for a poor response to AChEIs in the crude analysis (OR =2.342, 95% CI 1.217–4.508) as well as in the age- and sex-adjusted analysis (OR =2.367, 95% CI 1.208–4.638) and in the age-, sex- and APOE-adjusted analysis (OR =2.488, 95% CI 1.231–5.028).
Estimated haplotype frequencies at FOXO1 locus according to the response to AChEI treatment are summarized in . In both responders and nonresponders, CGA and CGG haplotypes were the most frequent, followed by haplotypes TCA and TGA. Notably, the most frequent C- haplotypes (CGA + CGG) accounted for about 70% of the overall haplotype frequencies estimated in both responder and nonresponder patients. Haplotype CGA was significantly overrepresented in nonresponder than in responder patients (0.346 versus 0.531; P=0.010). Conversely, CGG haplotype was underrepresented in nonresponder than in responder patients (0.212 versus 0.368; P=0.014). No significant differences were observed for haplotypes TCA and TGA. In both responder and nonresponder patients, a high value of LD coefficient r2 at FOXO1 locus (according to the response to AChEI treatment) was observed between SNPs rs2721069 and rs4943794 (r2 =0.53 and r2 =0.32, respectively). Conversely, a high value of LD coefficient between SNPs rs2721069 and rs7981045 was observed in nonresponder but not in responder patients (r2 =0.17 versus r2 =0.09, respectively) as shown in .
Table 4 Estimated haplotype frequencies at FOXO1 locus according to the response to AChEI treatment
Discussion
The influence of unbalances in biological mechanisms underlying AD pathogenesis, such as oxidative stress, may add an important contribution in considering the overall response to AChEI treatment. Indeed, recent data suggested that AChEIs might act directly on oxidative stress by enhancing antioxidant activity.Citation13 Thus, imbalances in oxidative stress/antioxidant activities may reduce the overall response to AChEIs by two ways. First of all, they may reduce the overall health of cholinergic neurons,Citation35,Citation36 thus limiting their capability to respond to the nervous signal. Second, they may directly contrast the efficacy of AChEI treatment by inducing an increased production of reactive oxygen species.
Personalized medicine is a medical model that proposes the customization of health care – with medical decisions, practices, and/or products being tailored to the individual patient. A method of targeting medication for an individual based on genetic characteristics would enable doctors to prescribe more effectively. Progress in this direction has been much slower than what the initial excitement suggested. A great deal of this delay relates to the fact that an individual’s response to drugs is multifactorial, resulting from multiple gene and environmental interactions.Citation37 Scientists also recognize that even as the knowledge base continues to expand, the clinical translation of that knowledge still requires empirical evidence, generated for a particular disease and drug combination, before treatment can be customized to a patient’s genotype.
In the present study we investigated the FOXO1 gene locus, encoding FOXO1 protein, as a potential genetic factor reducing the overall therapeutic response to AChEIs in patients with sporadic AD.
FOXO1 is one of the fox proteins playing pivotal roles in several human intracellular pathways. These proteins are transcription factors acting as nuclear regulator of transcriptional activity of a number of metabolic processes such as the cellular response to oxidative stress.Citation38 Indeed, it has been recently proposed that FOXO1 protein response to oxidative stress may be linked to AD by means of insulin brain resistance.Citation14 For this reason, in the present study, we enrolled only highly selected AD patients free from any form of insulin metabolism imbalance.
In the present study, we selected three SNPs showing a sufficient level of heterozygosity spanning 65 kb, ie, 60% of the full length of FOXO1 gene, thus suitable for a genetic analysis of FOXO1 locus. In the analysis, we observed a significant association of rs7981045 G/G with a poor response to AChEI treatment and important differences in LD coefficient values among these SNPs producing significant differences in haplotype distribution between responder and nonresponder patients. The correct recruitment of our sample is also warranted by the HWE that was correctly checked for each SNP in both responder and nonresponder groups. Notably, in the evaluation of the APOE polymorphism, we observed a deviation from the HWE in responder patients. This may be a limitation of the study. However the correct frequencies of the APOE polymorphism observed in our AD sample do not differ from those observed in AD Caucasians. Moreover, the association of rs7981045 G/G genotype with a poor response to AChEIs treatment was observed in both crude and APOE-adjusted analyses. In the estimation of the LD coefficients across 65 kb spanning FOXO1, we observed important differences in values of the LD coefficient between the study groups. As expected, these different LD values produced significant differences in the estimated haplotype frequency distribution between responders and nonresponders. Indeed, we observed a haplotype that may be at risk for a poor response to AChEI treatment as well as a haplotype that may be protective against a poor response to AChEI treatment. This condition is expected from a classical haplotype analysis showing both at risk and protective associations, thus confirming the quality of our analysis. We can speculate that the contribution of FOXO1 to the overall response to AChEI treatment may be due to a different genetic background at FOXO1 locus.
Current data on the clinical response to AChEIs in AD patients are not homogeneous, mainly due to the inclusion of patients with different degrees of disease severity, duration of treatments, and criteria to identify responder or nonresponder patients. For these reasons, in this study, we enrolled only highly selected patients with mild to moderate AD who were treated for 6 months, and responders to treatment were defined conservatively according to the NICE criteria as patients who showed improvement or no deterioration in cognition and improvement in functional status.Citation18 After 6 months of treatment with AChEIs, we showed that responder patients reached significant improvements of the clinical assessment, in particular better values of ADL, IADL, and ADAS-cog.
At 6 months we observed response to AChEI treatment in more than 60% of patients. This rate is in agreement with meta-analyses of randomized clinical trials of AChEIs donepezil, rivastigmine, and galantamine reporting 30% to 68% of patients responding to treatment after 6 months of follow-up.Citation6,Citation39 Moreover, these patients showed the typical AD-related APOE genotype distribution. Recent studies suggested that the ε4 allele of the APOE polymorphism seems to improve the responsiveness to specific AChEIs, such as donepezil.Citation40,Citation41 Indeed, we observed significant differences in the distribution of APOE ε2/ε4 and ε3/ε4 genotypes between the study groups. However, these differences may be due to the non-HWE distribution of the APOE genotype frequencies in responder patients. In fact, these differences did not produce differences in the APOE allele distribution. Thus, in agreement with other authors,Citation8,Citation42,Citation43 our study failed to find a significant role of the ε4 allele in improving the clinical response to AChEIs. Multivariate analyses demonstrated that the significant role of FOXO1 polymorphism in influencing the clinical response to donepezil was independent from the age, sex and MMSE score at baseline as well as the APOE polymorphism. In particular, multivariate analysis did not show a significant role of APOE polymorphism in improving clinical responsiveness to donepezil, even after adjustment for sex, age, MMSE at baseline, and FOXO1 genotypes. All these findings suggest that the APOE gene is unrelated to the AChEI metabolism and do not support the hypothesis of a direct interaction between APOE and FOXO1 polymorphisms.
The genotype frequencies of the SNP rs7981045 in the study cohort were comparable to the FOXO1 genotype distribution reported in Caucasians.Citation31 Moreover, the observed genotype frequencies at the FOXO1 and APOE loci did not differ from the expected HWE frequencies nor did they differ after dividing patients according to sex. These conditions minimize the risk of a genetic bias in patient enrollment.
A limitation of this study is the potential lack of generalizability of our findings, since our AD patients were selected according to strict inclusion criteria. Moreover, the large confidence interval associated with the G/G genotype in both crude (range from 1.706 to 52.108) and adjusted analysis (range from 2.510 to 70.362) could reflect imprecise OR values. However, given the high OR values associated with the G/G genotype (9.429 and 10.308 for the crude and the adjusted analysis, respectively), it is difficult to draw negative conclusions.
Clearly, FOXO1 is a minor actor in the theater of the events underlying the response to AChEI treatment since it is not an AChEI-metabolizing enzyme such as cytochrome P450 (CYP) 2D6, which has been demonstrated to play a major role in such response.Citation8–Citation10 Nevertheless, our results, if confirmed on large samples of highly selected patients, may suggest the presence of factors playing a background role in AChEI efficacy, which must be considered in the prediction of the overall clinical response to drug treatments. Further studies are needed to evaluate the role of FOXO1 in the response to AChEI treatment in AD patients with different CYP metabolizer phenotypes.
Acknowledgments
This work was fully supported by “Ministero della Salute”, IRCCS Research Program, Ricerca Corrente 2012–2014, Linea n. 2 “Malattie complesse” and by the “5×1,000” voluntary contribution.
Supplementary materials
Figure S1 Estimated values of linkage disequilibrium coefficient r2 at FOXO1 locus according to the response to AChEI treatment.
Abbreviation: AChEI, acetylcholinesterase inhibitor.
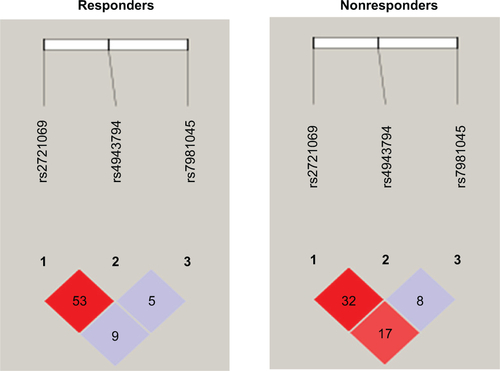
Table S1 Association of FOXO1 genotypes with the response to AChEI treatment assuming different genetic models of inheritance
Disclosure
The authors report no conflicts of interest in this work.
References
- Alzheimer’s Disease Education Referral Center [homepage on the Internet]Bethesda, MDNational Institute on Aging2014 Available from: http://www.nia.nih.gov/alzheimersAccessed March 1, 2014
- alz.org [homepage on the Internet]Chicago, ILAlzheimer’s Association2014 Available from: http://www.alz.orgAccessed March 1, 2014
- HampelHPrvulovicDTeipelSThe future of Alzheimer’s disease: the next 10 yearsProg Neurobiol20119571872822137045
- ImbimboBPPanzaFFrisardiVTherapeutic intervention for Alzheimer’s disease with γ-secretase inhibitors: still a viable option?Expert Opin Investig Drugs201120325341
- PanzaFFrisardiVImbimboBPSeripaDSolfrizziVPilottoAMonoclonal antibodies against β-amyloid (Aβ) for the treatment of Alzheimer’s disease: the Aβ target at a crossroadsExpert Opin Biol Ther20111167968621501112
- BirksJCholinesterase inhibitors for Alzheimer’s diseaseCochrane Database Syst Rev20061CD00559316437532
- AtriAEffective pharmacological management of Alzheimer’s diseaseAm J Manag Care201117suppl 13S346S35522214392
- PilottoAFranceschiMD’OnofrioGEffect of a CYP2D6 polymorphism on the efficacy of donepezil in patients with Alzheimer diseaseNeurology20097376176719738170
- AlbaniDBoneschiFMBiellaGReplication study to confirm the role of CYP2D6 polymorphism rs1080985 on donepezil efficacy in Alzheimer’s disease patientsJ Alzheimers Dis201230474574922465999
- SeripaDBizzarroAPilottoARole of cytochrome P4502D6 functional polymorphisms in the efficacy of donepezil in patients with Alzheimer’s diseasePharmacogenet Genomics20112122523020859244
- von BernhardiREugenínJAlzheimer’s disease: redox dysregulation as a common denominator for diverse pathogenic mechanismsAntioxid Redox Signal201216974103122122400
- CaiZZhaoBRatkaAOxidative stress and β-amyloid protein in Alzheimer’s diseaseNeuromolecular Med20111322325021901428
- KlugmanANaughtonDPIsaacMShahIPetrocziATabetNAntioxidant enzymatic activities in Alzheimer’s disease: the relationship to acetylcholinesterase inhibitorsJ Alzheimers Dis20123046747422451323
- ManolopoulosKNKlotzLOKorstenPBornsteinSRBarthelALinking Alzheimer’s disease to insulin resistance: the FoxO response to oxidative stressMol Psychiatry2010151046105220966918
- World Medical Association [homepage on the Internet]Ferney-Voltaire, FranceWorld Medical Association, Inc2014 Available from: http://www.wma.net/enAccessed March 1, 2014
- European Medicines Agency [homepage on the Internet]London, UKEuropean Medicines Agency2014 Available from: http://www.ema.europa.euAccessed March 1, 2014
- STROBE StatementStrengthening the Reporting of Observational Studies in EpidemiologyBern, SwitzerlandUniversity of Bern, Institute of Social and Preventive Medicine2009 Available from: http://www.strobe-statement.orgAccessed March 1, 2014
- National Institute for Health and Care ExcellenceLondon, UKNational Institute for Health and Care Excellence2014 Available from: http://www.nice.org.ukAccessed March 1, 2014
- FolsteinMFolsteinSMcHughPRMinimental state: a practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res1975121891981202204
- RosenWGMohsRCDavisKLA new rating scale for Alzheimer’s diseaseAm J Psychiatry1984141135613646496779
- HughesCPBergLDanzigerWLCobenLAMartinRLA new clinical scale for the staging of dementiaBr J Psychiatry19821405665727104545
- MorrisJCThe Clinical Dementia Rating (CDR): current version and scoring rulesNeurology199343241224148232972
- SeripaDFranceschiMD’OnofrioGPolymorphism C in the serotonin transporter gene (SLC6A4) in questionable dementia and Alzheimer’s diseaseNeurosci Lett200843833533918490109
- PetersenRCDoodyRKurzACurrent concepts in mild cognitive impairmentArch Neurol2001581985199211735772
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer Disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Service Task Force on Alzheimer’s DiseaseNeurology1984349399446610841
- RománGCTatemichiTKErkinjunttiTVascular dementia: diagnostic criteria for research studies Report of the NINDS-AIREN International WorkshopNeurology1993432502608094895
- HachinskiVCIliffLDZilhkaECerebral blood flow in dementiaArch Neurol1975326326371164215
- KatzSFordABMoskowitzRWJacksonBAJaffeMWStudies of illness in the aged: the index of ADL – a standardized measure of biological and psychological functionJAMA196318591491914044222
- LawtonMPBrodyEMAssessment of older people: self-maintaining and instrumental activities of daily livingGerontologist196991791865349366
- MillerSADykesDDPoleskyHFA simple salting out procedure for extracting DNA from human nucleated cellsNucleic Acids Res19981612153344216
- Database of single nucleotide polymorphisms (dbSNPs) [database on the Internet]Bethesda, MDNational Center for Biotechnology Information2009 Available from: http://www.ncbi.nlm.nih.gov/snpAccessed March 1, 2014
- SeripaDSignoriEGravinaCMateraMGRinaldiMFazioVMSimple and effective determination of apolipoprotein E genotypes by positive/negative polymerase chain reaction productsDiagn Mol Pathol20061518018516932075
- WiggintonJECutlerDJAbecasisGRA note on exact tests of Hardy-Weinberg equilibriumAm J Hum Genet20057688789315789306
- BarrettJCFryBMallerJDalyMJHaploview: analysis and visualization of LD and haplotype mapsBioinformatics20052126326515297300
- GuanZZCross-talk between oxidative stress and modifications of cholinergic and glutaminergic receptors in the pathogenesis of Alzheimer’s diseaseActa Pharmacol Sin20082977378018565274
- KrishnaswamyACooperEReactive oxygen species inactivate neuronal nicotinic acetylcholine receptors through a highly conserved cysteine near the intracellular mouth of the channel: implications for diseases that involve oxidative stressJ Physiol2012590pt 1394721969449
- HagaSBBurkeWUsing pharmacogenetics to improve drug safety and efficacyJAMA2004291232869287115199039
- GrossDNWanMBirnbaumMJThe role of FOXO in the regulation of metabolismCurr Diab Rep2009920821419490822
- HansenRAGartlehnerGLohrKNKauferDIFunctional outcomes of drug treatment in Alzheimer‘s disease: A systematic review and meta-analysisDrugs Aging20072415516717313203
- BizzarroAMarraCAcciarriAApolipoprotein E ε4 allele differentiates the clinical response to donepezil in Alzheimer’s diseaseDement Geriatr Cogn Disord20052025426116103669
- ChoiSHKimSYNaHREffect of ApoE genotype on response to donepezil in patients with Alzheimer’s diseaseDement Geriatr Cogn Disord20082544545018401173
- RigaudASTraykovLLatourFPresence or absence of at least one ε4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer’s diseasePharmacogenetics20021241542012142731
- SantoroASivieroPMinicuciNEffects of donepezil, galantamine and rivastigmine in 938 Italian patients with Alzheimer’s disease: a prospective, observational studyCNS Drugs20102416317620088621
