Abstract
With the practice-shifting changes made with the most recent guidelines for treating blood cholesterol, more older patients may be prescribed statin therapy. Therefore, it is imperative that practitioners have not only a working knowledge of information related to statins, but more specifically to their efficacy and safety in elderly populations. Pitavastatin is the most recent statin to receive regulatory approval. It is indicated for the treatment of primary hyperlipidemia or mixed dyslipidemia as an adjunctive therapy to diet. The overall body of evidence for the efficacy and safety of pitavastatin in elderly patients is small. The available data suggest that the ability of pitavastatin to lower low-density lipoprotein cholesterol in elderly patients is at least similar, and may be greater than that seen in comparatively younger cohorts. Taken together, the limited available data suggest that pitavastatin is effective at improving lipid parameters in elderly patients with a similar safety profile to other agents in the class. Until data become available distinguishing pitavastatin from the other available options, its ultimate role in the hyperlipidemia treatment armamentarium remains unclear.
Keywords:
Introduction to management of hypercholesterolemia in elderly patients
It has been well established that elevated serum cholesterol levels are associated with increasing cardiovascular risk as the population ages.Citation1–Citation3 Meta-analyses have suggested significantly higher cardiovascular mortality rates in older individuals compared with their younger cohorts, with a 10-fold higher risk seen with every 38.6 mg/dL increase in total cholesterol (TC) seen.Citation2 Trials with statins in older patients (>65 years of age) with clinical atherosclerotic cardiovascular disease show significant reductions in total mortality (relative risk 0.78, 95% confidence interval 0.65 to 0.89).Citation4 Thus, prescribing cholesterol-lowering therapy in the form of statins to reduce cardiovascular risk in older patients is of considerable benefit. With the practice-shifting changes made with the most recent American College of Cardiology/American Heart Association guidelines for treating blood cholesterol, more elderly patients may be prescribed statin therapy.Citation5 These guidelines expand the use of statins for primary prevention; a change that is most notable in older persons. Statins are recommended in patients who have 10-year atherosclerotic cardiovascular disease risk of ≥7.5%.Citation6 This means that most men over 60 years and women over 70 years of age will be eligible to receive statin therapy.Citation5 Of concern to some is the safety of cholesterol-lowering drug therapy in the aging population; an issue likely to increase in prevalence with these expanded use guideline recommendations. This can be particularly troublesome with the development of frailty which increases patients’ susceptibility to adverse events.Citation1,Citation6 Therefore, it is imperative that practitioners have not only a working knowledge of information related to statins, but more specifically to their efficacy and safety in older populations.
Pitavastatin (Livalo®; Kowa Pharmaceuticals America, Montgomery, AL, USA) is the most recent statin to receive regulatory approval. It is indicated for the treatment of primary hyperlipidemia or mixed dyslipidemia as an adjunctive therapy to diet.Citation7,Citation8 The aim of this article is to review the pharmacologic, pharmacokinetic, efficacy, and safety data of pitavastatin specifically in older populations.
Review of pharmacology, mode of action, and pharmacokinetics of pitavastatin in the elderly
Pharmacology of pitavastatin
Pitavastatin mimics other statins by inhibiting HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase to reduce low-density lipoprotein cholesterol (LDL-C), elevated TC, Apo B, and triglycerides (TG) and to increase high-density lipoprotein cholesterol (HDL-C).Citation7 In contrast with other statins, pitavastatin contains a cyclopropyl group that fits within the hydrophobic areas of the HMG-CoA reductase enzyme, partially explaining its potent inhibitory activities.Citation8 Studies have described pitavastatin to have a 2.4- to 6.8-fold greater potency than simvastatin and pravastatin, respectively.Citation9 Further, pitavastatin inhibits cholesterol synthesis greater than simvastatin (2.9-fold) and atorvastatin (5.7-fold), and its inhibitory effects on sterol synthesis appear to be liver selective and significantly stronger than simvastatin.Citation10,Citation11
Pharmacokinetics of pitavastatin
Pitavastatin is a potent and synthetic HMG-CoA reductase inhibitor, with a structure especially similar to fluvastatin and rosuvastatin.Citation12 Comparison of pharmacokinetic attributes () shows that pitavastatin has the highest bioavailability amongst all statins, and that it reaches peak plasma concentration (Cmax) within 1 hour after oral administration, the fastest of the seven approved statins in the United States.Citation8,Citation13 Administration of pitavastatin with a high-fat meal decreases the Cmax by 43%; however, the area under the curve (AUC) is not significantly reduced.Citation7 Further, the AUC of pitavastatin remains unchanged whether it is administered in the morning or evening.Citation7
Table 1 Pharmacokinetic characteristics of statinsCitation8,Citation13
Pitavastatin is highly protein bound, more than 99%, predominantly to albumin and alpha1-acid glycoprotein.Citation7 Pitavastatin is rapidly metabolized primarily via hepatic glucuronidation with UDP glucuronyltransferase (UGT1A3 and UGT2B7), forming the major inactive metabolite – pitavastatin lactone – with minimal CYP2C9 and 2C8 metabolism of clinical concern.Citation13 Pitavastatin is rapidly taken up into the liver through OATP, including OATP1B1 and OATP2.Citation14,Citation15 The 24-hour AUC of pitavastatin was increased 4.6-fold when it was concurrently administered with a potent OATP2 inhibitor (eg, cyclosporine), and is thought to be the major rate-limiting step in the hepatobiliary clearance of pitavastatin.Citation16 The plasma elimination half-life of pitavastatin is between 11 and 12 hours, with approximately 15% of the dose excreted in the urine, and a mean of 79% of the dose excreted in the feces within 7 days.Citation7,Citation13
To date, pharmacokinetic studies have not demonstrated clinically significant differences based either on sex (men versus [vs] women) or age (younger vs elderly, age >65 years). Morgan et al studied the effects of severe renal impairment not on hemodialysis on pitavastatin metabolism, transport, and clearance.Citation17 The study looked at age, body mass index, and sex-matched healthy adult subjects, using an US Food and Drug Administration (FDA)-approved cut-off of “healthy” estimated GFR of ≥80 mL/min/1.73 m2 to align the study with a previous pitavastatin analysis conducted in Europe.Citation17 Elevations in AUC and Cmax were not found to be significantly different from their healthy comparators. They concluded that the relationship between renal function and pitavastatin exposure may not be evident, partly due to limited urinary excretion, though they admitted that there was a small sample size (n=16) and single dosing (4 mg) in determining that pitavastatin was safe and well tolerated in patients with severe renal impairment not on hemodialysis.Citation17
Pharmacokinetic studies have not demonstrated clinically significant differences based either on age (younger vs elderly – age >65 years) or sex (men vs women).Citation7 Significant increases in Cmax have been seen in cirrhotic patients when compared with healthy subjects.Citation18 Similarly, the elimination half-life was 8.3 hours and 14.4 hours in patients with Child-Pugh A and Child-Pugh B classes, respectively.
Efficacy studies of pitavastatin
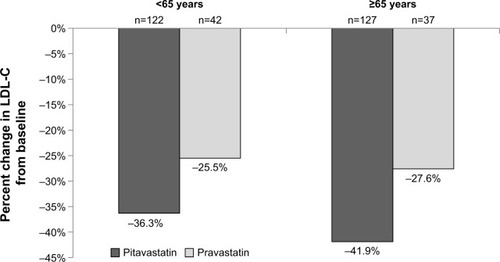
Information on the lipid-lowering efficacy of pitavastatin in elderly patients comes either from subgroup analysis of age-diverse studiesCitation19,Citation20 or those performed specifically in older adults.Citation21–Citation23 A Phase 3, randomized, double-blind, parallel-group, active-controlled study of 355 patients by Eriksson et al compared pitavastatin (4 mg/day) to simvastatin (40 mg/day) over a 12-week period.Citation19 Patients ≥65 years of age represented 23% of the population. While no significant difference in percent lowering of LDL-C from baseline was seen between pitavastatin (−44%) and simvastatin (−42%) in those <65 years, a greater reduction was shown with simvastatin (−48%) vs pitavastatin (−43%; P=0.024) in those ≥65 years of age although the overall reduction between age groups was similar.Citation19 A recently published study by Sponseller et al randomized 328 subjects with primary hyperlipidemia or mixed (combined) dyslipidemia to either pitavastatin 4 mg/day or pravastatin 40 mg/day for 12 weeks.Citation20 The reduction in LDL-C from baseline in the overall population was −38.1% with pitavastatin and −26.4% with pravastatin (P<0.001). In subjects <65 years of age, the median percent change in LDL-C from baseline was −36.3% with pitavastatin and −25.5% with pravastatin while the reduction in those ≥65 years of age was −41.9% with pitavastatin and −27.6% for pravastatin (). The exact rationale for the greater response in the older population is unknown. Whether differences in baseline LDL-C concentrations could have resulted in varying reductions is also not clear. The magnitude of within-treatment effect for pravastatin was similar between those < or ≥65 years of age, the age effect on reduction in LDL-C was greater for pitavastatin vs simvastatin (Pinteraction =0.02).Citation20
Figure 1 Percent LDL-C lowering ability of pitavastatin versus pravastatin by age.
Abbreviation: LDL-C, low-density lipoprotein cholesterol.
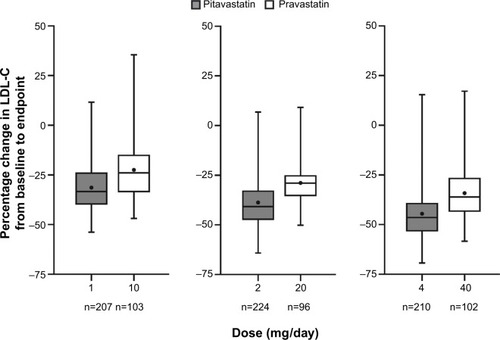
The efficacy of pitavastatin exclusively in a population ≥65 years of age was evaluated by Stender et al in 942 patients with primary hypercholesterolemia or combined (mixed) dyslipidemia.Citation21 Participants, who had a mean age of 70 years (range 65–89), were randomized to receive either pitavastatin (1, 2, or 4 mg/day) or pravastatin (10, 20, or 40 mg/day) for 12 weeks in a double-blind, double-dummy fashion. The primary efficacy outcome was the percent change in LDL-C from baseline with analyses performed to demonstrate the noninferiority of pitavastatin versus pravastatin. Additional lipoprotein parameters of interest included changes in TC, HDL-C, TG, and Apo-B. The following dosage comparisons were made: pitavastatin 1 mg vs pravastatin 10 mg; pitavastatin 2 mg vs pravastatin 20 mg; and pitavastatin 4 mg vs pravastatin 40 mg. The mean baseline LDL-C across the various dosing groups ranged from 163 mg/dL to 167 mg/dL. The mean decrease in LDL-C from baseline decreased across all doses in both arms in a dose-related fashion (). The percent LDL-C reduction was approximately 10% greater with pitavastatin versus pravastatin, which met the criteria for noninferiority (P<0.001). Pitavastatin nearly uniformly improved secondary outcome parameters vs pravastatin, including TC, HDL-C (except pitavastatin 1 mg vs pravastatin 10 mg; P=0.425), TG (except pitavastatin 2 mg vs pravastatin 20 mg; P=0.063), Apo-B, and non-HDL-C (P<0.05 for all, except where specified).
Figure 2 Change in LDL-C with pitavastatin versus pravastatin in patients aged ≥65 years.
Abbreviation: LDL-C, low-density lipoprotein cholesterol.
The same author group concurrently published results of an open-label, 60-week extension study for those who had completed the 12-week core study.Citation22 All patients, irrespective of their original randomization group, were started on open-label pitavastatin 2 mg/day, then up-titrated to 4 mg/day after 8 weeks if they did not attain their LDL-C target. The specific LDL-C target was not provided, but was based on the individual patients’ risk and followed the National Cholesterol Education Program Adult Treatment Panel III guidelines. Following 60 weeks of pitavastatin treatment, the LDL-C was 43% lower than at baseline of the 12-week core study.Citation21 All other secondary lipid parameters were also lower after 60 weeks compared with baseline. While 85.8% and 72.9% of pitavastatin and pravastatin (respectively) patients had attained their LDL-C goals after the 12-week core study,Citation21 the value increased to 93.8% after 60 weeks of pitavastatin.Citation22
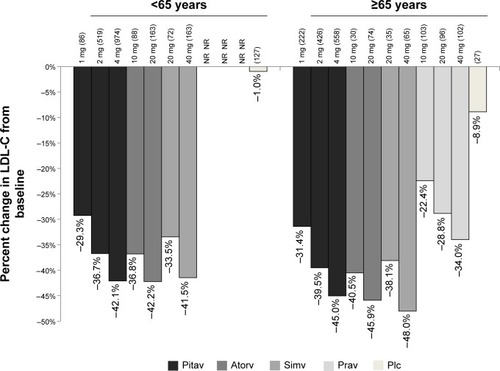
Comparative efficacy information for pitavastatin as well as other statins in patients <65 years and ≥65 years of age was included when the agent was submitted for approval to the FDA.Citation23 The percent LDL-C reductions in LDL-C for patients in the ≥65 versus <65 years groups are shown in .Citation23 It should be noted that direct statistical comparisons between these groups were not conducted, nor inferred.
Figure 3 Mean percent LDL-C lowering ability of statins by age.
Abbreviations: Atorv, atorvastatin; Pitav, pitavastatin; Plc, placebo; Prav, pravastatin; Simv, simvastatin; LDL-C, low-density lipoprotein cholesterol; NR, not reported.
Information on the non-lipid characteristics of pitavastatin in elderly patients is also available. The effect of pitavastatin on prevention of atrial fibrillation as well as cardiac structure and function was evaluated in 220 patients ≥65 years of age.Citation24 Individuals with hypertension and established left-ventricular (LV) hypertrophy and preserved systolic function (LV ejection fraction ≥50%) were included. Patients who required lipid lowering therapy (n=110) according to Japanese guidelines were started on pitavastatin (1–2 mg/day) in addition to antihypertensive treatment, while the control group (n=110) continued on their baseline antihypertensive regimens. Treatment and follow-up was continued for a 1-year period. All baseline clinical, laboratory, and echocardiographic parameters were similar between the two groups, with the exception of LDL-C. Patients receiving pitavastatin had a significantly lower rate of developing new-onset atrial fibrillation (5/110, 4.5%) than the non-statin group (15/110, 13.6%; P=0.019). In a multivariate Cox regression model, pitavastatin use (P=0.002) and an echocardiographic finding (left atrial peak strain; P=0.013) were independent predictors of reduced atrial fibrillation risk while prior coronary artery disease (P<0.001) was associated with elevated risk. Moreover, pitavastatin use resulted in a 6% reduction in LV mass index from baseline with no difference seen in the non-statin group (P<0.05). This study shows that pitavastatin not only reduces lipid parameters, but also improves echocardiographic parameters in patients with hypertension and LV hypertrophy and lowers their risk of developing new-onset atrial fibrillation.
Safety and tolerability of pitavastatin in elderly patients
The manufacturer reports that of the 2,800 patients randomized to pitavastatin 1 mg to 4 mg in controlled clinical studies, 1,209 (43%) were ≥65 years of age. No significant differences in efficacy or safety were observed between elderly patients and younger patients when pitavastatin was approved by the FDA.Citation7
Stender et al reported that 82% of pitavastatin patients in their 60-week open-label extension trial developed adverse events; however, only 14.3% of these were deemed to be specifically related to drug treatment.Citation22 The most common treatment-related adverse events included myalgia (2.6%), elevated serum creatine kinase (1.9%), and nausea (1.5%). Fewer than 7% of patients discontinued pitavastatin therapy because of the adverse events. Two patients died during the study, although neither death was attributed to pitavastatin therapy.
Stender et al reported 48 cases of myalgias; however, only 14 of these were considered treatment related, and none were severe (21 were moderate, 27 were mild).Citation22 Only four patients discontinued pitavastatin due to myalgia, and no cases of myopathy, myositis, or rhabdomyolysis were identified.
Patients receiving pitavastatin 4 mg/day had a higher incidence of elevations in liver enzymes (AST and ALT) by week 60.Citation22 One patient had an AST elevation three times the upper limit of normal (ULN), and one more than five times the ULN. Two patients had ALT increases more than three times the ULN, one more than five, and one more than ten. Two patients were said to have discontinued the study due to increases in ALT and AST, both of which were assessed as mild intensity and only one identified as treatment-related. Taken together, despite elevations in liver function tests (LFTs) in a handful of patients, significant liver injury requiring treatment discontinuation was rare.
Other studies have included elderly patients in their assessments of pitavastatin safety and tolerability; however, limited subgroup analysis data exist specific to those patients aged 65 years and older.Citation25–Citation27 The significant majority of adverse events were reported as mild and less frequent than with other potent statins, and compare similarly to the data from Stender et al.Citation22
Older patients who have been deemed frail may be at increased risk for adverse events.Citation1 This includes individuals with a combination of weakness, slowness, exhaustion, inactivity, and shrinking.Citation1 Unfortunately, data on the safety of pitavastatin in frail older patients are lacking and requires additional study.
Patient focused perspectives (quality of life, patient satisfaction, and acceptability/adherence)
Information on the impact of pitavastatin on humanistic outcomes, such as health-related quality of life, patient satisfaction, and treatment adherence is limited. No data on the impact of pitavastatin on health-related quality of life could be found. Patient satisfaction was evaluated in a Spanish study of 6,489 patients from primary or specialized health clinics in Spain who had been using pitavastatin for at least 12 weeks.Citation28 The mean age of the population was 60.9±11.2 years, with no age-related subgroup data provided. Patients who completed their prescribed treatment course (65% of the included population) showed improved satisfaction with the drug and with the drug efficacy than those who did not finish the treatment (P<0.001 for both). Treatment compliance in elderly patients was reported in an extension study by Stender et al.Citation22 Compliance, defined as taking between 80%–120% of the prescribed treatment regimen, was achieved in 97.5% of the 60-month follow-up population.
Conclusions, benefit–risk assessment
The overall body of evidence for the efficacy and safety of pitavastatin in elderly patients is small. The available data suggest that the ability of pitavastatin to lower LDL-C in elderly patients is at least similar, and may be greater than that seen in comparatively younger cohorts. A similar trend was seen with other statins, including atorvastatin, pravastatin, and simvastatin. Percent reductions ranging from 31%–45% were seen with pitavastatin compared with 22%–34% with pravastatin, 38%–48% with simvastatin, and 40%–46% with atorvastatin in patients ≥65 years of age.Citation23 Studies using rosuvastatin 10 mg/day in patients ≥65 years of age have shown 50% reductions in LCL-C from baseline, proportionally greater than pitavastatin.Citation29 Real-world studies of elderly patients have shown rosuvastatin to result in greater LDL-C reductions from baseline compared with other drugs in the class, including atorvastatin, pravastatin, and simvastatin.Citation30 Pitavastatin was not available at the time of this investigation; thus, the comparative efficacy of it with rosuvastatin is not currently known.
Clinical trials have shown that statins significantly reduce all-cause mortality, nonfatal myocardial infarction, and stroke in elderly patients.Citation4 The Study Assessing Goals in the Elderly (SAGE) of patients 66–85 years of age showed that more aggressive lipid lowering with atorvastatin reduced cholesterol and mortality to a greater extent than moderate intensity therapy with pravastatin.Citation31 Unfortunately, data on the impact of pitavastatin on terminal outcomes such as mortality and myocardial infarction are not available. Pitavastatin has been shown to have similar pleiotropic effects to other statins, including decreasing platelet activation and improving cardiac and endothelial function.Citation32 Thus, it is plausible that its use may confer similar clinical benefits, but these have yet to be elucidated. It should, therefore, be selected based on its lipid lowering ability and safety profile alone.
The safety of statins in elderly patients is a question frequently encountered by clinicians and patients alike. One of the biggest concerns, from a patient perspective, is the risk of statins adversely affecting cognition. A systematic review suggested that no causal link existed between statin use and development of Alzheimer disease or dementia.Citation33 Similarly, no decline in cognitive performance was seen with statin use. A task force commissioned by the National Lipid Association concluded that no association with the statin class and adverse effects on cognition are known and routine screening should not be performed.Citation34 While no information on the specific effects of pitavastatin on cognition in elderly patients is currently available, this adverse effect should not be of concern.
Muscle issues have also been a concern with statins.Citation35 Meta-analyses of observational studies in general populations have reported greater than a two-fold increase in the odds of myopathy with statin use (odds ratio 2.63, 95% confidence interval 1.50 to 4.61).Citation35 While statin-associated myalgia may be difficult to differentiate from that caused by other sources, tests can be run on individuals who have exhibited symptoms to provide a definitive diagnosis.Citation36 Stender et al reported that myalgia (2.6%) and increased serum creatine kinase (1.9%) did occur in elderly patients receiving pitavastatin.Citation22 This is compared with an approximate 3% incidence with moderately-potent comparators.Citation23
Liver toxicity is another safety issue routinely encountered with statin use.Citation35 Few of the elderly patients given pitavastatin by Stender et al exhibited signs or symptoms suggestive of liver damage.Citation22 Elevations in LFTs are seen at a similar rate between pitavastatin and other agents in the class.Citation23 While LFTs are not recommended to be routinely screened following statin initiation, they can be checked in individuals with symptoms suggestive of liver damage.Citation37
Taken together, the limited available data suggest that pitavastatin is effective at improving lipid parameters in elderly patients with a similar safety profile to other agents in the class. Current guidelines recommend its use when moderate-intensity statin therapy is indicated.Citation6 A drawback to its use is likely to be its cost. Since pitavastatin is still available only as a branded product in the United States, other agents with similar efficacy and safety and lower cost are likely to be used with greater frequency. Until data become available distinguishing pitavastatin from the other available options, its ultimate role in the hyperlipidemia treatment armamentarium remains unclear.
Disclosure
The authors declare no relevant conflicts of interest.
References
- StrandbergTEKolehmainenLVuorioAEvaluation and treatment of older patients with hypercholesterolemia: a clinical reviewJAMA2014312111136114425226479
- Prospective Studies Collaboration; LewingtonSWhitlockGBlood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deathsLancet200737096021829183918061058
- SchatzIJMasakiKYanoKCholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort studyLancet2001358927935135511502313
- AfilaloJDuqueGSteeleRStatins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysisJ Am Coll Cardiol2008511374518174034
- StoneNJRobinsonJGLichtensteinAH2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risks in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation201412925 Suppl 2S1S4524222016
- CleggAYoungJLliffeSFrailty in elderly peopleLancet2013381986875276223395245
- Livalo® (pitavastatin) [package insert]Montgomery, ALKowa Pharmaceuticals America, Inc2013
- BakerWLDattaRPitavastatin: a New 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitor for the Treatment of HyperlipidemiaAdv Ther2011281132721170619
- AokiTNishimuraHNagakawaSPharmacologic profile of a novel synthetic inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductaseArzneimittelforschung19974789049099296275
- MorikawaSUmetaniMNakagawaSRelative induction of mRNA for HMG CoA reductase and LDL receptor by five different HMGCoA reductase inhibitors in cultured human cellsJ Atheroscler Thromb20007313814411480454
- SuzukiHAokiTTamakiTHypolipidemic effect of NK-104, a potent HMG-CoA reductase inhibitor, in guinea pigsAtherosclerosis1999146225927010532682
- SaitoYCritical appraisal of the role of pitavastatin in treating dyslipidemias and achieving lipid goalsVasc Health Risk Manag2009592193619997573
- AlagonaPPitavastatin: evidence for its place in treatment of hypercholesterolemiaCore Evid201059110521468365
- ShimadaSFujinoHHojimaJMoriyasuMKojimaJUptake mechanism of pitavastatin, a new inhibitor of HMG-CoA reductase, in rat hepatocytesDrug Metab Pharmacokinet200318424525115618742
- HiranoMMaedaKShitaraYSugiyamaYContribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humansJ Pharmacol Exp Ther2004311113914615159445
- HasanumaTNakamuraMYajiTThe drug-drug interactions of pitavastatin (NK-104), a novel HMG-CoA reductase inhibitor and cyclosporineJ Clin Therap Med2003194381389
- MorganRECampbellSEYuCYSponsellerCAMusterHAComparison of the safety, tolerability, and pharmacokinetic profile of a single oral dose of pitavastatin 4 mg in adult subjects with severe renal impairment not on hemodialysis versus healthy adult subjectsJ Cardiovasc Pharmacol2012601424822472908
- HuiCKCheungMYLauGKPharmacokinetics of pitavastatin in subjects with Child-Pugh A and B cirrhosisBr J Clin Pharmacol200459329129715752374
- ErikssonMBudinskiDHounslowNComparative efficacy of pitavastatin and simvastatin in high-risk patients: a randomized controlled trialAdv Ther201128981182321874538
- SponsellerCAMorganREKryzhanovskiVACampbellSEDavidsonMHComparison of the lipid-lowering effects of pitavastatin 4 mg versus pravastatin 40 mg in adults with primary hyperlipidemia or mixed (combined) dyslipidemia: a phase IV, prospective, US, multicenter, randomized, double-blind, superiority trialClin Ther20143681211122224998014
- StenderSBudinskiDGoshoMHounslowNPitavastatin shows greater lipid-lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolemia or combined (mixed) dyslipidemiaEur J Prev Cardiol2013201405322679249
- StenderSBudinskiDHounslowNPitavastatin demonstrates long-term efficacy, safety and tolerability in elderly patients with primary hypercholesterolemia or combined (mixed) dyslipidaemiaEur J Prev Cardiol2013201293922345687
- US Food Drug AdministrationClinical Review PitavastatinCenter for Drug Evaluation and Research2009 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdfAccessed January 29, 2015
- WaritaSKawasakiMTanakaREffect of pitavastatin on cardiac structure and function and on prevention of atrial fibrillation in elderly hypertensive patients. A prospective study of 2-years’ follow-upCirc J201276122755276222878405
- OseLBudinskiDHounslowNArnesonVComparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemiaCurr Med Res Opin200925112755276519785568
- OseLBudinskiDHounslowNArnesonVLong-term treatment with pitavastatin is effective and well tolerated by patients with primary hypercholesterolemia or combined dyslipidemiaAtherosclerosis2010210120220820080236
- GumprechtJGoshoMBudinskiDHounslowNComparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemiaDiabetes Obes Metab20111311047105521812889
- Rodriquez ArroyoLADiaz RodriquezAPinto SataXCoca PaverasARius TaruellaJEffectivity and satisfaction with the treatment of dyslipidemia with pitavastatin. Multicentric, descriptive, post authorized and observational study (REINA study)Clin Investig Arterioscler2014265205217 Spanish
- BlasettoJWSteinEABrownWVChitraRRazaAEfficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groupsAm J Cardiol2003915A3C10C
- HarleyCRGandhiSBlasettoJLow-density lipoprotein cholesterol (LDL-C) levels and LDL-C goal attainment among elderly patients treated with rosuvastatin compared with other statins in routine clinical practiceAm J Geriatr Pharmacother20075318519417996658
- DeedwaniaPStonePHBairey MerzCNEffects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE)Circulation2007115670070717283260
- DavignonJPleiotropic effects of pitavastatinBr J Clin Pharmacol2011734S18S35
- RichardonKSchoenMFrenchBStatins and cognitive function: a systematic reviewAnn Intern Med20131591068869724247674
- Rojas-FernandezCHGoldsteinLBLeveyAITaylorBABittnerVThe National Lipid Association’s Safety Task ForceAn assessment by the Statin Cognitive Safety Task Force: 2014 updateJ Clin Lipidol201483 SupplS5S1624793442
- MacedoAFTaylorFCCasasJPAdlerAPrieto-MerinoDEbrahimSUnintended effects of statins from observational studies in the general population: systematic review and meta-analysisBMC Med2014225124655568
- RosensenRSBakerSKJacobsonTAKopeckySLParkerBAThe National Lipid Association’s Muscle Safety Expert PanelAn assessment by the Statin Muscle Safety Task Force: 2014 updateJ Clin Lipidol201483 SupplS58S7124793443
- BaysHCohenDEChalasaniNHarrisonSAThe National Lipid Association’s Statin Safety Task ForceAn assessment by the Statin Liver Safety Task Force: 2014 updateJ Clin Lipidol201483 SupplS47S5724793441
